Effects of Substrate on Movement Patterns and Behavior of Stream Fish through Culverts: An Experimental Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Experimental Design
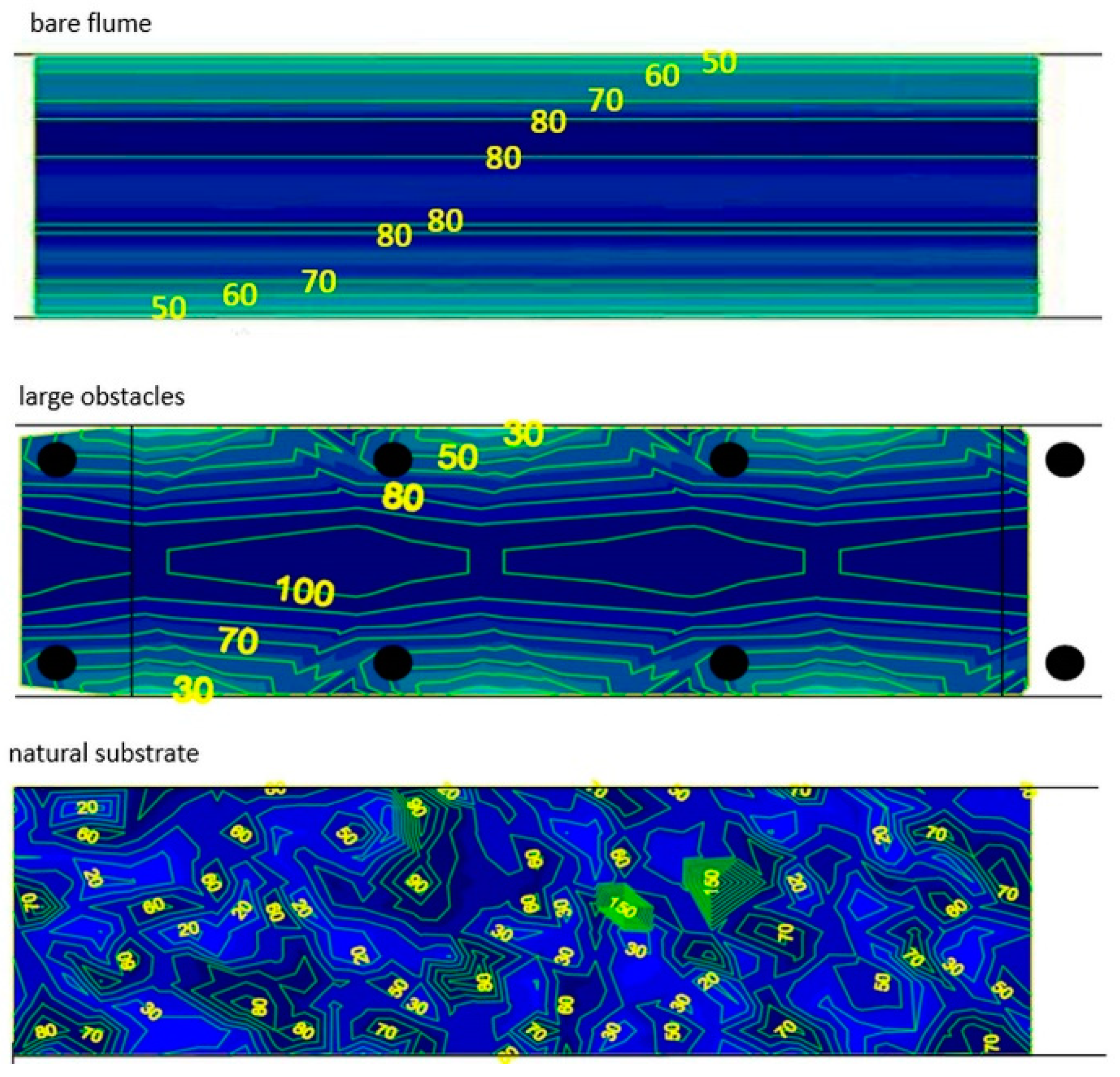
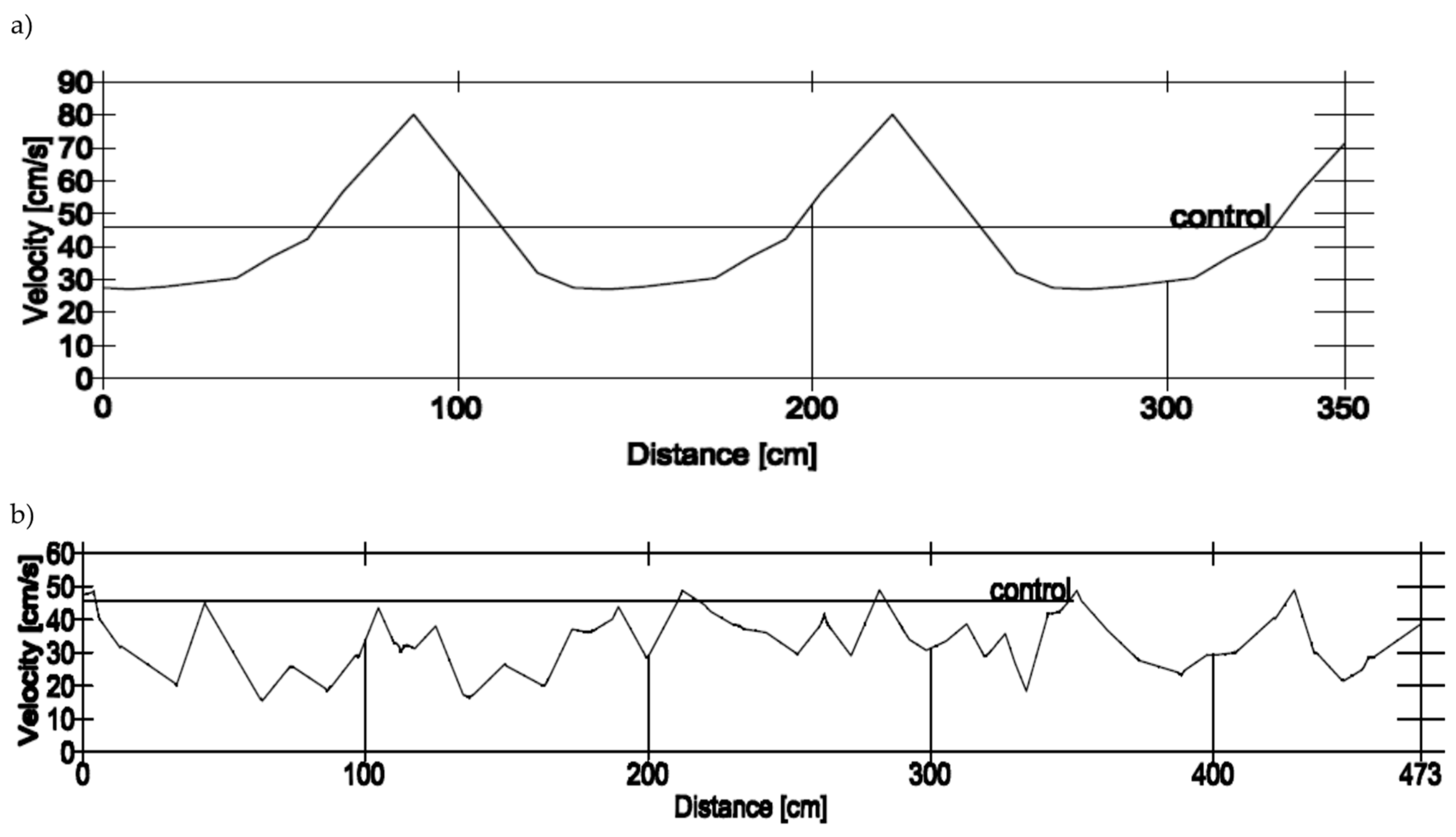
2.3. Velocity Profiles and Swim Paths
2.4. Statistical Analyses
3. Results
3.1. Swim Paths
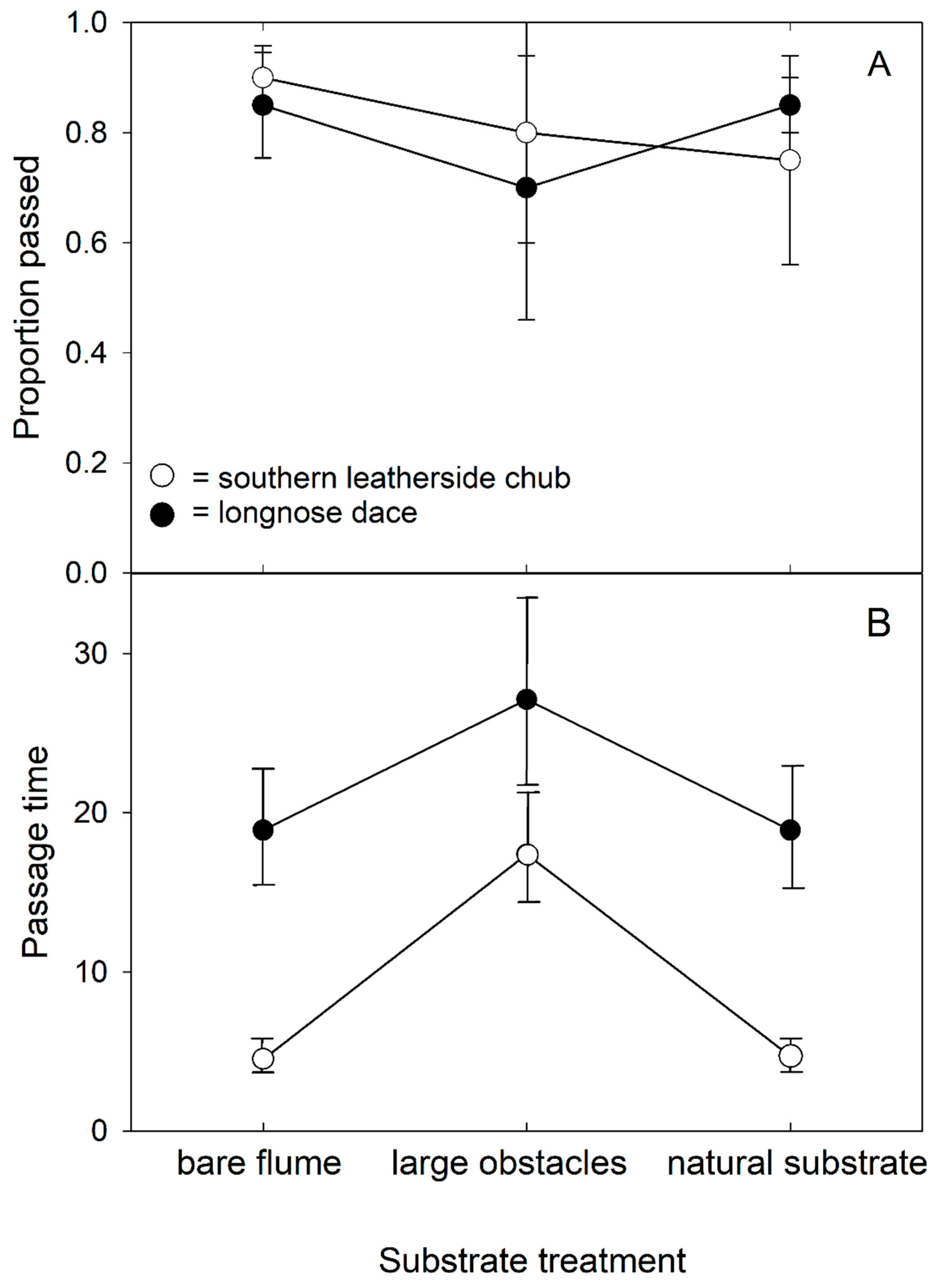
3.2. Passage Success and Time
3.3. Velocity Profiles and Swim Paths
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Starrs, D.; Ebner, B.; Lintermans, M.; Fulton, C. Using sprint swimming performance to predict upstream passage of the endangered Macquarie perch in a highly regulated river. Fish. Manag. Ecol. 2011, 18, 360–374. [Google Scholar] [CrossRef]
- Price, D.M.; Quinn, T.; Barnard, R.J. Fish passage effectiveness of recently constructed road crossing culverts in the Puget Sound region of Washington State. N. Am. J. Fish. Manag. 2010, 30, 1110–1125. [Google Scholar] [CrossRef]
- Warren Jr, M.L.; Pardew, M.G. Road crossings as barriers to small-stream fish movement. Trans. Am. Fish. Soc. 1998, 127, 637–644. [Google Scholar] [CrossRef]
- Blank, M.; Cahoon, J.; Burford, D.; McMahon, T.; Stein, O. Studies of Fish Passage through Culverts in Montana; Road Ecology Center: Berkeley, CA, USA, 2005. [Google Scholar]
- Clark, S.P.; Toews, J.S.; Tkach, R. Beyond average velocity: Modelling velocity distributions in partially filled culverts to support fish passage guidelines. Int. J. River Basin Manag. 2014, 12, 101–110. [Google Scholar] [CrossRef]
- MacPherson, L.M.; Sullivan, M.G.; Foote, A.L.; Stevens, C.E. Effects of culverts on stream fish assemblages in the Alberta foothills. N. Am. J. Fish. Manag. 2012, 32, 480–490. [Google Scholar] [CrossRef]
- Park, D.; Sullivan, M.; Bayne, E.; Scrimgeour, G. Landscape-level stream fragmentation caused by hanging culverts along roads in Alberta’s boreal forest. Can. J. For. Res. 2008, 38, 566–575. [Google Scholar] [CrossRef]
- Gibson, R.J.; Haedrich, R.L.; Wernerheim, C.M. Loss of fish habitat as a consequence of inappropriately constructed stream crossings. Fisheries 2005, 30, 10–17. [Google Scholar] [CrossRef]
