2. Waste Management in Different Countries
The waste generated from MSWI usually ends up in two ways, disposed as landfill or for reuse as secondary raw materials. In most developed countries where land is scarce and environmental controls are tight, environmental policies tend to reduce landfill disposals as much as possible [
1,
2,
3]. In Japan, about 80% of MSW is incinerated and the recycling and reuse of MSWI ash in different ways have been described [
4,
5,
6,
7]. In China, more than 80% of the MSW ends up as landfill, and compost production ranks as the second major application; only few processes involving the recycling of ashes have been undertaken [
8]. The best management strategy for waste ashes is recycling and reusing. Different kinds of utilization methods are used in developed countries. Although the ashes contain high concentrations of heavy metals, salts, chloride and organic pollutants, which may limit the applications of reuse, the treatment of ashes will improve the environmental characteristics and enhance the possibility for reuse [
9,
10,
11,
12,
13].
3. MSW Incineration Processes
The incineration process is separated into three main parts: incineration, energy recovery and air pollution control [
14,
15].
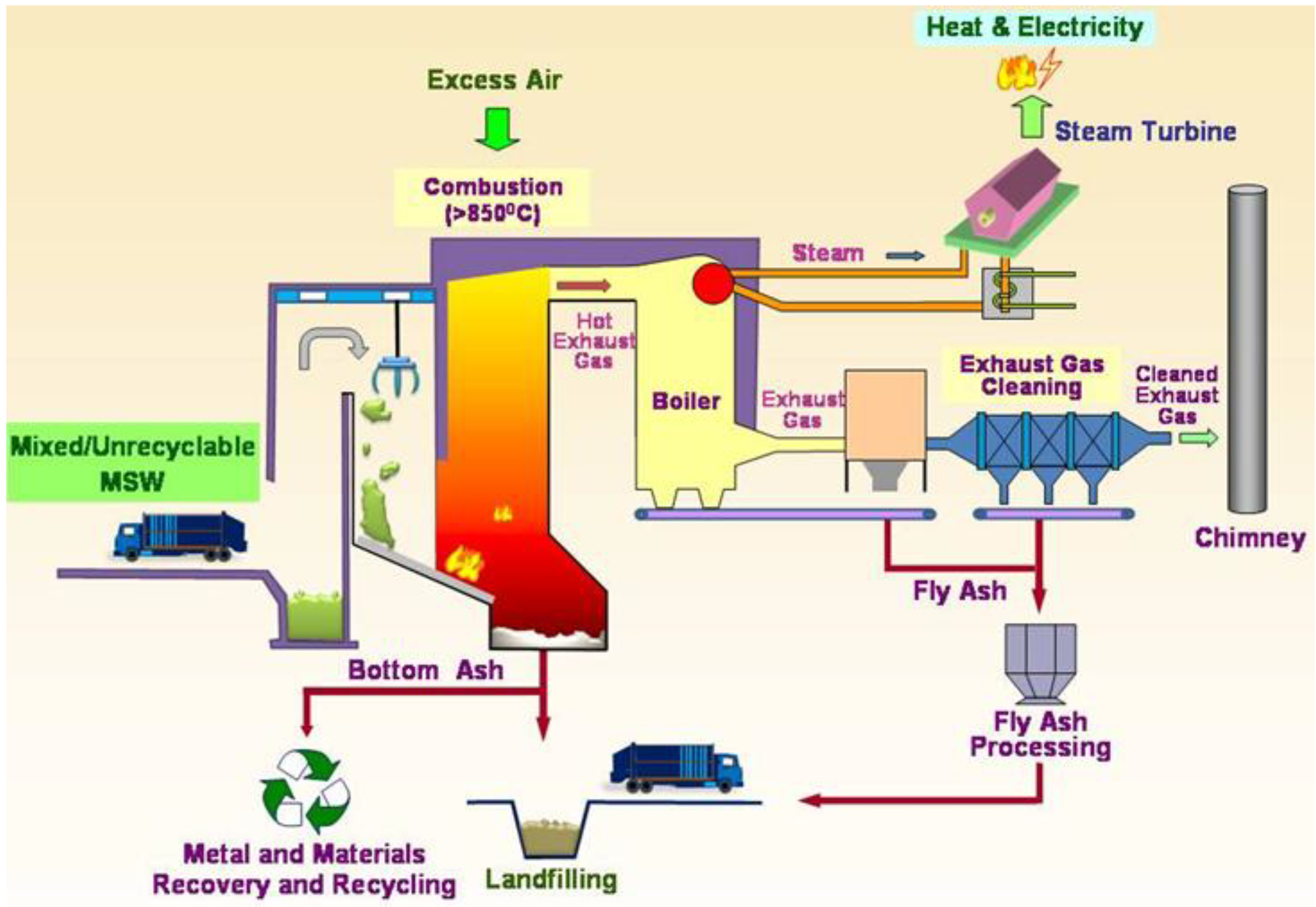
Figure 1 shows a schematic diagram depicting the common MSW incineration process.
