The Search for Sustainable Subsurface Habitats on Mars, and the Sampling of Impact Ejecta
Abstract
:1. Introduction
The Subsurface as a Protected Haven for Life
2. Earth
2.1. The Deep Subsurface Biosphere on Earth
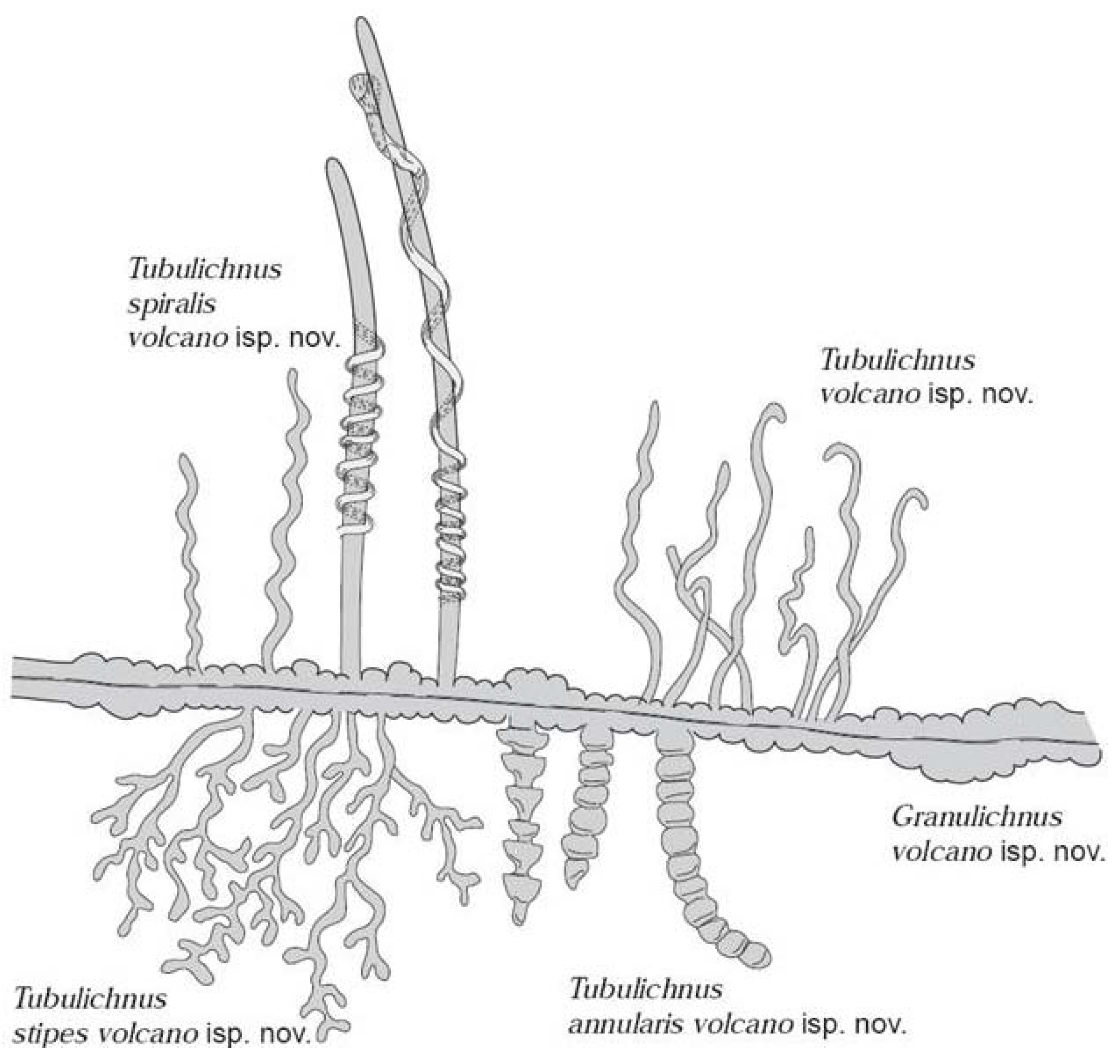
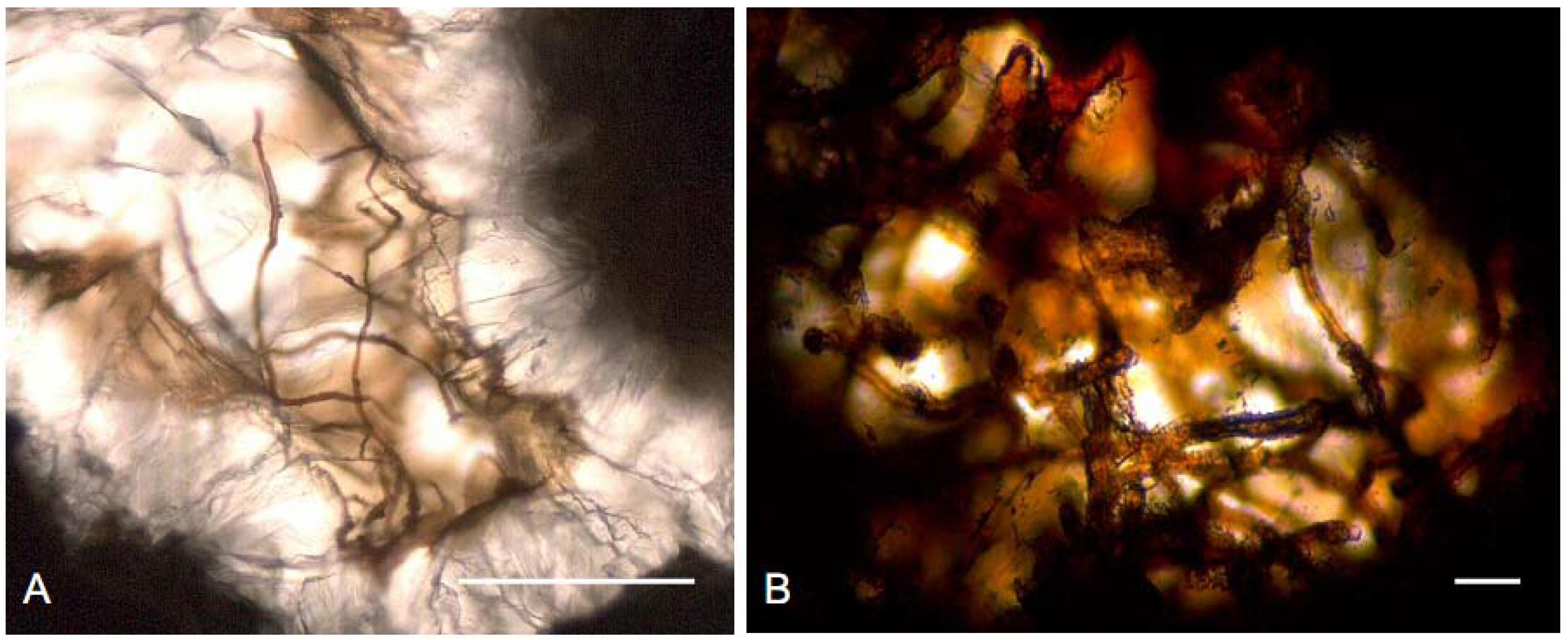
2.2. Sustainability of the Deep Biosphere
2.3. Endoliths
3. Mars
3.1. The Martian Subsurface
3.2. Hydrothermal Activity on Mars
3.3. Sustainability of a Potential Subsurface Biosphere on Mars
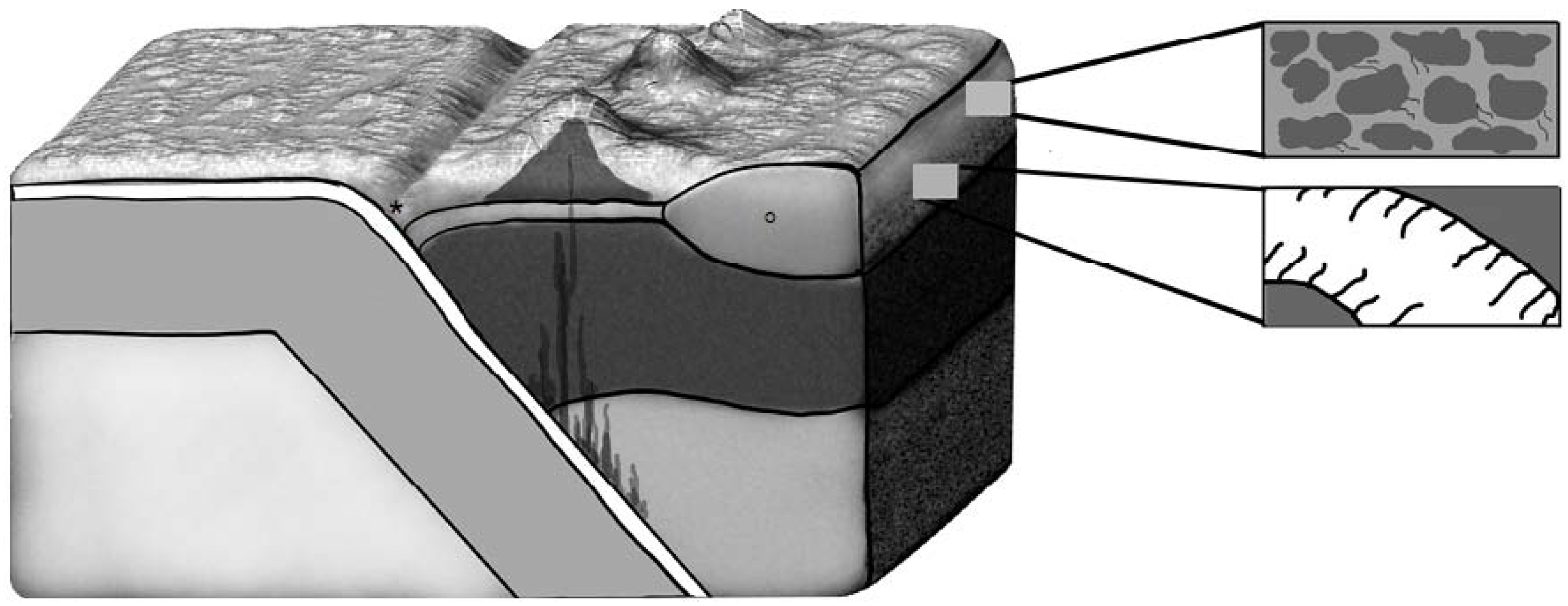
4. Impact Deposits: A Window to the Subsurface

Acknowledgement
References
- Allwood, A.C.; Walter, M.R.; Kamber, B.S.; Marshall, C.P.; Burch, I.W. Stromatolite reef from the Early Archaean era of Australia. Nature 2006, 441, 714–718. [Google Scholar] [CrossRef]
- Furnes, H.; Banerjee, N.R.; Muehlenbachs, K.; Staudigel, H.; de Wit, M. Early life recorded in Archean pillow lavas. Science 2004, 304, 578–581. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Yamada, K.; Yoshida, N.; Maruyama, S.; Isozaki, Y. Evidence from fluid inclusions for microbial methanogenesis in the early Archaean era. Nature 2006, 440, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Rosing, M.T. C-depleted carbon microparticles in >3700-Ma sea-floor sedimentary rocks from west Greenland. Science 1999, 283, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Han, T.M.; Runnegar, B. Megascopic eukaryoticalgae from the 2.1-billion-year-old Negaunee iron-formation, Michigan. Science 1992, 257, 232–235. [Google Scholar]
- Schneider, D.A.; Bickford, M.E.; Cannon, W.F.; Schulz, K.J.; Hamilton, M.A. Age of volcanic rocks and syndepositional iron formations, Marquette Range Supergroup: Implications for the tectonic setting of paleoproterozoic iron formations of the Lake Superior region. Can. J. Earth Sci. 2002, 39, 999–1012. [Google Scholar] [CrossRef]
- Holland, H.D. The oxygenation of the atmosphere and oceans. Phil. T. Roy. Soc. B. 2006, 361, 903–915. [Google Scholar] [CrossRef]
- Cockell, C.S.; Kaltenegger, L.; Raven, J.A. Cryptic photosynthesis-extrasolar planetary oxygen without a surface biological signature. Astrobiology 2009, 9, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Cockell, C.H.; Barlow, N.G. Impact excavation and the search for subsurface life on Mars. Icarus 2002, 155, 340–349. [Google Scholar] [CrossRef]
- Trimarco, E.; Balkwill, D.; Davidson, M.; Onstott, T.C. In situ enrichment of a diverse community of bacteria from 4–5 km deep fault zone in South Africa. Geomicrobiol. J. 2006, 23, 463–473. [Google Scholar] [CrossRef]
- Santelli, C.M.; Orcutt, B.N.; Banning, E.; Bach, W.; Moyer, C.L.; Sogin, M.L.; Staudigel, H.; Edwards, K.J. Abundance and diversity of microbial life in ocean crust. Nature 2008, 453, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Furnes, H.; McLoughlin, N.; Muehlenbachs, K.; Banerjee, N.; Staudigel, H.; Dilek, Y.; de Wit, M.; Van Kranendonk, M.; Schiffman, P. Oceanic pillow lavas and hyaloclastites as habitats for microbial life through time—A review. In Links between Geological Processes, Microbial Activities and Evolution of Life; Dilek, Y., Furnes, H., Muehlenbachs, K., Eds.; Springer: Berlin, Germany, 2008; pp. 1–68. [Google Scholar]
- Rasmussen, B. Filamentous microfossils in a 3,235-million-year-old volcanogenic massive sulphide deposit. Science 2000, 405, 676–679. [Google Scholar]