- Rodríguez, T.T.; Agudo, J.P.; Mosquera, L.P.; González, E.P. Evaluating vertical-slot fishway designs in terms of fish swimming capabilities. Ecol. Eng. 2006, 27, 37–48. [Google Scholar] [CrossRef]
- Winston, M.R.; Taylor, C.M.; Pigg, J. Upstream extirpation of four minnow species due to damming of a prairie stream. Trans. Am. Fish. Soc. 1991, 120, 98–105. [Google Scholar] [CrossRef]
- Roscoe, D.W.; Hinch, S.G. Effectiveness monitoring of fish passage facilities: Historical trends, geographic patterns and future directions. Fish Fish. 2010, 11, 12–33. [Google Scholar] [CrossRef]
- Mallen-Cooper, M.; Brand, D. Non-salmonids in a salmonid fishway: What do 50 years of data tell us about past and future fish passage? Fish. Manag. Ecol. 2007, 14, 319–332. [Google Scholar] [CrossRef]
- Franklin, P.A.; Bartels, B.; Ecosystems, F. Restoring connectivity for migratory native fish in a New Zealand stream: Effectiveness of retrofitting a pipe culvert. Aquat. Conserv. Mar. 2012, 22, 489–497. [Google Scholar] [CrossRef]
- House, M.R.; Pyles, M.R.; White, D. Velocity distributions in streambed simulation culverts used for fish passage. J. Am. Water Resour. Assoc. 2005, 41, 209–217. [Google Scholar] [CrossRef]
- Richmond, M.C.; Deng, Z.; Guensch, G.R.; Tritico, H.; Pearson, W.H. Mean flow and turbulence characteristics of a full-scale spiral corrugated culvert with implications for fish passage. Ecol. Eng. 2007, 30, 333–340. [Google Scholar] [CrossRef]
- Belford, D.A.; Gould, W.R. An evaluation of trout passage through six highway culverts in Montana. N. Am. J. Fish. Manag. 1989, 9, 437–445. [Google Scholar] [CrossRef]
- Fitch, G.M. Nonanadromous Fish Passage in Highway Culverts. Final Report; The National Academies of Sciences, Engineering, and Medicine: Washington, DC, USA, 1995. [Google Scholar]
- Mallen-Cooper, M.; Stuart, I. Optimising Denil fishways for passage of small and large fishes. Fish. Manag. Ecol. 2007, 14, 61–71. [Google Scholar] [CrossRef]
