Role of Scirpus mariqueter on Methane Emission from an Intertidal Saltmarsh of Yangtze Estuary
Abstract
:1. Introduction
2. Methods and Materials
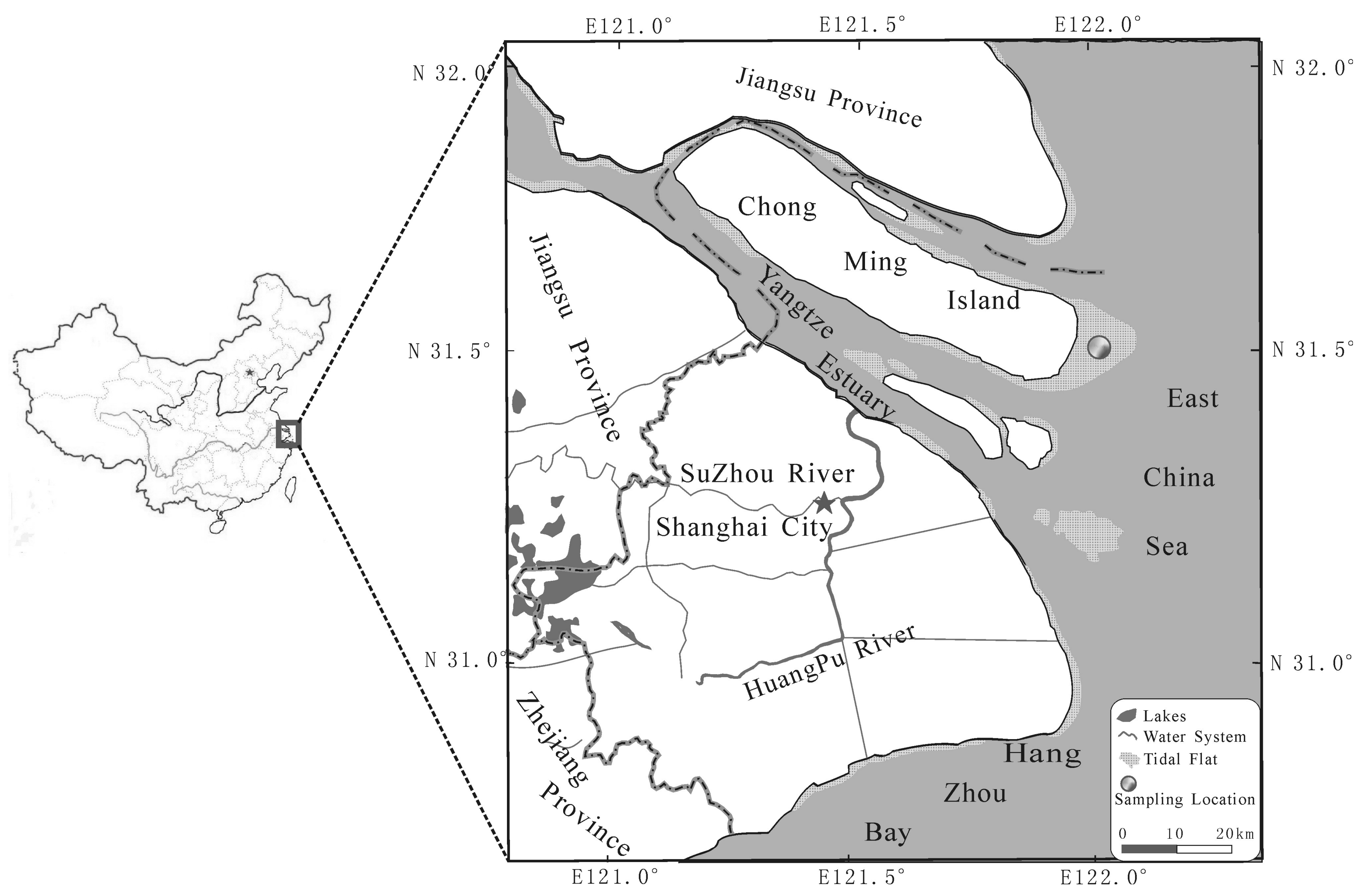
2.1. Study Site
2.2. Sampling
2.3. Pore-Water CH4 Extraction
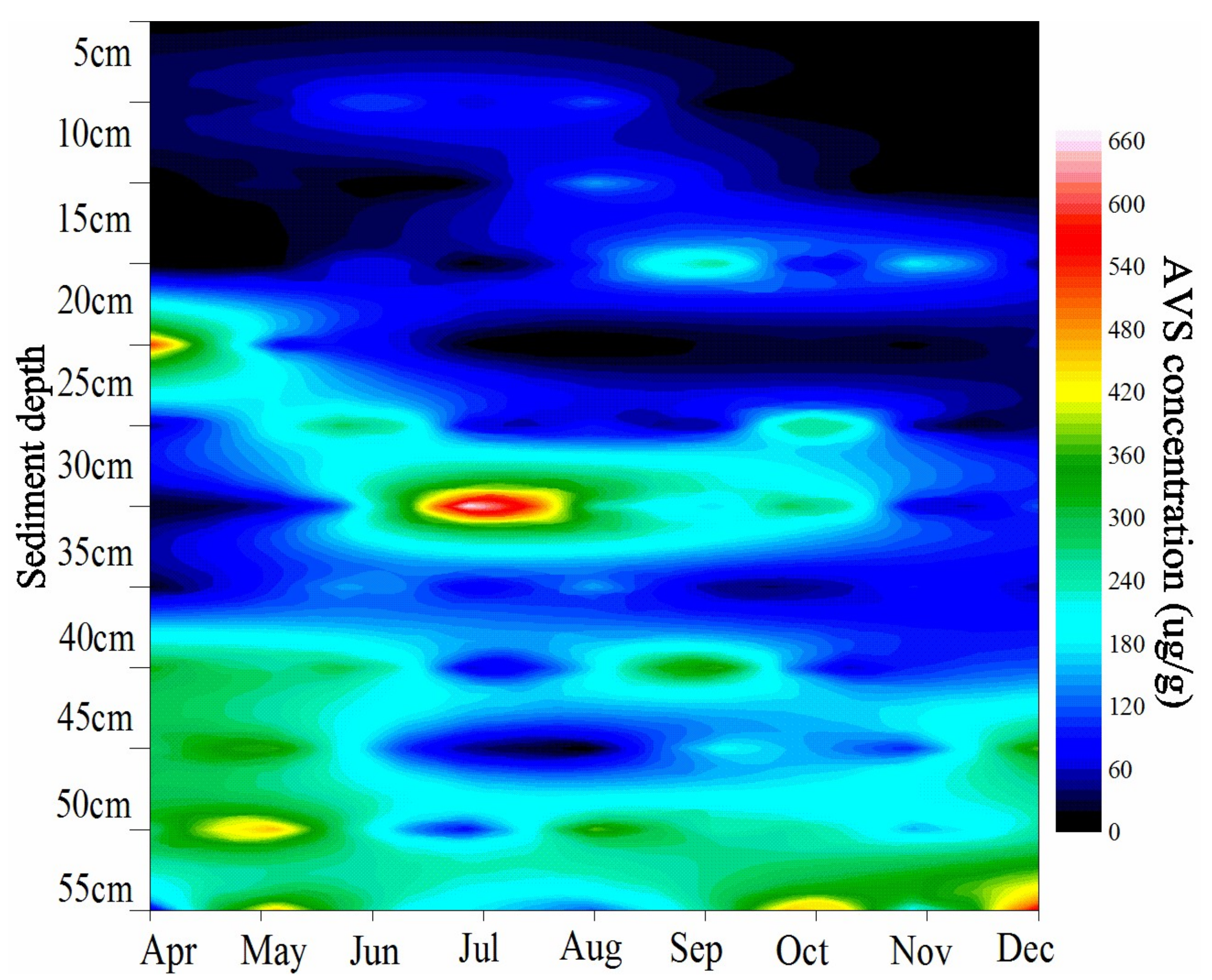
2.4. Sediment Properties
2.5. Data Calculating
2.6. Statistical Analysis
3. Results
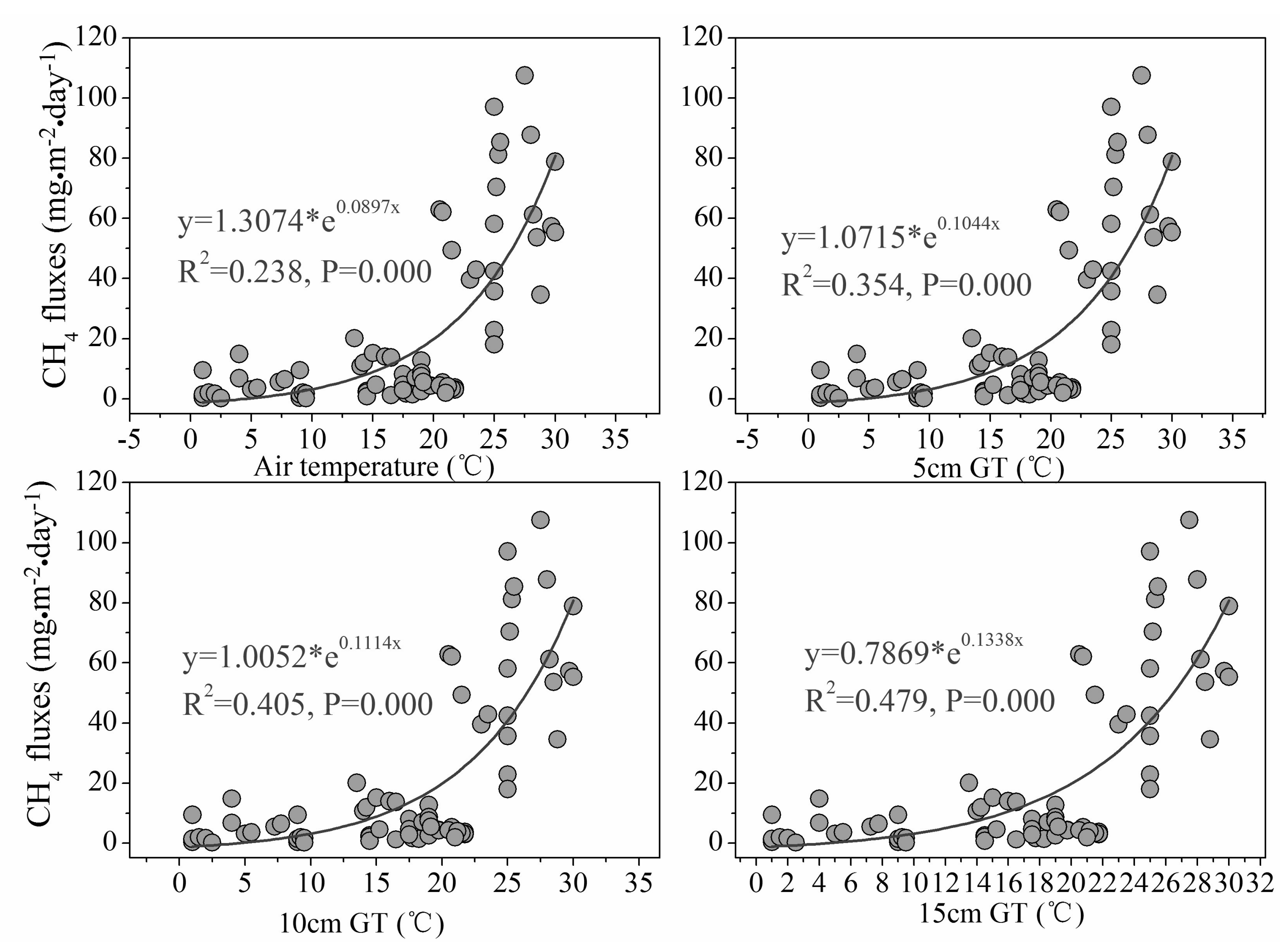
3.1. Environmental Factors
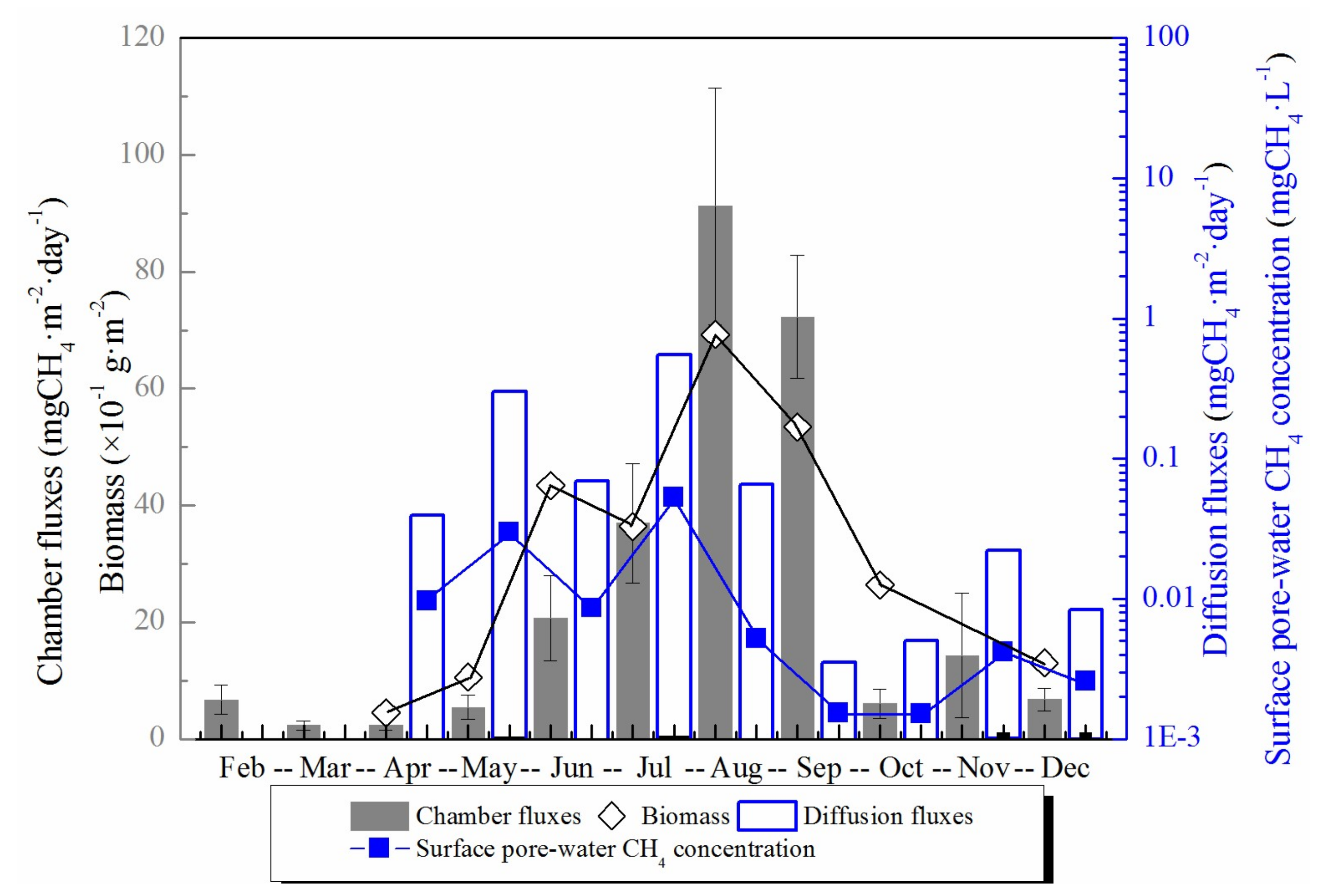
3.2. CH4 Fluxes
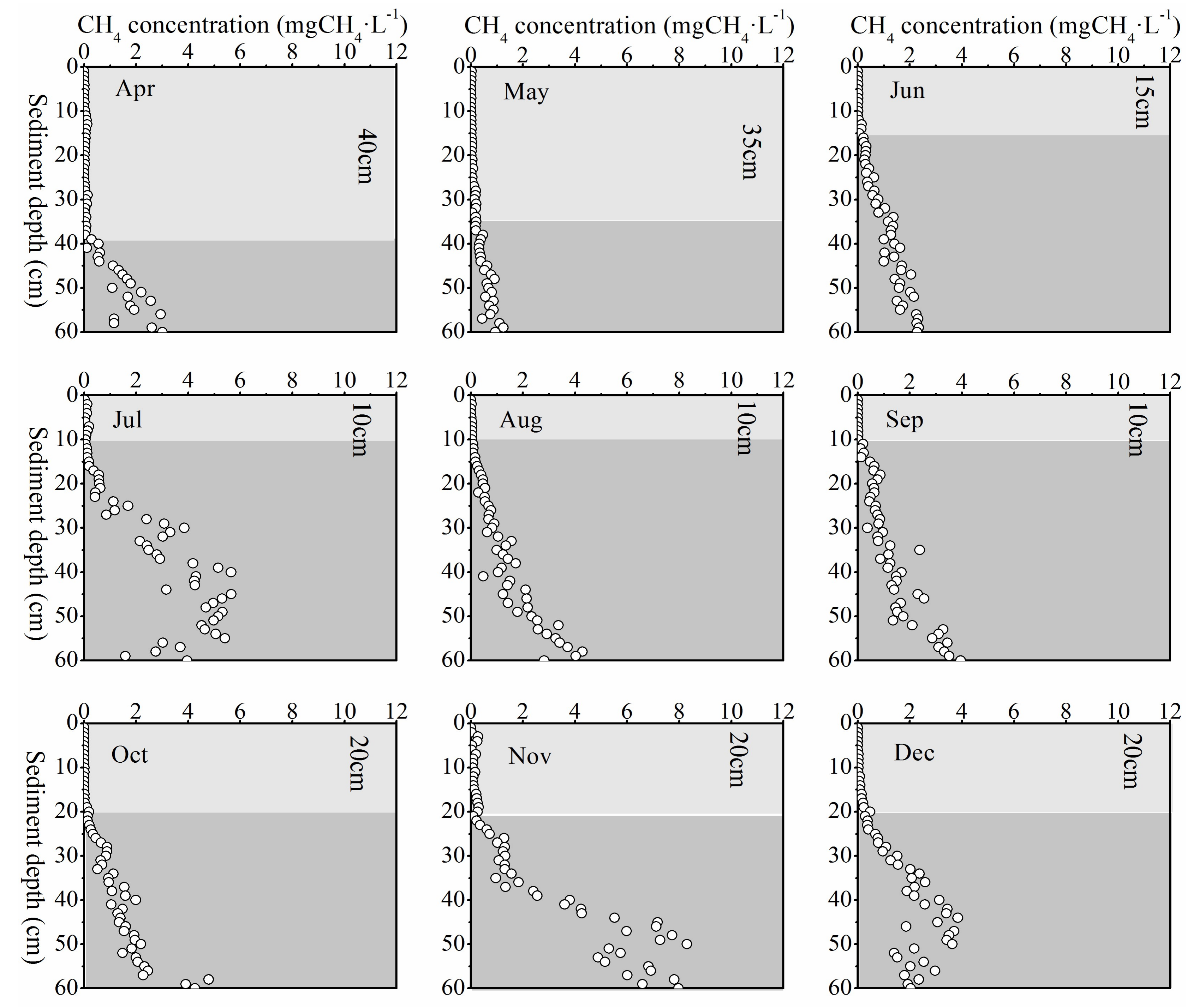
3.3. Pore-Water CH4 Concentration
4. Discussion
4.1. Dominant Mechanism of CH4 Emission
4.2. The Implication of Pore-Water CH4 Concentration for CH4 Emission
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- IPCC. Climate Change 2013: The Physical Science Basis; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Keller, J.K.; Sutton-Grier, A.E.; Bullock, A.L.; Megonigal, J.P. Anaerobic metabolism in tidal freshwater wetlands: I. Plant removal effects on iron reduction and methanogenesis. Estuaries Coasts 2013, 36, 457–470. [Google Scholar] [CrossRef]
- Whiticar, M.J. Diagenetic relationships of methanogenesis, nutrients, acoustic turbidity, pockmarks and freshwater seepages in EckernfordeBay. Mar. Geol. 2002, 182, 29–53. [Google Scholar] [CrossRef]
- Moore, T.R.; Dalva, M. Methane and carbon dioxide exchange potentials of peat soils in aerobic and anaerobic laboratory incubations. Soil Biol. Biochem. 1997, 29, 1157–1164. [Google Scholar] [CrossRef]
- Segers, R. Methane production and methane consumption: A review of processes underlying wetland methane fluxes. Biogeochemistry 1998, 41, 23–51. [Google Scholar] [CrossRef]
- Tang, J.; Zhuang, Q.; Shannon, R.D.; White, J.R. Quantifying wetland methane emissions with process-based models of different complexities. Biogeosciences 2010, 7, 6121–6171. [Google Scholar] [CrossRef]
- Watson, A.; Stephen, K.D.; Nedwell, D.B.; Arah, J.R. Oxidation of methane in peat: Kinetics of CH4 and O2 removal and the role of plant roots. Soil Biol. Biochem. 1997, 29, 1257–1267. [Google Scholar] [CrossRef]