Figure 1.
Schematic Diagram of the MSW incineration process (Reproduced with permission from [
16], published by Environmental Protection Department, the Government of the Hong Kong Special Administrative Region, 2009).
Figure 1.
Schematic Diagram of the MSW incineration process (Reproduced with permission from [
16], published by Environmental Protection Department, the Government of the Hong Kong Special Administrative Region, 2009).
The MSW is fed into the furnace continually for incineration. Based on the Best Practical Means for the combustion of wastes, the combustion is enhanced by following the three T’s guideline—high Temperature increases burnout, increased Turbulence exposes more waste surface and increases burnout, and a longer residence Time for the flue gas (>2 seconds) and the MSW increases burnout—the temperature for incineration should be at least 850 ○C with a residence time of more than two seconds. During the process, the air supply must be sufficient to ensure complete combustion of waste and to prevent the formation of dioxins and carbon monoxide.
For energy recovery, the heat generated from waste is used to produce steam in the boiler. Then the steam drives the turbine to generate electricity. The excess heat generated can also be used for other purposes, e.g., heating for swimming pools.
Air pollution is a major problem for incineration. In modern incinerators, an advanced pollution control system is designed to minimize the pollution and ensure compliance with environmental standards. A dry/wet scrubber is used to spray fine atomized slurry or lime powder into the hot exhaust gas in order to neutralize the acidic gases such as sulfur oxides and hydrogen chloride. An activated carbon column or injected activated carbon spray is used to adsorb the heavy metals and organic pollutants such as PCB and VOC in the exhaust gas [
17]. A selective non-catalytic reduction system is used to remove nitrogen oxides by adding urea or ammonia for reaction to nitrogen, carbon dioxide and water. The bag filter system acts to filter and remove the fine particulates and dust particles [
16].
5. Characterization of Incineration Ashes
The composition of municipal solid waste varies over time and from country to country, due to the differences in lifestyle and waste recycling processes of a country; the ash content will vary too. Generally, the chemical and physical characterization of ash will depend on the compositions of the raw MSW, the operational conditions, the type of incinerator and air pollution control system design [
20]. The chemical composition shows that the major elements are Si, Al, Fe, Mg, Ca, K, Na and Cl. Further, SiO
2, Al
2O
3, CaO, Fe
2O
3, Na
2O, K
2O are the common oxides found in ash (
Table 1 and
Table 2). CaO is the most abundant compound that exists in MSWI fly ash, which constitutes up to 46%, but SiO
2 is the most abundant compound that exists in MSWI bottom ash, containing up to 49%.
Table 1.
Oxide compositions in MSWI fly ash (FA) (wt%).
Table 1.
Oxide compositions in MSWI fly ash (FA) (wt%).
| Authors | [21] | [22] | [23] | [24] | [25] | [26] | [27] | [28] |
|---|
| Type | FA | FA | FA | FA | FA | FA | FA | FA |
|---|
| SiO2 | 18.8 | 11.47 | 19.4 | 13.6 | 18.5 | 20.5 | 6.35 | 27.52 |
| Al2O3 | 12.7 | 5.75 | 10.1 | 0.92 | 7.37 | 5.8 | 3.5 | 11 |
| CaO | 24.3 | 29.34 | 19.7 | 45.42 | 37.5 | 35.8 | 43.05 | 16.6 |
| Fe2O3 | 1.6 | 1.29 | 1.8 | 3.83 | 2.26 | 3.2 | 0.63 | 5.04 |
| MgO | 2.6 | 3.02 | 2.8 | 3.16 | 2.74 | 2.1 | 1.38 | 3.14 |
| K2O | 4.3 | 7.02 | 8.1 | 3.85 | 2.03 | 4 | 4.59 | 8.24 |
| Na2O | 5.8 | 8.7 | 8.9 | 4.16 | 2.93 | 3.7 | 5.8 |
| SO3 | 6.4 | N/A | N/A | 5.18 | 14.4 | N/A | 4.64 | 8.34 |
| P2O5 | 2.7 | 1.69 | N/A | N/A | 1.56 | N/A | N/A | N/A |
| TiO2 | 1.5 | 0.85 | 1.9 | 3.12 | 1.56 | N/A | N/A | 1.88 |
Table 2.
Oxide compositions MSWI bottom ash (wt%).
Table 2.