- Cooke, S.J.; Bunt, C.M.; Hamilton, S.J.; Jennings, C.A.; Pearson, M.P.; Cooperman, M.S.; Markle, D.F. Threats, conservation strategies, and prognosis for suckers (Catostomidae) in North America: Insights from regional case studies of a diverse family of non-game fishes. Biol. Conserv. 2005, 121, 317–331. [Google Scholar] [CrossRef]
- Hard, A.; Kynard, B. Video evaluation of passage efficiency of American shad and sea lamprey in a modified Ice Harbor fishway. N. Am. J. Fish. Manag. 1997, 17, 981–987. [Google Scholar] [CrossRef]
- Laine, A.; Kamula, R.; Hooli, J. Fish and lamprey passage in a combined Denil and vertical slot fishway. Fish. Manag. Ecol. 1998, 5, 31–44. [Google Scholar] [CrossRef]
- Moser, M.L.; Matter, A.L.; Stuehrenberg, L.C.; Bjornn, T.C. Use of an extensive radio receiver network to document Pacific lamprey (Lampetra tridentata) entrance efficiency at fishways in the Lower Columbia River, USA. In Aquatic Telemetry; Springer: New York, NY, USA, 2002; pp. 45–53. [Google Scholar]
- Tudorache, C.; Viaene, P.; Blust, R.; Vereecken, H.; De Boeck, G. A comparison of swimming capacity and energy use in seven European freshwater fish species. Ecol. Freshw. Fish 2008, 17, 284–291. [Google Scholar] [CrossRef]
- Knaepkens, G.; Baekelandt, K.; Eens, M. Fish pass effectiveness for bullhead (Cottus gobio), perch (Perca fluviatilis) and roach (Rutilus rutilus) in a regulated lowland river. Ecol. Freshw. Fish 2006, 15, 20–29. [Google Scholar] [CrossRef]
- Facey, D.; Grossman, G. The relationship between water velocity, energetic costs, and microhabitat use in four North American stream fishes. Hydrobiologia 1992, 239, 1–6. [Google Scholar] [CrossRef]
- Peake, S.; Beamish, F.W.; McKinley, R.; Scruton, D.; Katopodis, C. Relating swimming performance of lake sturgeon, Acipenser fulvescens, to fishway design. Can. J. Fish. Aquat. Sci. 1997, 54, 1361–1366. [Google Scholar] [CrossRef]
- Downie, A.T.; Kieffer, J.D. A split decision: The impact of substrate type on the swimming behaviour, substrate preference and UCrit of juvenile shortnose sturgeon (Acipenser brevirostrum). Environ. Biol. Fishes 2017, 100, 17–25. [Google Scholar] [CrossRef]