- Fritz, C.; Pancotto, V.A.; Elzenga, J.; Visser, E.J.; Grootjans, A.P.; Pol, A.; Iturraspe, R.; Roelofs, J.G.M.; Smolders, A.J. Zero methane emission bogs: Extreme rhizosphere oxygenation by cushion plants in Patagonia. New Phytol. 2011, 190, 398–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smemo, K.A.; Yavitt, J.B. Anaerobic oxidation of methane: An underappreciated aspect of methane cycling in peatland ecosystems? Biogeosciences 2011, 8, 779–793. [Google Scholar] [CrossRef]
- Liu, D.Y.; Ding, W.X.; Jia, Z.J.; Cai, Z.C. Relation between methanogenic archaea and methane production potential in selected natural wetland ecosystems across China. Biogeosciences 2011, 8, 329–338. [Google Scholar] [CrossRef]
- Chowdhury, T.R.; Dick, R.P. Ecology of aerobic methanotrophs in controlling methane fluxes from wetlands. Appl. Soil Ecol. 2013, 65, 8–22. [Google Scholar] [CrossRef]
- Chanton, J.P. The effect of gas transport on the isotope signature of methane in wetlands. Org. Geochem. 2005, 36, 753–768. [Google Scholar] [CrossRef]
- Dingemans, B.J.; Bakker, E.S.; Bodelier, P.L. Aquatic herbivores facilitate the emission of methane from wetlands. Ecology 2011, 92, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Kao-Kniffin, J.; Freyre, D.S.; Balser, T.C. Methane dynamics across wetland plant species. Aquat. Bot. 2010, 93, 107–113. [Google Scholar] [CrossRef]
- Lai, D.Y.F. Methane Dynamics in Northern Peatlands: A Review. Pedosphere 2009, 19, 409–421. [Google Scholar] [CrossRef]
- Belger, L.; Forsberg, B.R.; Melack, J.M. Carbon dioxide and methane emissions from interfluvial wetlands in the upper Negro River basin, Brazil. Biogeochemistry 2011, 105, 171–183. [Google Scholar] [CrossRef]
- Laanbroek, H.J. Methane emission from natural wetlands: Interplay between emergent macrophytes and soil microbial processes. A mini-review. Ann. Bot. 2010, 105, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Larmola, T.; Tuittila, E.S.; Tiirola, M.; Nykänen, H.; Martikainen, P.J.; Yrjälä, K.; Tuomivirta, T.; Fritze, H. The role of Sphagnum mosses in the methane cycling of a boreal mire. Ecology 2010, 91, 2356–2365. [Google Scholar] [CrossRef] [PubMed]
- Cho, R.; Schroth, M.H.; Zeyer, J. Circadian methane oxidation in the root zone of rice plants. Biogeochemistry 2012, 111, 317–330. [Google Scholar] [CrossRef]