Oxide compositions MSWI bottom ash (wt%).
| Authors | [29] | [30] | [31] | [32] | [24] | [25] | [27] |
|---|
| Type | BA (150–200 mesh) | MSWI ash | MSWI ash | MSWI ash | BA | BA | BA |
|---|
| SiO2 | 27.8 | 29.4 | 12.01 | 5.44 | 13.44 | 46.7 | 49.38 |
| Al2O3 | 9.9 | 18 | 8.1 | 3.1 | 1.26 | 6.86 | 6.58 |
| CaO | 25.9 | 27.2 | 13.86 | 42.55 | 50.39 | 26.3 | 14.68 |
| Fe2O3 | 4 | 13.3 | 1.21 | 1.69 | 8.84 | 4.69 | 8.38 |
| MgO | 3.3 | 1.6 | 2.62 | 1.83 | 2.26 | 2.22 | 2.32 |
| K2O | 1.8 | 0.9 | 7.41 | 4.31 | 1.78 | 0.888 | 1.41 |
| Na2O | 3.3 | 3.6 | 17.19 | 4.82 | 12.66 | 4.62 | 7.78 |
| SO3 | N/A | N/A | N/A | 12.73 | 0.5 | 2.18 | 0.57 |
| P2O5 | 6.9 | N/A | N/A | 1.62 | N/A | 0.855 | N/A |
| TiO2 | 2 | N/A | N/A | 0.92 | 2.36 | 0.77 | N/A |
For heavy metals, Cr, Cu, Hg, Ni, Cd, Zn and Pb are the most commonly found in MSWI ash, and Zn and Pb usually exist in the largest amounts (fly ash and bottom ash shown in
Table 3 and
Table 4, respectively). These metals may cause leaching problems and are harmful to the environment without proper treatment. Generally, the heavy metals content in fly ash is higher than in bottom ash due to the vaporization of metals during combustion and the process of metals adsorption on the surface of fly ash particles.
MSWI fly ash contains a much higher chloride content than MSWI bottom ash (
Table 5 and
Table 6, respectively). This may be due to the lime scrubber in the air pollution control system, which removes acidic gases such as HCl, thus resulting in a high amount of chloride content remaining in fly ash after the air pollution control system. The loss on ignition of ashes is around 4–13% for fly ash and 3–5% for bottom ash (
Table 7).
Table 3.
Heavy metals found in MSWI fly ash (FA) (mg/kg).
Table 3.
Heavy metals found in MSWI fly ash (FA) (mg/kg).
| Authors | [10] | [33] | [34] | [35] | [36] |
|---|
| Type | FA | FA | FA | FA | FA |
|---|
| Ag | 31–95 | ND–700 | N/A | N/A | N/A |
| As | 31–95 | 15–751 | N/A | 93 | N/A |
| Ba | 920–1,800 | 88–9,001 | N/A | 4,300 | 539 |
| Cd | 250–450 | 5–2211 | 25.5 | 470 | 95 |
| Co | 29–69 | 2.3–1,671 | N/A | N/A | 14 |
| Cr | 140–530 | 21–1,901 | 118 | 863 | 72 |
| Cu | 860–1,400 | 187–2,381 | 313 | 1,300 | 570 |
| Hg | 0.8–7 | 0.9–73 | 52 | N/A | N/A |
| Mn | 0.8–1.7 | 171–8,500 | N/A | 1,600 | 309 |
| Ni | 95–240 | 10–1,970 | 60.8 | 124 | 22 |
| Pb | 7,400–19,000 | 200–2,600 | 1496 | 10,900 | 2,000 |
| Se | 6.1–31 | 0.48–16 | N/A | 41 | N/A |
| Zn | 19,000–41,000 | 2,800–152,000 | 4,386 | 25,800 | 6,288 |
| Sn | 1,400–1,900 | N/A | N/A | N/A | N/A |
| Sr | 80–250 | N/A | N/A | 433 | 151 |
| V | 32–150 | N/A | N/A | 37 | N/A |
Table 4.
Heavy metals found in MSWI bottom ash (BA) (mg/kg).
Table 4.