- Kieffer, J.; Arsenault, L.; Litvak, M. Behaviour and performance of juvenile shortnose sturgeon Acipenser brevirostrum at different water velocities. J. Fish Biol. 2009, 74, 674–682. [Google Scholar] [CrossRef] [PubMed]
- May, L.; Kieffer, J. The effect of substratum type on aspects of swimming performance and behaviour in shortnose sturgeon Acipenser brevirostrum. J. Fish Biol. 2017, 90, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Branco, P.; Santos, J.M.; Katopodis, C.; Pinheiro, A.; Ferreira, M.T. Pool-type fishways: Two different morpho-ecological cyprinid species facing plunging and streaming flows. PLoS ONE 2013, 8, e65089. [Google Scholar] [CrossRef]
- Romão, F.; Quaresma, A.L.; Branco, P.; Santos, J.M.; Amaral, S.; Ferreira, M.T.; Katopodis, C.; Pinheiro, A.N. Passage performance of two cyprinids with different ecological traits in a fishway with distinct vertical slot configurations. Ecol. Eng. 2017, 105, 180–188. [Google Scholar] [CrossRef]
- Doehring, K.; Young, R.G.; McIntosh, A.R. Factors affecting juvenile galaxiid fish passage at culverts. Mar. Freshw. Res. 2011, 62, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Johnson, G.E.; Pearson, W.H.; Southard, S.L.; Mueller, R.P. Upstream movement of juvenile coho salmon in relation to environmental conditions in a culvert test bed. Trans. Am. Fish. Soc. 2012, 141, 1520–1531. [Google Scholar] [CrossRef]
- Pearson, W.; Richmond, M.; Johnson, G.; Sargeant, S.; Mueller, R.; Cullinan, V.; Deng, Z.; Dibrani, B.; Guensch, G.; May, C. Protocols for Evaluation of Upstream Passage of Juvenile Salmonids in an Experimental Culvert Test Bed; Report No. PNWD-3525; Battelle Memorial Institute: Richland, WA, USA, 2005. [Google Scholar]
- Kristensen, E.A.; Baattrup-Pedersen, A.; Thodsen, H. An evaluation of restoration practises in lowland streams: Has the physical integrity been re-created? Ecol. Eng. 2011, 37, 1654–1660. [Google Scholar] [CrossRef]