- Koelbener, A.; Ström, L.; Edwards, P.J.; Venterink, H.O. Plant species from mesotrophic wetlands cause relatively high methane emissions from peat soil. Plant Soil 2010, 326, 147–158. [Google Scholar] [CrossRef]
- Ding, W.; Cai, Z.; Tsuruta, H. Plant species effects on methane emissions from freshwater marshes. Atmos. Environ. 2005, 39, 3199–3207. [Google Scholar] [CrossRef]
- Bubier, J.L.; Moore, T.R.; Bellisario, L.; Comer, N.T.; Crill, P.M. Ecological controls on methane emissions from a northern peatland complex in the zone of discontinuous permafrost, Manitoba, Canada. Glob. Biogeochem. Cycles 1995, 9, 455–470. [Google Scholar] [CrossRef]
- Koh, H.S.; Ochs, C.A.; Yu, K. Hydrologic gradient and vegetation controls on CH4 and CO2 fluxes in a spring-fed forested wetland. Hydrobiologia 2009, 630, 271–286. [Google Scholar] [CrossRef]
- Juszczak, R.; Augustin, J. Exchange of the greenhouse gases methane and nitrous oxide between the atmosphere and a temperate peatland in central Europe. Wetlands 2013, 33, 895–907. [Google Scholar] [CrossRef]
- Chen, X.; Zong, Y. Coastal erosion along the Changjiang deltaic shoreline, China: History and prospective. Estuar. Coast. Shelf Sci. 1998, 46, 733–742. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Z.; Xu, S. Methane emission from Yangtze estuarine wetland, China. J. Geophys. Res. 2009, 114, G02011. [Google Scholar] [CrossRef]
- Bu, N.S.; Qu, J.F.; Zhao, H.; Yan, Q.W.; Zhao, B.; Fan, J.L.; Fang, C.M.; Li, G. Effects of semi-lunar tidal cycling on soil CO2 and CH4 emissions: A case study in the Yangtze River estuary, China. Wetl. Ecol. Manag. 2015, 23, 727–736. [Google Scholar] [CrossRef]
- Yin, S.; An, S.; Deng, Q.; Zhang, J.; Ji, H.; Cheng, X. Spartina alterniflora invasions impact CH4 and N2O fluxes from a salt marsh in eastern China. Ecol. Eng. 2015, 81, 192–199. [Google Scholar] [CrossRef]
- Sun, S.; Cai, Y.; An, S. Differences in morphology and biomass allocation of Scirpus mariqueter between creekside and inland communities in the Changjiang estuary, China. Wetlands 2002, 22, 786–793. [Google Scholar] [CrossRef]