Heavy metals found in MSWI bottom ash (BA) (mg/kg).
| Authors | [37] | [33] | [38] | [31] | [39] |
|---|
| Type | BA | BA | BA | BA | BA |
|---|
| Ag | 4.1–14 | 2–38 | 8.5–10.7 | N/A | N/A |
| As | 19–80 | 1.3–45 | 209–227 | 160 | 13 |
| Ba | 900–2,700 | 47–2,000 | 1,104–1,166 | N/A | N/A |
| Cd | 1.4–40 | 0.3–61 | 6.8–7.8 | 110 | 3 |
| Co | <10–40 | 22–706 | 49.6–53.1 | N/A | N/A |
| Cr | 230–600 | 13–1,400 | 323–439 | 260 | 900 |
| Cu | 900–4,800 | 80–10,700 | 4,139–4,474 | N/A | 500 |
| Hg | <0.01–3 | 0.003–2 | N/A | N/A | 2.6 |
| Mn | <0.7–1.7 | 50–3,100 | 869–894 | N/A | 280 |
| Ni | 60–190 | 9–430 | 216–242 | N/A | 180 |
| Pb | 1,300–5,400 | 98–6,500 | 2,474–2,807 | N/A | 2,700 |
| Se | 0.6–8 | ND–3.4 | 230–265 | 130 | N/A |
| Zn | 1,800–6,200 | 200–12,400 | 4,261–4,535 | N/A | 600 |
| Sn | <100–1,300 | N/A | N/A | 840 | 960 |
| Sr | 170–350 | N/A | N/A | N/A | N/A |
| V | 36–90 | N/A | N/A | N/A | N/A |
Table 5.
Chloride Content of MSWI fly ash (FA) (mg/kg).
Table 5.
Chloride Content of MSWI fly ash (FA) (mg/kg).
| Authors | [24] | [25] | [37] | [40] | [32] | [41] | [27] | [28] | [36] | [42] |
|---|
| Type | FA | FA | FA | FA | FA | FA | FA | FA | FA | FA |
|---|
| 5,749 | 8,670 | 45,000–100,000 | 19,000–210,000 | 120,000–200,000 | 131,000 | 83,800 | 103,200 | 157,200 | 215,000 |
Table 6.
Chloride Content of MSWI bottom ash (BA) (mg/kg).
Table 6.
Chloride Content of MSWI bottom ash (BA) (mg/kg).
| Authors | [24] | [31] | [32] | [39] | [25] |
|---|
| Type | BA | BA | BA | BA | BA |
|---|
| 2,876 | 149,500 | 201,100 | 2,300 | 1,760 |
Table 7.
Loss on Ignition of MSWI fly ash (FA) and bottom ash (BA) (wt%).
Table 7.
Loss on Ignition of MSWI fly ash (FA) and bottom ash (BA) (wt%).
| Author | [10] | [24] | [28] | [24] | [31] |
|---|
| Type | FA | FA | FA | BA | BA |
|---|
| 4.3 | 9.73 | 13.36 | 3.24 | 4.59 |
Generally, the dioxin levels in fly ash in most countries has demonstrated values higher than 1 ng I-TEQ/g (
Table 8), which is the Japan Ministry of the Environment (2001) Environmental Quality Standard for Soils. The international emission standard limit for dioxin concentration in flue gas is 0.1 ng I-TEQ/m
3. In order to minimize the flue gas dioxin concentration; the dioxin is removed from vapor phase to solid phase by lime scrubbing and activated carbon injection to adsorb dioxin [
43,
44,
45]. Thus, the remaining air pollution control fly ash contains relatively high amounts of dioxin and is classified as hazardous waste [
46,
47,
48]. There are a number of ways of minimizing dioxin/furan formation during MSW incineration which can significantly reduce the dioxin/furan. A two-stage approach system was designed to achieve complete combustion and minimize formation by a well controlled combustion system; and a well designed end-of-pipe treatment system to remove dioxins. In the combustion system, the combustion temperature should be above 1,000
○C, the combustion residence time should be greater than 1 s and the combustion chamber turbulence should be represented by a Reynolds number greater than 50,000, with good MSW feed preparation and controlled feed rate. In the end-of-pipe treatment systems, very rapid gas cooling from 400 to 250
○C should be achieved. Semi-dry lime scrubbing and bag filtration coupled with activated carbon injection adsorption as end-of-pipe treatments can all play a role in prevention or minimization of dioxins in the final flue gas emission to the atmosphere [
46].
Table 8.
Dioxin in MSWI fly ash (FA) and bottom ash (BA) (ng I-TEQ/g).
Table 8.
Dioxin in MSWI fly ash (FA) and bottom ash (BA) (ng I-TEQ/g).
| Country | China | Korea | Japan | Taiwan |
|---|
| Authors | [49] | [20] | [44] | [50] | [51] | [52,53] |
|---|
| Type | FA | FA | FA | FA | FA | FA |
|---|
| PCDD/F | | 7.53 | 0.98–1.5 | 0.798 | 0.13–21 | 6.7 | 0.47–2.3 |
7. Applications of MSWI Ashes
After the above treatments, the ashes are much more usable. To determine the possibility of application, there are three main factors to address: suitability for processing, technical performance and environmental impact [
99].