- Rodgers, E.M.; Heaslip, B.M.; Cramp, R.L.; Riches, M.; Gordos, M.A.; Franklin, C.E. Substrate roughening improves swimming performance in two small-bodied riverine fishes: Implications for culvert remediation and design. Conserv. Physiol. 2017, 5, cox034. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.L.; Kristensen, E.A.; Kronvang, B.; Thodsen, H. Ecological effects of re-introduction of salmonid spawning gravel in lowland Danish streams. River Res. Appl. 2009, 25, 626–638. [Google Scholar] [CrossRef]
- Santos, J.M.; Branco, P.; Katopodis, C.; Ferreira, T.; Pinheiro, A. Retrofitting pool-and-weir fishways to improve passage performance of benthic fishes: Effect of boulder density and fishway discharge. Ecol. Eng. 2014, 73, 335–344. [Google Scholar] [CrossRef]
- Heimerl, S.; Hagmeyer, M.; Echteler, C. Numerical flow simulation of pool-type fishways: New ways with well-known tools. Hydrobiologia 2008, 609, 189. [Google Scholar] [CrossRef]
- Liao, J.C. A review of fish swimming mechanics and behaviour in altered flows. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007, 362, 1973–1993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blake, R. The biomechanics of intermittent swimming behaviours in aquatic vertebrates. In Biomechanics in Animal Behaviour; CRC Press: Boca Raton, FL, USA, 2000; pp. 79–103. [Google Scholar]
- Nursall, J. Some behavioral interactions of spottail shiners (Notropis hudsonius), yellow perch (Perca flavescens), and northern pike (Esox lucius). J. Fish. Board Can. 1973, 30, 1161–1178. [Google Scholar] [CrossRef]
- Webb, P.W. Control of posture, depth, and swimming trajectories of fishes. Integr. Comp. Biol. 2002, 42, 94–101. [Google Scholar] [CrossRef]
- Webb, P.W. Entrainment by river chub Nocomis micropogon and smallmouth bass Micropterus dolomieu on cylinders. J. Exp. Biol. 1998, 201, 2403–2412. [Google Scholar]
- Ward, D.L.; Schultz, A.A.; Matson, P.G. Differences in swimming ability and behavior in response to high water velocities among native and nonnative fishes. Environ. Biol. Fishes 2003, 68, 87–92. [Google Scholar] [CrossRef]
- Mullen, D.M.; Burton, T.M. Size-related habitat use by longnose dace (Rhinichthys cataractae). Am. Midl. Nat. 1995, 133, 177–183. [Google Scholar] [CrossRef]