- Sun, S.; Gao, X.; Cai, Y. Variations in sexual and asexual reproduction of Scirpus mariqueter along an elevational gradient. Ecol. Res. 2001, 16, 263–274. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Y.; Deng, H.; Wang, D.; Chen, Z.; Xu, S. Effect of Scirpus mariqueter on nitrous oxide emissions from a subtropical monsoon estuarine wetland. J. Geophys. Res. 2012, 117. [Google Scholar] [CrossRef]
- Khalil, M.A.K.; Rasmussen, R.A.; Wang, M.X.; Ren, L. Emissions of trace gases from Chinese rice fields and biogas generators: CH4, N2O, CO, CO2, chlorocarbons, and hydrocarbons. Chemosphere 1990, 20, 207–226. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E.; Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Sumner, M.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis. Part 3—Chemical Methods; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Grasshof, K.; Ehrhard, M.; Kremling, K. Methods of Seawater Analysis, 2nd ed.; Verlag Chemie: Weiheim, Germany, 1983. [Google Scholar]
- Lin, Y.H.; Guo, M.X.; Zhuang, Y. Determination of acid volatilesulfide and simultaneously extracted metals in sediment. Acta Sci. Circumstantiae 1997, 17, 353–358. (In Chinese) [Google Scholar]
- Lee, B.G.; Griscom, S.B.; Lee, J.S.; Choi, H.J.; Koh, C.H.; Luoma, S.N.; Fisher, N.S. Influences of dietary uptake and reactive sulfides onmetal bioavailability from aquatic sediments. Science 2000, 287, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Hornibrook, E.R.C.; Bowes, H.L.; Culbert, A.; Gallego-Sala, A.V. Methanotrophy potential versus methane supply by pore water diffusion in peatlands. Biogeosciences 2009, 6, 1491–1504. [Google Scholar] [CrossRef] [Green Version]
- Lerman, A. Geochemical Processes: Water and Sediment Environments; John Wiley: New York, NY, USA, 1979; 481p. [Google Scholar]
- Rose, C.; Crumpton, W.G. Spatial patterns in dissolved oxygen and methane concentrations in a prairie pothole wetland in Iowa, USA. Wetlands 2006, 26, 1020–1025. [Google Scholar] [CrossRef]