Table 9.
Applications of MSWI ashes.
Table 9.
Applications of MSWI ashes.
| Type | Application | Composition% | Country | Authors |
|---|
| BA | Aggregate in concrete | up to 50% | France | [100] |
| BA | Aggregate in concrete | replace up to 15% of cement | Slovenia | [101] |
| BA | Road base | | Spain | [39] |
| BA | Adsorbent for dyes | | India | [29] |
| BA | Concrete | | Italy | [102] |
| Mixed ash | Cement clinker | up to 50% | Portugal | [103] |
| Mixed ash | Cement clinker | 44% | Japan | [31] |
| Mixed ash | Cement clinker | 15% | Taiwan | [30] |
| Mixed ash | Cement clinker | 1.75% FA 3.5% BA | Taiwan | [24] |
| Mixed ash | Aggregate in concrete | | Spain | [27] |
| FA | Concrete | 50% | France | [104] |
| FA | Eco cement | 50% | Japan | [105] |
| FA | Ceramic tile | | China | [26] |
| FA | Binder for stabilizing sludge | 45% | China | [32] |
| FA | Glass ceramic | 75% FA, 20% SiO2, 5% MgO, 2% TiO2 | Korea | [106] |
| FA | Glass ceramic (low melting temperature) | China | [28] |
| FA | Cement clinker | replace up to 30% of raw material | China | [107] |
| FA | Blended cement | up to 45% | UK | [108] |
Here, seven methods for the utilization of MSWI ash are reviewed, namely, cement and concrete production, road pavement, glasses and ceramics, agriculture, stabilizing agent, adsorbents and zeolite production.
7.1. Cement and Concrete Production
Since MSWI ash contains CaO, SiO
2, Fe
2O
3, and Al
2O
3, and the fact that a considerable amount of cement was used for the production of mortar and concrete, the composition of fly ash and bottom ash is similar to the composition of raw materials for cement production. Thus, it could be a possible replacement of raw material in Portland cement production [
24,
109,
110]. R. Kikuchi has shown that the addition of MSWI ash for clinker production will shorten the setting time and decrease workability; he suggested that a delaying agent like gypsum should be added [
103].
Cement production (
Figure 4) indeed consumes huge quantities of energy and emits large amounts of carbon dioxide, which is the major cause of global warming industry activities. One of the advantages of using MSWI ash as cement raw material is the reduction in carbon dioxide emissions, thus minimizing the global warming effect. As a large amount of energy is used to decompose the calcium carbonate (CaCO
3) to lime (CaO), a huge amount of carbon dioxide is emitted during the process. Due to the fact that MSWI bottom ash and fly ash is composed of lime instead of calcium carbonate, it can reduce the carbon dioxide emission. There are several technical problems discouraging this application; the high chloride content will affect the product quality, and the cycling effect in the cement kilns will cause rapid clogging and corrosion inside the heat exchangers [
111,
112]. The high concentration of heavy metals will also be an environmental concern.
Pre-treatment of fly ash is recommended to remove the chloride and heavy metals content, also the quantities of MSWI ash added to the process should be carefully controlled in order to ensure the process safety as well as product quality [
58,
62,
113].
Figure 4.
Schematic Diagram of the Cement Production Process.
Figure 4.
Schematic Diagram of the Cement Production Process.
For the hydration behavior of cement clinker, it is found that alkali metal content enhances hydration and contents of Zn, Pb and Cd retards the rate of hydration of cement. After washing pre-treatment of MSWI ash, the alkali metal content is reduced and the hydration rate of washed MSW ash containing clinkers is lower than the raw MSWI ash containing clinkers [
31,
114,
115,
116]. Fly ash contains high chloride and sulfate content, which reveal the formation of a ettringite phase in the hydration period, thus the hydration reaction slows down with the increasing fly ash content [
117].
Based on S/S technology, the MSWI fly ash can be potentially applied as a replacement of cement or as an aggregate [
118]. The addition of up to 50% treated fly ash will not affect the strength and hardness, and the leaching property is acceptable for the use in road construction. However, the long-term durability has not yet been determined [
104,
119].
It is possible to use MSWI bottom ash as concrete aggregate. The results show that treated (immersion in sodium hydroxide for 15 days) bottom ash can replace up to 50% of gravel in concrete without affecting the durability. Cracking and swelling occur if the ash is not treated, due to the reaction between metallic aluminum and cement. MSWI fly ash could also be used as lightweight concrete aggregate by processing into pellets. This could be suitable for non-structural applications such as interior walls for insulating purposes [
100]. Also, the use of cement-solidified MSWI fly ash has been proved to be suitable for safety reuse as artificial aggregate in Portland cement mortars. It showed low leaching rates of heavy metals, high compressive strengths (up to 36 N/mm
2 after 90 days of curing) without delay in mechanical strength development for the mortars incorporating into aggregate [
120]. Water washing treatment can enhance the reuse of MSW fly ash as a concrete aggregate, under the condition of a compact pressure of 28 N/mm
2 and sintering temperature of 1,140
○C for 60 minutes [
121].