- Webb, P.W.; Gerstner, C.L.; Minton, S.T. Station-holding by the mottled sculpin, Cottus bairdi (Teleostei: Cottidae), and other fishes. Copeia 1996, 488–493. [Google Scholar] [CrossRef]
- Edwards, E.A.; Li, H.; Schreck, C.B. Habitat Suitability Index Models: Longnose Dace; Western Energy and Land Use Team: Fort Collins, CO, USA, 1983. [Google Scholar]
- Aedo, J.; Belk, M.C.; Hotchkiss, R.H. Swimming Performance and Morphology of Utah Fishes: Critical Information for Culvert Design in Utah Streams; The National Academies of Sciences, Engineering, and Medicine: Washington, DC, USA, 2009. [Google Scholar]
- Wilson, K.W.; Belk, M.C. Habitat characteristics of leatherside chub (Gila copei) at two spatial scales. West. N. Am. Nat. 2001, 61, 36–42. [Google Scholar]
- Belk, M.; Johnson, J.; Wilson, K.; Smith, M.; Houston, D. Variation in intrinsic individual growth rate among populations of leatherside chub (Snyderichthys copei Jordan & Gilbert): Adaptation to temperature or length of growing season? Ecol. Freshw. Fish 2005, 14, 177–184. [Google Scholar]
- Ward, D.; Maughan, O.; Bonar, S. A Variable-Speed Swim Tunnel for Testing the Swimming Ability of Age-0 Fish. N. Am. J. Aquac. 2002, 64, 228–231. [Google Scholar] [CrossRef]
- Peake, S. An evaluation of the use of critical swimming speed for determination of culvert water velocity criteria for smallmouth bass. Trans. Am. Fish. Soc. 2004, 133, 1472–1479. [Google Scholar] [CrossRef]
- Peake, S.J.; Farrell, A.P. Postexercise physiology and repeat performance behaviour of free-swimming smallmouth bass in an experimental raceway. Physiol. Biochem. Zool. 2005, 78, 801–807. [Google Scholar] [CrossRef]
- Castro-Santos, T. Optimal swim speeds for traversing velocity barriers: An analysis of volitional high-speed swimming behavior of migratory fishes. J. Exp. Biol. 2005, 208, 421–432. [Google Scholar] [CrossRef]
- Schlichting, H. Boundary-Layer Theory, 7th ed.; McGraw-Hill Book Co.: New York, NY, USA, 1979. [Google Scholar]