- Sleep, N.H.; Zahnle, K. Refugia from asteroid impacts on early Mars and the early Earth. J. Geophys. Res. 1998, 103, 28529–28544. [Google Scholar] [CrossRef]
- Farmer, J.D.; Des Marais, D.J. Exploring for a record of ancient Martian life. J. Geophys. Res. 1999, 104, 26977–26995. [Google Scholar] [CrossRef] [PubMed]
- Kminek, G.; Bada, J.L. The effect of ionizing radiation on the preservation of amino acids on Mars. Earth Planet Sci. Lett. 2006, 245, 1–5. [Google Scholar] [CrossRef]
- Dartnell, L.R.; Desorgher, L.; Ward, J.M.; Coates, A.J. Martian sub-surface ionising radiation: Biosignatures and geology. Biogeosciences 2007, 4, 545–558. [Google Scholar] [CrossRef] [Green Version]
- Lammer, H.; Lichtenegger, H.I.M.; Kolb, C.; Ribas, I.; Guinan, E.F.; Abart, R.; Bauer, S.J. Loss of water from Mars: Implications for the oxidation of the soil. Icarus 2003, 165, 9–25. [Google Scholar] [CrossRef]
- Zacny, K.; Bar-Cohen, Y.; Brennan, M.; Briggs, G.; Cooper, G.; Davis, K.; Dolgin, B.; Glaser, D.; Glass, B.; Gorevan, S.; Guerrero, J.; McKay, C.; Paulsen, G.; Stanley, S.; Stoker, C. Drilling systems for extraterrestrial subsurface exploration. Astrobiology 2008, 8, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Farmer, J.D. Hydrothermal systems on Mars: An assessment of present evidence. In Evolution of Hydrothermal Ecosystems on Earth (and Mars?); Bock, G.R., Goode, J.A., Eds.; Wiley and Sons: Chichester, UK, 1996. [Google Scholar]
- Bowden, S.A.; Court, R.W.; Milner, D.; Baldwin, E.C.; Lindgren, P.; Crawford, I.A.; Parnell, J.; Burchell, M.J. The thermal alteration by pyrolysis of the organic component of small projectiles of mudrock during capture at hypervelocity. J. Anal. Appl. Pyrol. 2008, 82, 312–314. [Google Scholar] [CrossRef]
- Lindgren, P.; Parnell, J.; Bowden, S.A.; Taylor, C.; Osinski, G.; Lee, P. Preservation of biological markers in clasts within impact melt breccias from the Haughton impact structure, Devon Island. Astrobiology 2009, 9, 391–400. [Google Scholar] [CrossRef] [PubMed]
- The MEPAG next decade science analysis group. Science priorities for Mars sample return. Astrobiology 2008, 8, 489–535. [Google Scholar]
- Holm, N.G. Why are hydrothermal systems proposed as plausible environments for the origin of life? Origins. Life. Evol. B. 1992, 22, 5–14. [Google Scholar] [CrossRef]
- Russel, M.J.; Hall, A.J.; Boyce, A.J.; Fallick, A.E. 100th anniversary special paper: On hydrothermal convection systems and the emergence of life. Econ. Geol. 2005, 100, 419–438. [Google Scholar]
- Sleep, N.H.; Zahnle, K.J.; Kasting, J.F.; Morowitz, H. Annihilation of ecosystems by large asteroid impacts on the early Earth. Nature 1989, 342, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Rampino, M.R. Role of the galaxy in periodic impacts and mass extinctions on the Earth. In Catastrophic Events and Extinctions: Impacts and Beyond; Koeberl, C., MacLeod, K.G., Eds.; GSA Special Paper 356; Geological Society of America: Boulder, CO, USA, 2002; pp. 667–678. [Google Scholar]
- Schultz, P.H.; D’Hondt, S. Cretaceous-Tertiary (Chicxulub) impact angle and its consequences. Geology 1996, 11, 963–967. [Google Scholar] [CrossRef]
- Erwin, D.H.; Bowring, S.A.; Yugan, J. End-Permian mass extinctions: A review. In Catastrophic Events and Extinctions: Impacts and Beyond; Koeberl, C., MacLeod, K.G., Eds.; GSA Special Paper 356; Geological Sociaety of America: Boulder, CO, USA, 2002; pp. 363–384. [Google Scholar]