The leaching problem is the major environmental concern of this application. Although many results show that the heavy metal leaching is not significant, unexpected heavy metal leaching may occur when the structure is demolished or comes in contact with rain [
122].
Attempts at using large amounts of ash in cement clinker have been developed in Japan: this is known as ecocement [
31,
105]. Another process, known as the Co-combustion process [
15,
111], initially uses the energy from MSW incineration to calcine limestone to lime in Portland cement production and then uses a blend of fly ash and bottom ash as part of the clinker raw meal feed.
7.2. Road Pavement
A typical road pavement consists of several layers, which are composed of different types of materials.
Figure 5 shows the structure of a road pavement. The uppermost layer of a sealed pavement is wearing course. It should be even, durable and highly skid resistant. The most common materials for wearing courses are bituminous surface dressing and asphalt concrete. The layer below the wearing course is the base course, which is the main load-spreading layer. The base may consist of premixed asphalt, cement concrete, graded granular gravel, crushed rock, or materials stabilized with lime or cement. The layer below the base course is the sub-base, which is usually constructed from natural gravel or from materials stabilized with cement or lime. The lowest layer is subgrade, which is the soil acting as a foundation for the pavement [
123].
Figure 5.
Typical pavement structure.
Figure 5.
Typical pavement structure.
A possible way to reuse the MSWI bottom ash is to replace the materials in the base course and sub-base. The use of MSWI bottom ash in road pavement provides a simple and direct method for reuse of the incineration ash [
124]. Several road sections have utilized MSWI bottom ash in road construction [
125,
126,
127]. A test road was built in Sweden and the bottom ash was used as a sub-base material [
128]. It was found that substituting gravel in the road base with the bottom ash did not affect the release of Ca, Co, Fe, Mn, Ni, NO
3-N (nitrate-nitrogen) and Pb to the environment. Another three-year study on the utilization of MSWI bottom ash in road pavement in France, showed the concentrations of heavy metals, fluorides and pH values in the leachate were below the limits authorized for potable water [
125]. It indicated that it is safe to use bottom ash for road construction.
In Japan, a melting and stone production plant was built to utilize MSWI fly ash for stone production. One of the applications of the stone produced is for permeable-pavements, with 85 wt% of the grounded stone. This technology has been proven to be commercially applicable, and in 1999, about 1800 m
2 of pavement blocks were used for a park in Chiba Prefecture [
129].
The main concern of these applicants is that the leaching of contaminants into soil and groundwater raise an environmental problem. A possible solution could be pre-treatment of ash by washing to reduce the concentration of contaminants [
39,
126]. It has been reported that the use of fly ash stabilized by cement, which would be used as a road base construction material, did not meet the leaching standards for construction material. In contrast, the washed stabilized fly ash can meet the most severe standards [
56].
7.3. Glasses, Glass-Ceramics and Ceramics
MSWI bottom ash and fly ash has also been used as raw materials for the production of glasses, glass-ceramics and ceramics under high temperature (>1,000
○C). As MSWI bottom ash and fly ash contains high content of SiO
2, Al
2O
3 and CaO, it is possible to use MSWI bottom and fly ash to replace part of the clay for the production of ceramics without pre-treatment. Andreola
et al. has studied the feasibility of using MSWI bottom ash and fly ash for the production of ceramic tile body [
130]. They found that introducing up to 20 wt % of bottom ash into the ceramic body did not substantially change the mineralogical and thermal behaviors of the ceramic body. However, the use of fly ash brought problems in the properties of the ceramic body produced and it may due to high chloride and organic content in the fly ash. Haiying
et al. showed different results for the utilization of fly ash in the production of ceramic tiles [
26]. They showed that with 20% MSWI fly ash added, the ceramic tile register a high compressive strength and low water absorption after 960
○C sintering. The amount of toxic heavy metal leaching was reduced to one-tenth of that in fly ash. The MSWI ash can be used for the production of ceramic tiles and acceptable durability is obtained even when the tiles contain 50% of MSWI ash.