- Shoemaker, J.K.; Varner, R.K.; Schrag, D.P. Characterization of subsurface methane production and release over 3 years at a New Hampshire wetland. Geochim. Cosmochim. Acta 2012, 91, 120–139. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Ye, C.; Chen, X.; Xie, B.; Huan, C.; Zhang, J.; Xu, M. Effects of plant species on soil microbial processes and CH4 emission from constructed wetlands. Environ. Pollut. 2013, 174, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Song, C.; Wang, X.; Sun, X.; Meng, H.; Sun, L. Greenhouse gas emissions from different wetlands during the snow-covered season in Northeast China. Atmos. Environ. 2012, 62, 328–335. [Google Scholar] [CrossRef]
- Aubertin, M.; Aachib, M.; Authier, K. Evaluation of diffusive gas flux through covers with a GCL. Geotext. Geomembr. 2000, 18, 215–233. [Google Scholar] [CrossRef]
- Rasmussen, H.; Jørgensen, B.B. Microelectrode studies of seasonal oxygen uptake in a coastal sediment: Role of molecular diffusion. Mar. Ecol. Prog. Ser. 1992, 81, 289–303. [Google Scholar] [CrossRef]
- Yun, J.; Yu, Z.; Li, K.; Zhang, H. Diversity, abundance and vertical distribution of methane-oxidizing bacteria (methanotrophs) in the sediments of the Xianghai wetland, Songnen Plain, northeast China. J. Soils Sediments 2013, 13, 242–252. [Google Scholar] [CrossRef]
- Liebner, S.; Schwarzenbach, S.P.; Zeyer, J. Methane emissions from an alpine fen in central Switzerland. Biogeochemistry 2012, 109, 287–299. [Google Scholar] [CrossRef]
- Chmura, G.L.; Kellman, L.; Guntenspergen, G.R. The greenhouse gas flux and potential global warming feedbacks of a northern macrotidal and microtidal salt marsh. Environ. Res. Lett. 2011, 6, 044016. [Google Scholar] [CrossRef]
- Askaer, L.; Elberling, B.; Glud, R.N.; Kühl, M.; Lauritsen, F.R.; Joensen, H.P. Soil heterogeneity effects on O2 distribution and CH4 emissions from wetlands: In situ and mesocosm studies with planar O2 optodes and membrane inlet mass spectrometry. Soil Biol. Biochem. 2010, 42, 2254–2265. [Google Scholar] [CrossRef]
- Song, H.; Liu, X. Anthropogenic effects on fluxes of ecosystem respiration and methane in the Yellow River Estuary, China. Wetlands 2016, 36, 113–123. [Google Scholar] [CrossRef]
- Jerman, V.; Metje, M.; Mandić-Mulec, I.; Frenzel, P. Wetland restoration and methanogenesis: The activity of microbial populations and competition for substrates at different temperatures. Biogeosciences 2009, 6, 1127–1138. [Google Scholar] [CrossRef]