- Berry, W.B.N.; Ripperdan, R.L.; Finney, S.C. Late Ordovician extinction: A Laurentian view. In Catastrophic Events and Extinctions: Impacts and Beyond; Koeberl, C., MacLeod, K.G., Eds.; GSA Special Paper 356; Geological Sociaety of America: Boulder, CO, USA, 2002; pp. 463–472. [Google Scholar]
- Hoffman, P.F.; Kaufman, A.J.; Halverson, G.P.; Schrag, D.P. A neoproterozoic snowball Earth. Science 1998, 281, 1342–1346. [Google Scholar] [CrossRef] [PubMed]
- Bastin, E.S. Microorganisms in oil fields. Science 1926, 63, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Bastin, E.S.; Greer, F.E. Additional data on sulfate-reducing bacteria in soils and waters of Illinois oil fields. Bull. Amer. Assoc. Petrol. Geol. 1930, 14, 153–159. [Google Scholar]
- Lieske, R.; Hoffmann, E. Untersuchungen über den Bakteriengehalt der Erde in grossen Tiefen. Centralbl. F. Bakt, II. Abt. 1929, 77, 305–309. [Google Scholar]
- Emery, K.O.; Dietz, R.S. Gravity coring instrument and mechanics of sediment coring. Bull. Geol. Soc. Am. 1941, 52, 1685–1714. [Google Scholar] [CrossRef]
- Chapelle, F.H.; Zelibor, J.L.J.; Grimes, D.J.; Knobel, L.L. Bacteria in deep coastal plain sediments of Maryland: A possible source of CO2 to groundwater. Water Resour. Res. 1987, 23, 1625–1632. [Google Scholar] [CrossRef]
- Sinclair, J.L.; Ghiorse, W.C. Distribution of aerobic bacteria, protozoa, algae and fungi in deep subsurface sediments. Geomicrobiol. J. 1989, 7, 15–31. [Google Scholar] [CrossRef]
- Pedersen, K. Preliminary investigations of deep ground water microbiology in Swedish granitic rock. SKB Tech. Rep. 1987, 88, 1–22. [Google Scholar]
- Pedersen, K.; Ekendahl, S. Distribution and activity of bacteria in deep granitic groundwaters of southeastern Sweden. Microb. Ecol. 1990, 20, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K. The deep subterranean biosphere. Earth-Sci. Rev. 1993, 34, 243–260. [Google Scholar] [CrossRef]
- Pedersen, K. Exploration of deep intraterrestrial microbial life: Current perspectives. FEMS Microbiol. Lett. 2000, 185, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, B.A.; Farmer, J.D.; von Blanckenburg, F.; Fallick, A.E. Subsurface filamentous fabrics: An evaluation of modes of origins based on morphological and geochemical criteria, with implications for exopalaeontology. Astrobiology 2008, 8, 87–117. [Google Scholar] [CrossRef] [PubMed]
- Kretzschmar, M. Fossile pilze in eisen-stromatolithen von warstein (rheinisches schiefergebirge). Facies 1982, 7, 237–260. [Google Scholar] [CrossRef]
- Trewin, N.H.; Knoll, A.H. Preservation of devonian chemotrophic filamentous bacteria in calcite veins. Palaios 1999, 14, 288–294. [Google Scholar] [CrossRef]
- D’Hondt, S.; Jørgensen, B.B.; Miller, J.; Batzke, A.; Blake, R.; Cragg, B.A.; Cypionka, H.; Dickens, G.R.; Ferdelman, T.; Hinrichs, K.U.; Holm, N.G.; Mitterer, R.; Spivack, A.; Wang, G.; Bekins, B.; Engelen, B.; Ford, K.; Gettemy, G.; Rutherford, S.D.; Sass, H.; Skilbeck, C.G.; Aiello, I.W.; Guerin, G.; House, C.H.; Inagaki, F.; Meister, P.; Naehr, T.; Niitsuma, S.; Parkes, R.J.; Schippers, A.; Smith, D.C.; Teske, A.; Wiegel, J.; Naranjo Padilla, C.; Solis Acosta, J.L. Distributions of microbial activities in deep subseafloor sediments. Science 2004, 306, 2216–2221. [Google Scholar] [CrossRef] [PubMed]
- Parkes, R.J.; Cragg, B.A.; Wellsbury, P. Recent studies on bacterial populations and processes in subseafloor sediments: A review. Hydrogeol. J. 2000, 8, 11–28. [Google Scholar] [CrossRef]