Vitrification, which is one of the most efficient techniques employed to treat hazardous wastes, is able to fix heavy metals or toxic substances into the amorphous structure of glass. At the same time, the toxic substances such as dioxins decompose when melted above 1,300
○C. The vitrified ash is used as road base material, blasting grit, embankments, in the production of construction and decorative materials like ceramic tiles, pavement bricks and water-permeable blocks. Cheng observed that the domestic waste incinerator fly ash sintered and heated at 950
○C for two hours has good potential to manufacture light-weight aggregates or bricks for engineering applications [
23].
Glass-ceramics are fine-grained polycrystalline materials formed when glasses of suitable compositions are heat-treated, and thus undergo controlled crystallization to the lower energy, crystalline state. The mechanical and thermal properties of glass-ceramics are superior to those of the parent glass. Due to its distinct properties, the glass-ceramics find a wide variety of applications. Numerous silicate based wastes have been considered for the production of glass-ceramics [
131]. It has been found that glass produced from the vitrification of incineration ash is suitable for the production of glass-ceramic materials due to its mechanical and thermal characteristics [
25]. Vitrified ashes have been successfully used as raw material for production of glass-ceramics. [
22,
28,
132,
133] and the properties of the glass-ceramics were greatly affected by the heat treatment time and temperature. Yang demonstrated the reuse of MSWI fly ash for glass-ceramic production at a relatively low melting temperature by the use of additives. The melting temperature can be decrease significantly from 1,500
○C to 1,200
○C, which makes the process less energy intensive and environmentally friendly as less energy is consumed [
28].
Glasses obtained from the vitrification process show lower percentages of metals release and less ion release compared to incinerator ash [
134]. However, the exposure of the glass to water may leach toxic substances from the glass matrix. The corrosion behavior of glass and glass-ceramic made of MSWI fly ash was investigated by Park and Heo [
135]. It was found that the leaching of heavy metal ions from the glass and glass-ceramic was well below environmental regulations.
7.4. Agriculture
Nitrogen, phosphorous and potassium are the three main nutrients for plant growth. Both MSWI fly ash and bottom ash have been tested to provide nutrients to soil in agricultural applications. As MSWI bottom ash contains acceptable amounts of phosphorous and potassium, it can be used as a partial replacement of commercial fertilizers. Also, the lime in fly ash can reduce the soil acidity, thus it can be used as a liming agent. However, there are many restrictions for these applications. The heavy metals in bottom ash will be toxic to plants and animals; the high salts content will induce salt stress in plants; the pH value in soil will affect the mobility of elements; and the leaching of heavy metal into ground water will cause environmental concerns. As a result, more research studies have to be done in this field [
136].
One of the studies showed that MSWI fly ash, bottom ash and combined ash can influence plant growth in a positive manner. Growth of alfalfa and Swiss chard in ash-amended soils was similar than that in soils amended with phosphorous and potassium fertilizer, indicating that MSW ash can supply essential nutrients for plant growth. However, the high Mo concentrations and uptake of Cd was significant enough to raise concern if the plant tissue was to be ingested by cattle, sheep, and swine. Also, the high soluble salt content can cause problems with sensitive plants under high amounts of ash amendments [
137].
7.5. Stabilizing Agent
MSWI fly ash has been examined for possible use as a landfill interim cover [
138]. The co-digestion of MSW with the proper amount of MSWI fly ash, could facilitate bacterial activity, digestion efficiency as well as methane gas production rate. It is found that the toxic heavy metals and released ions such as chloride do not have significant impact on anaerobic digestion.
Furthermore, the potential for utilization of MSWI fly ash as S/S binder to treat heavy metal-bearing sludge has been examined. An optimum mix comprising 45% of fly ash, 50% of sludge and 5% of cement could provide the required stabilization and solidification for disposal. This co-disposal approach can minimize the enlargement of landfill volume and effectively stabilize the heavy metals [
32].
7.6. Adsorbents
Adsorption techniques are widely used to remove pollutants from waters and people are looking for low-cost alternatives to activated carbon [
139,
140,
141]. Bottom ash from MSWI has been employed for removing dye and heavy metals from wastewater [
29,
142]. One of the major concerns for using incinerator ash for wastewater treatment is the leachability of heavy metals, as the presence of heavy metals in the water is a serious problem due to their high toxicity. The use of MSW fly ash as the adsorbent is less common than the bottom ash [
143]. This may due to the fact there are more toxic heavy metals in the leachate of fly ash, which reduces the possibility to use it as an adsorbent. In contrast, the bottom ash rarely releases hazardous heavy metal in the leachate.