- Brix, H. Gas exchange through dead culms of reed, Phragmites australis (Cav.) Trin. ex Steudel. Aquat. Bot. 1989, 35, 81–98. [Google Scholar] [CrossRef]
- Holzapfel-Pschorn, A.; Conrad, R.; Seiler, W. Effects of vegetation on the emission of methane from submerged paddy soil. Plant Soil 1986, 92, 223–233. [Google Scholar] [CrossRef]
- Schütz, H.; Holzapfel-Pschorn, A.; Conrad, R.; Rennenberg, H.; Seiler, W. A 3-year continuous record on the influence of daytime, season, and fertilizer treatment on methane emission rates from an Italian rice paddy. J. Geophys. Res. Atmos. 1989, 94, 16405–16416. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Papen, H.; Rennenberg, H. Impact of gas transport through rice cultivars on methane emission from rice paddy fields. Plant Cell Environ. 1997, 20, 1175–1183. [Google Scholar] [CrossRef]
- King, J.Y.; Reeburgh, W.S.; Regli, S.K. Methane emission and transport by arctic sedges in Alaska: Results of a vegetation removal experiment. J. Geophys. Res. Atmos. 1998, 103, 29083–29092. [Google Scholar] [CrossRef]
- Kelker, D.; Chanton, J. The effect of clipping on methane emissions from Carex. Biogeochemistry 1997, 39, 37–44. [Google Scholar] [CrossRef]
- Green, S.M.; Baird, A.J. A mesocosm study of the role of the sedge Eriophorum angustifolium in the efflux of methane—including that due to episodic ebullition-from peatlands. Plant Soil 2012, 351, 207–218. [Google Scholar] [CrossRef]
- Waddington, J.M.; Roulet, N.T.; Swanson, R.V. Water table control of CH4 emission enhancement by vascular plants in boreal peatlands. J. Geophys. Res. Atmos. 1996, 101, 22775–22785. [Google Scholar] [CrossRef]
- Stanley, E.H.; Ward, A.K. Effects of vascular plants on seasonal pore water carbon dynamics in a lotic wetland. Wetlands 2010, 30, 889–900. [Google Scholar] [CrossRef]
- Martens, C.S.; Albert, D.B.; Alperin, M.J. Biogeochemical processes controlling methane in gassy coastal sediments—Part 1. A model coupling organic matter flux to gas production, oxidation and transport. Cont. Shelf Res. 1998, 18, 1741–1770. [Google Scholar] [CrossRef]