- Toner, B.M.; Santelli, C.M.; Marcus, M.A.; Wirth, R.; Chan, C.S.; McCollom, T.; Bach, W.; Edwards, K.J. Biogenic iron oxyhydroxide formation at mid-ocean ridge hydrothermal vents: Juan de Fuca Ridge. Geochim. Cosmochim. Ac. 2009, 73, 388–403. [Google Scholar] [CrossRef]
- Lysnes, K.; Thorseth, I.H.; Steinsbu, B.O.; Øvreås, L.; Torsvik, T.; Pedersen, R.B. Microbial community diversity in seafloor basalt from the Arctic spreading ridges. FEMS Microbiol. Ecol. 2004, 50, 213–230. [Google Scholar] [CrossRef] [PubMed]
- Takami, H.; Ioue, A.; Fuji, F.; Horikoshi, K. Microbial flora in the deepest sea mud of the Mariana Trench. FEMS Microbiol. Lett. 1997, 152, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Staudigel, H.; Tebo, B.; Yayanos, A.; Furnes, H.; Kelley, K.; Plank, T.; Muehlenbachs, K. The oceanic crust as a bioreactor. In The Subseafloor Biosphere at Mid-Ocean Ridges; Geophysical Monograph Series 144; Wilcock, W.S.D., DeLong, E.F., Kelley, D.S., Baross, J.A., Cary, S.C., Eds.; American Geophysical Union: Washington, DC, USA, 2004; pp. 325–341. [Google Scholar]
- Ivarsson, M.; Lausmaa, J.; Lindblom, S.; Broman, C.; Holm, N.G. Fossilized microorganisms from the Emperor Seamounts: Implications for the search for a sub-surface fossil record on Earth and Mars. Astrobiology 2008, 8, 1139–1157. [Google Scholar] [CrossRef] [PubMed]
- Ivarsson, M.; Lindblom, S.; Broman, C.; Holm, N.G. Fossilized microorganisms associated with zeolite-carbonate interfaces in sub-seafloor hydrothermal environments. Geobiology 2008, 6, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Peckmann, J.; Bach, W.; Behrens, K.; Reitner, J. Putative cryptoendolithic life in Devonian pillow basalt, Rheinisches Schiefergebirge, Germany. Geobiology 2008, 6, 125–135. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, N.; Brasier, M.D.; Wacey, D.; Green, O.R.; Perry, R.S. On biogenicity criteria for endolithic microborings on early Earth and beyond. Astrobiology 2007, 7, 10–26. [Google Scholar] [CrossRef] [PubMed]
- McLoughlin, N.; Furnes, H.; Banerjee, N.H.; Muehlenbachs, K.; Staudigel, H. Ichnotaxonomy of microbial trace fossils in volcanic glass. J. Geol. Soc. 2009, 166, 159–169. [Google Scholar] [CrossRef]
- Ehrlich, H.L. Geomicrobiology, 3rd ed.; Marcel Dekker: New York, NY, USA, 1996; p. 719. [Google Scholar]
- Furnes, H.; Thorseth, I.H.; Tumyr, O.; Torsvik, T.; Fisk, M.R. Microbial activity in the alteration of glass from pillow lavas from hole 896A. Proc. Oc. Drill. Prog. Sci. Res. 1996, 148, 191–206. [Google Scholar]
- Furnes, H.; Staudigel, H. Biological mediation in ocean crust alteration: How deep is the deep biosphere? Earth Planet Sci. Lett. 1999, 166, 97–103. [Google Scholar] [CrossRef]
- Thorseth, I.H.; Pedersen, R.B.; Christie, D.M. Microbial alteration of 0–30-Ma seafloor basaltic glasses from the Australian Antarctic Discordance. Earth Planet. Sci. Lett. 2003, 215, 237–247. [Google Scholar] [CrossRef]
- Hallbeck, L.; Pedersen, K. Characterization of microbial processes in deep aquifers of the Fennoscandian Shield. Appl. Geochem. 2008, 23, 1796–1819. [Google Scholar] [CrossRef]
- Gadd, G.M. Geomycology: Biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycol. Res. 2007, 111, 3–49. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, P.; Rades-Rohkohl, E.; Kölbel-Boelke, J.; Nehrkorn, A. Morphological and taxonomic diversity of groundwater micro-organisms. In Progr Hydrogeochem; Matthess, G., Frimmel, F.H., Hirsch, P., Schulz, H.D., Usdowski, E., Eds.; Springer-Verlag: Berlin, Germany, 1992. [Google Scholar]