The cation exchange capacity of MSWI bottom ash is the total amount of exchangeable capacity (milliequivalents per 100 grams of adsorbing materials). It is important to compare adsorption capacities for different materials. It has been found that the cation exchange capacity (CEC) greatly depends on the particle size of the bottom ash. The adsorption characteristics of heavy metals by different MSW bottom ash particle sizes has been investigated, the smaller the particle size, the higher the CEC value and surface area. The adsorption rate of heavy metals increases with decreasing bottom ash particle size, which means the adsorption rate is proportional to the particle size and specific surface area of bottom ash. Decreasing the particle size from +120 to −100 mesh increases the CEC of the bottom ash from 8.0 to 24.6 meq/100 g. It has been used for removing Ni and Cu from plating rinse water with pH of 3.8 [
142].
Bottom ash was also used as an adsorbent for the removal of dyes from wastewater. Gupta
et al. [
29] studied the removal of dyes from wastewater using bottom ash from MSWI from Belgium. The results showed that the removals of dyes are up to 98% by the batch method and the adsorption capacity is comparable to other available adsorbents. The optimum pH for the dye adsorption ranged from 5 to 8 and depended on the chemical structure of the dye molecules. The adsorption capacities of alizarin yellow, fast green and methyl violet onto the bottom ash were found to be 0.083, 0.068, 0.043 mmol/g respectively. It suggests that the low-cost bottom ash can be used for the removal of dye from wastewater.
Besides use as an adsorbent for wastewater treatment, the bottom ash was also used for gas purification. The bottom ash was used for the removal of reduced sulfur compounds from a real landfill gas [
144]. It was found that the bottom ash was able to remove hydrogen sulfide, methyl mercaptan, dimethyl sulfide without affecting the energetic content of the landfill gas as methane is not retained in the bottom ash. It was found that one kilogram bottom ash is able to sequestrate more than 3.0 g of hydrogen sulfide, 44 mg of methyl mercaptan, and 86 mg of dimethyl sulfide. Hydrogen sulfide and methyl mercaptan retention is probably due to acid-basic reactions involving sulfur mineralization under the form of low solubility metal sulfides. The retention mechanism for dimethyl sulfide is most likely by physical adsorption.
Several studies have reported the use of fly ash as an adsorbent for dyes [
143,
145], heavy metals [
146,
147,
148,
149] and organics [
150,
151]. One of the advantages of using fly ash for wastewater treatment is that the fly ash has neutralizing ability; therefore the fly ash can be used in the treatment of acidic industrial wastewater. It is because excess of lime was always added to remove the HCl and SO
2 in the flue gas in MSW incinerators [
113]. However, the final pH of the treated water greatly depends on the liquid to solid ratio used.
7.7. Zeolite Production
Both synthetic and natural zeolites have been employed for environmental and catalytic applications [
152,
153]. As the main components of coal fly ash are SiO
2 and Al
2O
3 (70–80%), which exhibit a similar composition to zeolite, coal fly ash has been used as a raw material to synthesize zeolite-like materials [
154,
155]. Although, MSWI fly ash has a lower content of SiO
2 and Al
2O
3 (15–30%), different types of zeolites have been synthesized by utilizing the MSWI fly ash by fusion or hydrothermal process [
156,
157]. The surface area and CEC of the zeolite produced was found significantly higher than the raw fly ash. Although the CEC of the zeolite produced (90 meq/100 g) is lower than the commercial zeolite (200–300 meq/100 g), it shows the feasibility of using MSWI fly ash to synthesis zeolite. However, it was found that the residual liquid after the synthesize contains high concentrations of heavy metals such as Pb and Zn needs further treatment before discharge [
156].
As MWSI fly ash has a lower content of SiO
2 and Al
2O
3, a mixed fly ash of MSW and coal may become a feasible way for improving the quality of the zeolite and the use of MSW ash for zeolite production. Fan
et al. used the waste fly ash from MSW and coal co-combustion power plant to synthesize zeolites [
158]. They demonstrated that the properties of the zeolite products mainly depend on NaOH concentration, reaction temperature and crystallization time. The development of Zeolite X is favorable under development at lower NaOH/ash ratio and operating temperature and zeolite HS can be synthesized at higher NaOH/ash ratio and operating temperature [
158]. The monolayer adsorption capacities of Zn
2+ onto the zeolite produced was found to be 121.97 mg/g, which is higher than coal fly ash based zeolite. Fan
et al. also modified the zeolite with iron (II) ions and used this product for Arsenic (V) adsorption as hydrated iron has a high affinity towards arsenic oxyanions [
149]. The adsorption capacity for As (V) was 13.04 mg/g. The adsorption capacity of modified zeolite, developed from fly ash, is comparable to the capacities of many of the other adsorbents [
159]. Zeolite was also successfully synthesized by alkaline hydrothermal treatment of MSWI bottom ash; however, the use of bottom ash from MSWI for zeolite synthesis is less common than using fly ash [
160].