- Borrel, G.; Jézéquel, D.; Biderre-Petit, C.; Morel-Desrosiers, N.; Morel, J.P.; Peyret, P.; Fonty, G.; Lehours, A.C. Production and consumption of methane in freshwater lake ecosystems. Res. Microbiol. 2011, 162, 832–847. [Google Scholar] [CrossRef] [PubMed]
- Frenzel, P.; Thebrath, B.; Conrad, R. Oxidation of methane in the oxic surface layer of a deep lake sediment (Lake Constance). FEMS Microbiol. Lett. 1990, 73, 149–158. [Google Scholar] [CrossRef]
- Nielsen, L.P.; Christensen, P.B.; Revsbech, N.P.; Sørensen, J. Denitrification and oxygen respiration in biofilms studied with a microsensor for nitrous oxide and oxygen. Microb. Ecol. 1990, 19, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Valentine, D.L.; Reeburgh, W.S. New perspectives on anaerobic methane oxidation. Environ. Microbiol. 2000, 2, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, S.L.; Laidler, J.R.; Brewer, E.A.; Eberly, J.O.; Sandborgh, S.C.; Colwell, F.S. Anaerobic oxidation of methane: Mechanisms, bioenergetics, and the ecology of associated microorganisms. Environ. Sci. Technol. 2008, 42, 6791–6799. [Google Scholar] [CrossRef] [PubMed]
- Baird, A.J.; Beckwith, C.W.; Waldron, S.; Waddington, J.M. Ebullition of methane-containing gas bubbles from near-surface Sphagnum peat. Geophys. Res. Lett. 2004, 31. [Google Scholar] [CrossRef]

| Month | Temperature (°C) | PAR (W·m−2) | Biomass | SOC (g·dm·kg) | SWC (%) | APS/MD (μm) | NH4+-N (mg·kg−1) | NO3−-N (mg·kg−1) | AVS (μg·g−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AAT | ATR | GT5 | GT10 | GT15 | (g·dm·m−2) | Number of Living Shoots in 50 cm × 50 cm Quadrats | ||||||||
| February | 4 | −2.0~9.0 | 3 | 2 | 1 | 8~266 | - | - | 7.27 | 43 | 16.81/23.81 | 3.04 | 0.15 | - |
| March | 12 | 7.5~14.5 | 10 | 9 | 9 | 17~233 | - | - | 6.91 | 39 | 15.06/20.23 | 4.88 | 0.19 | 7.46 |
| April | 25 | 16.0~28.0 | 20 | 20 | 17 | 15~304 | 44.6 | 66 | 7.59 | 36 | 14.31/20.07 | 7.54 | 0.12 | 1.37 |
| May | 23 | 15.5~29.5 | 23 | 21 | 20 | 35~317 | 105.9 | 223 | 6.78 | 55 | 12.51/16.86 | 4.82 | 0.98 | 14.3 |
| June | 27 | 25.0~28.5 | 26 | 26 | 26 | 30~145 | 433.9 | 403 | 7.86 | 54 | 7.747/9.527 | 5.65 | 0.49 | 6.35 |
| July | 33 | 30.0-35.5 | 31 | 32 | 29 | 38~297 | 364.1 | 445 | 8.42 | 66 | 7.129/8.744 | 4.33 | ND | 8.28 |