- Krauss, G.; Sridhar, K.R.; Jung, K. Aquatic hyphomycetes in polluted groundwater habitats of central Germany. Microbial. Ecol. 2003, 45, 329–339. [Google Scholar]
- Pedersen, K.; Arlinger, J.; Ekendahl, S.; Hallbeck, L. 16S rRNA gene diversity of attached and unattached bacteria in boreholes along the access tunnel to the Äspö Hard Rock Laboratory. FEMS Microbiol. Ecol. 1996, 19, 249–262. [Google Scholar]
- Ekendahl, S.; O’Neill, A.H.; Thomsson, E.; Pedersen, K. Characterisation of yeasts isolated from deep igneous rock aquifers of the Fennoscandian Shield. Microb. Ecol. 2003, 46, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Bidle, K.D.; Lee, S.; Marchant, D.R.; Falkowski, P.G. Fossil genes and microbes in the oldest ice on earth. PNAS 2007, 104, 13455–13460. [Google Scholar] [CrossRef] [PubMed]
- Teske, A.P. The deep subsurface biosphere is alive and well. Trends Microbiol. 2005, 13, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.J.; Bach, W.; McCollom, T.M. Geomicrobiology in oceanography: Microbe-mineral interactions at and below the seafloor. Trends Microbiol. 2005, 13, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Golubic, S.; Friedmann, I.; Schneider, J. The lithobiontic ecological niche, with special reference to microorganisms. J. Sediment. Petrol. 1981, 51, 475–478. [Google Scholar]
- Fisk, M.R.; Popa, R.; Mason, O.U.; Storrie-Lombardi, M.C.; Vicenzi, E.P. Iron-magnesium silicate bioweathering on Earth (and Mars?). Astrobiology 2006, 6, 48–68. [Google Scholar] [CrossRef] [PubMed]
- Eickmann, B.; Bach, W.; Kiel, S.; Reitner, J.; Peckmann, J. Evidence for cryptoendolithic life in Devonian pillow basalts of variscan orogens, Germany. Palaeogeogr. Palaeocl. Palaeoecol. 2009, 283, 120–125. [Google Scholar] [CrossRef]
- Ivarsson, M.; Holm, N.G. Microbial colonization of various habitable niches during alteration of oceanic crust. In Links between Geological Processes, Microbial Activities and Evolution of Life; Dilek, Y., Furnes, H., Muehlenbachs, K., Eds.; Springer: Berlin, Germany, 2008; pp. 69–111. [Google Scholar]
- Honnorez, J. Generation of phillipsites by palagonitization of basaltic glass in sea water and the origin of K-rich deep-sea sediments. In Natural Zeolites, Occurrence, Properties, Use; Sand, L.B., Mumpton, F.A., Eds.; Pergamon Press: London, UK, 1978; pp. 245–258. [Google Scholar]
- Boynton, W.V.; Ming, D.W.; Kounaves, S.P.; Young, S.M.M.; Arvidson, R.E.; Hecht, M.H.; Hoffman, J.; Niles, P.B.; Hamara, D.K.; Quinn, R.C.; Smith, P.H.; Sutter, B.; Catling, D.C.; Morris, R.V. Evidence for calcium carbonate at the Mars Phoenix landing site. Science 2009, 325, 61–64. [Google Scholar] [PubMed]
- Mumma, M.J.; Villanueva, G.L.; Novak, R.E.; Hewagama, T.; Bonev, B.P.; DiSanti, M.A.; Mandell, A.M.; Smith, M.D. Strong release of methane on Mars in northern summer 2003. Science 2009, 323, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Cockell, C.S.; Lee, P.; Broady, P.; Lim, D.S.S.; Osinski, G.R.; Parnell, J.; Koeberl, C.; Pesonen, L.; Salminen, J. Effects of asteroid and comet impacts on habitats for lithophytic organisms—A synthesis. Meteorit. Planet. Sci. 2005, 40, 1901–1914. [Google Scholar] [CrossRef]