- Belk, M.C.; Benson, L.J.; Rasmussen, J.; Peck, S.L. Hatchery-induced morphological variation in an endangered fish: A challenge for hatchery-based recovery efforts. Can. J. Fish. Aquat. Sci. 2008, 65, 401–408. [Google Scholar] [CrossRef]
- Song, T.; Chiew, Y. Turbulence measurement in nonuniform open-channel flow using acoustic Doppler velocimeter (ADV). J. Eng. Mech. 2001, 127, 219–232. [Google Scholar] [CrossRef]
- SonTek. ADV Principles of Operation; Sontek Inc.: San Diego, CA, USA, 2001. [Google Scholar]
- Lockhart, S. Tutorial Guide to AutoCad; Schroff Development Corporation: Mission, KS, USA, 2012. [Google Scholar]
- SAS Institute. SAS-STAT Software: Changes and Enhancements through Release 6.12; SAS Institute: Cary, NC, USA, 1997. [Google Scholar]
- Littell, R.C.; Milliken, G.A.; Stroup, W.W.; Wolfinger, R.D.; Schabenberger, O. SAS for Mixed Models; SAS Institute: Cary, NC, USA, 2007. [Google Scholar]
- Stoll, S.; Kail, J.; Lorenz, A.W.; Sundermann, A.; Haase, P. The importance of the regional species pool, ecological species traits and local habitat conditions for the colonization of restored river reaches by fish. PLoS ONE 2014, 9, e84741. [Google Scholar] [CrossRef] [PubMed]
- Sigler, W.F.; Sigler, J.W. Fishes of Utah: A Natural History; University of Utah Press Salt: Lake City, UT, USA, 1996. [Google Scholar]
- Ead, S.; Rajaratnam, N.; Katopodis, C.; Ade, F. Turbulent open-channel flow in circular corrugated culverts. J. Hydraul. Eng. 2000, 126, 750–757. [Google Scholar] [CrossRef]
- Chin, C.-L.; Murray, D.W. Variation of velocity distribution along nonuniform open-channel flow. J. Hydraul. Eng. 1992, 118, 989–1001. [Google Scholar] [CrossRef]
- Billman, E.J.; Pyron, M. Evolution of form and function: Morphology and swimming performance in North American minnows. J. Freshw. Ecol. 2005, 20, 221–232. [Google Scholar] [CrossRef]
- Boubée, J.; Jowett, I.; Nichols, S.; Williams, E. Fish Passage at Culverts: A Review, with Possible Solutions for New Zealand Indigenous Species; Department of Conservation: Wellington, New Zealand, 1999.