| August | 25 | 23.5~26.0 | 25 | 25 | 25 | 15~98 | 692.6 | 972 | 7.12 | 91 | 6.636/7.891 | 4.98 | 0.59 | 3.43 |
| September | 20 | 16.0~25.0 | 25 | 24 | 23 | 38~313 | 534.1 | 869 | 7.65 | 46 | 7.402/9.479 | 4.48 | 0.49 | 11.5 |
| October | 22 | 16.0~24.0 | 21 | 21 | 20 | 24~284 | 263.9 | 623 | 8.01 | 55 | 8.317/10.81 | 4.06 | 0.10 | 7.32 |
| November | 11 | 5.5.0~14.0 | 14 | 14 | 14 | 19~222 | - | - | 7.78 | 69 | 8.082/10.28 | 5.25 | 0.16 | 12.5 |
| December | 7 | −1.0~9.5.0 | 7 | 6 | 6 | 8~188 | 130.3 | - | 7.63 | 65 | 9.096/11.92 | 4.07 | 0.20 | - |
| Depth | CH4 Fluxes b | Depth | CH4 Fluxes | Depth | CH4 Fluxes |
|---|---|---|---|---|---|
| 0–5 cm | −0.084 | 21–25 cm | 0.590 | 41–45 cm | −0.034 |
| 6–10 cm | 0.146 | 26–30 cm | 0.192 | 46–50 cm | −0.065 |
| 11–15 cm | 0.732 * | 31–35 cm | 0.279 | 51–55 cm | 0.219 |
| 16–20 cm | 0.777 * | 36–40 cm | 0.101 | 56–60 cm | 0.190 |
| Vegetation Type | Proportion of Plant Emitted CH4 (%) a | Plant Treatment b | References |
|---|---|---|---|
| C. lasiocarpa | 73~82 | clipping c | [21] |
| C. meyeriana | 75~86 | clipping | [21] |
| Rice | 94 | clipping | [52] |
| Rice | 97 | clipping | [53] |
| Rice | 90 | clipping | [54] |
| Reed | 60 | clipping | [52] |
| Weeds | 84 | clipping | [52] |
| Sedge | 79 | uprooting d | [55] |
| Eriophorum latifolium | 80 | uprooting | [20] |
| Sedge | 94 | clipping | [56] |
| Sedge | 83 | clipping | [57] |
| E. vaginatum | 88 | clipping | [58] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, D.; Chen, Z.; Jin, H.; Hu, H.; Chen, J.; Yang, Z. Role of Scirpus mariqueter on Methane Emission from an Intertidal Saltmarsh of Yangtze Estuary. Sustainability 2018, 10, 1139. https://doi.org/10.3390/su10041139
Li Y, Wang D, Chen Z, Jin H, Hu H, Chen J, Yang Z. Role of Scirpus mariqueter on Methane Emission from an Intertidal Saltmarsh of Yangtze Estuary. Sustainability. 2018; 10(4):1139. https://doi.org/10.3390/su10041139
Chicago/Turabian StyleLi, Yangjie, Dongqi Wang, Zhenlou Chen, Haiyan Jin, Hong Hu, Jianfang Chen, and Zhi Yang. 2018. "Role of Scirpus mariqueter on Methane Emission from an Intertidal Saltmarsh of Yangtze Estuary" Sustainability 10, no. 4: 1139. https://doi.org/10.3390/su10041139