- Cockell, C.S. The origin and emergence of life under impact bombardment. Phil. T. Roy. Soc. B. 2006, 361, 1845–1856. [Google Scholar] [CrossRef]
- Cockell, C.S.; Osinski, G.R. Impact-induced impoverishment and transformation of a sandstone habitat for lithophytic microorganisms. Meteorit. Planet. Sci. 2007, 42, 1985–1993. [Google Scholar] [CrossRef]
- Sleep, N.H. Martian plate tectonics. J. Geophys. Res. 1994, 99, 5639–5655. [Google Scholar] [CrossRef]
- McSween, H.Y.J. What we have learned about Mars from SNC meteorites. Meteoritics 1994, 29, 757–779. [Google Scholar] [CrossRef]
- Carr, M. Water on Mars; Oxford University Press: New York, NY, USA, 1996. [Google Scholar]
- Smith, P.H.; Tamppari, L.K.; Arvidson, R.E.; Bass, D.; Blaney, D.; Boynton, W.V.; Carswell, A.; Catling, D.C.; Clark, B.C.; Duck, T.; DeJong, E.; Fisher, D.; Goetz, W.; Gunnlaugsson, H.P.; Hecht, M.H.; Hipkin, V.; Hoffman, J.; Hviid, S.F.; Keller, H.U.; Kounaves, S.P.; Lange, C.F.; Lemmon, M.T.; Madsen, M.B.; Markiewicz, W.J.; Marshall, J.; McKay, C.P.; Mellon, M.T.; Ming, D.W.; Morris, R.V.; Pike, W.T.; Renno, N.; Staufer, U.; Stoker, C.; Taylor, P.; Whiteway, J.A.; Zent, A.P. H2O at the Phoenix landing site. Science 2009, 325, 58–61. [Google Scholar]
- Whiteway, J.A.; Komguem, L.; Dickinson, C.; Cook, C.; Illnicki, M.; Seabrook, J.; Popovici, V.; Duck, T.J.; Davy, R.; Taylor, P.A.; Pathak, J.; Fisher, D.; Carswell, A.I.; Daly, M.; Hipkin, V.; Zent, A.P.; Hecht, M.H.; Wood, S.E.; Tamppari, L.K.; Renno, N.; Moores, J.E.; Lemmon, M.T.; Daerden, F.; Smith, P.H. Mars water-ice clouds and precipitation. Science 2009, 325, 68–70. [Google Scholar] [PubMed]
- Byrne, S.; Dundas, C.M.; Kennedy, M.R.; Mellon, M.T.; McEwen, A.S.; Cull, S.C.; Daubar, I.J.; Shean, D.E.; Seelos, K.D.; Murchie, S.L.; Cantor, B.A.; Arvidson, R.E.; Edgett, K.S.; Reufer, A.; Thomas, N.; Harrison, T.N.; Posiolova, L.V.; Seelos, F.P. Distribution of mid-latitude ground ice on mars from new impact craters. Science 2009, 325, 1674–1676. [Google Scholar] [CrossRef] [PubMed]
- Kerr, R.A. On Mars, a Second Chance for Life. Science 2004, 306, 2010–2012. [Google Scholar] [CrossRef] [PubMed]
- Bibring, J.P.; Langevin, Y.; Mustard, J.F.; Poulet, F.; Arvidson, R.; Gendrin, A.; Gondet, B.; Mangold, N.; Pinet, P.; Forget, F.; The OMEGA Team. Global mineralogical and aqueous mars history derived from Omega/mars express data. Science 2006, 312, 400–404. [Google Scholar]
- Jakosky, B.M. Mars volatile evolution: Evidence from stable isotopes. Icarus 1991, 94, 14–31. [Google Scholar] [CrossRef]
- Jakosky, B.M.; Jones, J.H. Evolution of water on mars. Nature 1994, 370, 328–329. [Google Scholar] [CrossRef]
- Gooding, J.L. Soil mineralogy and chemistry on Mars: Possible clues from salts and clays in SNC meteorites. Icarus 1992, 99, 28–41. [Google Scholar] [CrossRef]
- Griffith, L.L.; Shock, E.L. A geochemical model for the formation of hydrothermal carbonates on mars. Nature 1995, 377, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Catling, D.C.; Moore, J.M. The nature of coarse-grained crystalline hematite and its implications for the early environment of mars. Icarus 2003, 165, 277–300. [Google Scholar] [CrossRef]
- Wyatt, M.B.; McSween, H.Y.J. Spectral evidence for weathered basalt as an alternative to andesite in the northern lowlands of mars. Nature 2002, 417, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.R.; Morris, R.V.; Lane, M.D.; Banfield, J.L.; Malin, M.C. Global mapping of martian hematite mineral deposits: Remnants of water-driven processes on early mars. J. Geophys. Res. 2001, 106, 23873–23885. [Google Scholar] [CrossRef]