- Mitchell, C. Swimming performances of some native freshwater fishes. N. Z. J. Mar. Freshw. Res. 1989, 23, 181–187. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, J.; Davies, P. Improving the upstream passage of two galaxiid fish species through a pipe culvert. Fish. Manag. Ecol. 2007, 14, 221–230. [Google Scholar] [CrossRef]
- Powers, P.; Bates, K. Culvert Hydraulics Related to Upstream Juvenile Salmon Passage; Washington Department of Fish and Wildlife, Land and Restoration Services Program, Environmental Engineering Services: Washington, DC, USA, 1997.
- Hotchkiss, R.H.; Frei, C.M. Design for Fish Passage at Roadway-Stream Crossings: Synthesis Report; Federal Highway Administration: Washington, DC, USA, 2007.
- Lacey, R.J.; Neary, V.S.; Liao, J.C.; Enders, E.C.; Tritico, H.M. The IPOS framework: Linking fish swimming performance in altered flows from laboratory experiments to rivers. River Res. Appl. 2012, 28, 429–443. [Google Scholar] [CrossRef]


| Source of Variation | df | Chi-Square | P-Value |
|---|---|---|---|
| species | 1 | 0.09 | 0.76 |
| substrate | 2 | 2.09 | 0.35 |
| species × substrate | 2 | 1.43 | 0.49 |
| Source of Variation | df | F Value | P-Value |
|---|---|---|---|
| species | 1/89 | 46.31 | <0.0001 |
| substrate | 2/89 | 11.94 | <0.0001 |
| species × substrate | 2/89 | 3.96 | 0.0224 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, K.; Wait, L.E.; Monk, S.K.; Rader, R.; Hotchkiss, R.H.; Belk, M.C. Effects of Substrate on Movement Patterns and Behavior of Stream Fish through Culverts: An Experimental Approach. Sustainability 2019, 11, 470. https://doi.org/10.3390/su11020470
Johnson K, Wait LE, Monk SK, Rader R, Hotchkiss RH, Belk MC. Effects of Substrate on Movement Patterns and Behavior of Stream Fish through Culverts: An Experimental Approach. Sustainability. 2019; 11(2):470. https://doi.org/10.3390/su11020470
Chicago/Turabian StyleJohnson, Kyla, Lindsay E. Wait, Suzanne K. Monk, Russell Rader, Rollin H. Hotchkiss, and Mark C. Belk. 2019. "Effects of Substrate on Movement Patterns and Behavior of Stream Fish through Culverts: An Experimental Approach" Sustainability 11, no. 2: 470. https://doi.org/10.3390/su11020470