- Klingelhöfer, G. Mössbauer In Situ Studies of the Surface of Mars. Hyperfine Interact. 2005, 158, 117–124. [Google Scholar] [CrossRef]
- Minitti, M.E.; Rutherford, M.J. Genesis of the mars pathfinder “sulfur-free” rock from SNC parental liquids. Geochim. Cosmochim. Ac. 2000, 64, 2535–2547. [Google Scholar] [CrossRef]
- Christensen, P.R.; Ruff, S.W. Formation of the hematite-bearing unit in Meridiani Planum: Evidence for deposition in standing water. J. Geophys. Res. 2004, 109, E08003. [Google Scholar] [CrossRef]
- Neukum, G.; Jaumann, R.; Hoffmann, H.; Hauber, E.; Head, J.W.; Basilevsky, A.T.; Ivanov, B.A.; Werner, S.C.; van Gasselt, S.; Murray, J.B.; McCord, T.; The HRSC Co-investigator Team. Recent and episodic volcanic and glacial activity on Mars revealed by the high resolution stereo camera. Nature 2004, 432, 971–979. [Google Scholar]
- Ames, D.E.; Watkinson, D.H.; Parrish, R.R. Dating of a regional hydrothermal system induced by the 1850 Ma Sudbury impact event. Geology 1998, 26, 447–450. [Google Scholar]
- Naumov, M.V. Impact-generated hydrothermal systems: Data from Popigai, Kara, and Puchezh-Katunki impact structures. In Impacts in Precambrian Shields; Plado, J., Peasonen, L.J., Eds.; Springer: Berlin, Germany, 2002; pp. 117–171. [Google Scholar]
- Osinski, G.R.; Spray, J.G.; Lee, P. Impact induced hydrothermal activity within the Haughton impact structure, arctic Canada: generation of a transient, warm, wet oasis. Meteorit. Planet. Sci. 2001, 36, 731–745. [Google Scholar]
- Newsom, H.E. Hydrothermal alteration of impact melt sheets with implications for Mars. Icarus 1980, 44, 207–216. [Google Scholar] [CrossRef]
- Pope, K.O.; Kieffer, S.W.; Ames, D.E. Impact melt sheet formation on Mars and its implication for hydrothermal systems and exobiology. Icarus 2006, 183, 1–9. [Google Scholar] [CrossRef]
- Squyres, S.W.; Arvidson, R.E.; Ruff, S.; Gellert, R.; Morris, R.V.; Ming, D.W.; Crumpler, L.; Farmer, J.D.; Des Marais, D.J.; Yen, A.; McLennan, S.M.; Calvin, W.; Bell, J.F., III; Clark, B.C.; Wang, A.; McCoy, T.J.; Schmidt, M.J.; McLennan, P.A., Jr. Detection of silica-richdeposits on mars. Science 2008, 320, 1063–1067. [Google Scholar]
- Bishop, J.L.; Noe Dobrea, E.Z.; McKeown, N.K.; Parente, M.; Ehlmann, B.L.; Michalski, J.R.; Milliken, R.E.; Poulet, F.; Swayze, G.A.; Mustard, J.F.; Murchie, S.L.; Bibring, J.P. Phyllosilicate diversity and past aqueous activity revealed at Mawrth Vallis, Mars. Science 2008, 321, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Mustard, J.F.; Murchie, S.L.; Pelkey, S.M.; Ehlmann, B.L.; Milliken, R.E.; Grant, J.A.; Bibring, J.P.; Poulet, F.; Bishop, J.; Noe Dobrea, E.; Roach, L.; Seelos, F.; Arvidson, R.E.; Wiseman, S.; Green, R.; Hash, C.; Humm, D.; Malaret, E.; McGovern, J.A.; Seelos, K.; Clancy, T.; Clark, R.; Des Marais, D.; Izenberg, N.; Knudson, A.; Langevin, Y.; Martin, T.; McGuire, P.; Morris, R.; Robinson, M.; Roush, T.; Smith, M.; Swayze, G.; Taylor, H.; Titus, T.; Wolff, M. Hydrated silicate minerals on Mars observed by the mars reconnaissance orbiter CRISM instrument. Nature 2008, 454, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Poulet, F.; Bibring, J.P.; Mustard, J.F.; Gendrin, A.; Mangold, N.; Langevin, Y.; Arvidson, R.E.; Gondet, B.; Gomez, C.; The Omega Team. Phyllosilicates on mars and implications for early martian climate. Nature 2005, 438, 623–627. [Google Scholar]
- Clifford, S.M. A model for the hydrologic and climatic behavior of water on mars. J. Geophys. Res. 1993, 88, 2456–2474. [Google Scholar] [CrossRef]
- McKinley, J.P.; Stevens, T.O.; Westall, F. Microfossils and paleoenvironments in deep subsurface basalt samples. Geomicrobiol. J. 2000, 17, 43–54. [Google Scholar] [CrossRef]
- Herrera, A.; Cockell, C.S.; Self, S.; Blaxter, M.; Reitner, J.; Thorsteinsson, T.; Arp, G.; Dröse, W.; Tindle, A.G. A cryptoendolithic community in volcanic glass. Astrobiology 2009, 9, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Laskar, J.; Correia, A.C.M.; Gastineau, M.; Joutel, F.; Levrard, B.; Robutel, P. Long term evolution and chaotic diffusion of the insolation quantities of Mars. Icarus 2004, 170, 343–364. [Google Scholar] [CrossRef]
- Balme, M.R.; Gallagher, C. An equatorial periglacial landscape on Mars. Earth Planet. Sci. Lett. 2009, 285, 1–15. [Google Scholar] [CrossRef]
- Osinski, G.R.; Spray, J.G.; Lee, P. Impactites of the Haughton impact structure, Devon island, Canadian high Arctic. Meteorit. Planet. Sci. 2005, 40, 1789–1812. [Google Scholar] [CrossRef]
- French, B.M. Traces of catastrophe: A handbook of Shock-Metamorphic Effects in Terrestrial Meteorite Impact Structures. In LPI Contribution No. 954; Lunar and Planetary Institute: Houston, TX, USA, 1998; p. 120. [Google Scholar]
- Malin, M.C.; Edgett, K.S.; Posiolova, L.V.; McColley, S.M.; Noe Dobrea, E.Z. Present-day cratering rate and contemporary gully activity on Mars. Science 2006, 314, 1573–1577. [Google Scholar] [CrossRef] [PubMed]
- Byrne, S.; Dundas, C.M.; Kennedy, M.R.; Mellon, M.T.; McEwan, A.S.; Cull, S.C.; Daubar, I.J.; Shean, D.E.; Seelos, K.D.; Murchie, S.L.; Cantor, B.A.; Arvidson, R.E.; Edgett, K.S.; Reufer, A.; Thomas, N.; Harrison, T.N.; Posiolova, L.V.; Seelos, F.P. Distribution of mid-latitude ground ice on Mars fromnew impact craters. Science 2009, 325, 1674–1676. [Google Scholar] [CrossRef] [PubMed]
- Blank, J.; Miller, G.H.; Ahrens, M.J.; Winans, R.E. Experimental shock chemistry of aqueous amino acid solutions and the cometary delivery of prebiotic compounds. Origins. Life. Evol. B. 2001, 31, 15–51. [Google Scholar] [CrossRef]
- Parnell, J.; Lee, P.; Osinski, G.R.; Cockell, C.S. Application of organic geochemistry to detect signatures of organic matter in the Haughton impact structure. Meteorit. Planet. Sci. 2005, 40, 1879–1885. [Google Scholar] [CrossRef]
- Hofmann, P.; Leythaeuser, D.; Schwark, L. Organic matter from the Bunte Breccia of the Ries Crater, southern Germany: Investigating possible thermal effects of the impact. Planet. Space Sci. 2001, 49, 845–851. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ivarsson, M.; Lindgren, P. The Search for Sustainable Subsurface Habitats on Mars, and the Sampling of Impact Ejecta. Sustainability 2010, 2, 1969-1990. https://doi.org/10.3390/su2071969
Ivarsson M, Lindgren P. The Search for Sustainable Subsurface Habitats on Mars, and the Sampling of Impact Ejecta. Sustainability. 2010; 2(7):1969-1990. https://doi.org/10.3390/su2071969
Chicago/Turabian StyleIvarsson, Magnus, and Paula Lindgren. 2010. "The Search for Sustainable Subsurface Habitats on Mars, and the Sampling of Impact Ejecta" Sustainability 2, no. 7: 1969-1990. https://doi.org/10.3390/su2071969



