Effect of Mechanical Pre-Treatment of the Agricultural Substrates on Yield of Biogas and Kinetics of Anaerobic Digestion
Abstract
:1. Introduction
2. Materials and Methodology
2.1. Substrates
2.2. Laboratory Measurements
2.3. The Method of Anaerobic Digestion
2.4. Cumulative Biogas Production
- Vo—normal volume of biogas, Nml;
- V—read out biogas volume, ml;
- pl—air pressure at the time of reading, hPa;
- pw—pressure of water vapor in the ambient temperature, hPa;
- To—the normal temperature To = 273 K;
- po—normal pressure po = 1013 hPa;
- T—ambient temperature, K.
- Vis—the volume of biogas produced from the inoculum, Nml;
- ΣVis—the total volume of biogas produced from the inoculum, Nml;
- mis—inoculum mass, g;
- mm—weight of inoculum in the control sample, g;
- Vn—net volume of biogas, Nml.
- Vs—unit biogas produced during the experiment;
- M—substrate mass, g;
- TS—total solid, %
- VS—volatile solid, % TS.
2.5. Kinetic Model of Biogas Production
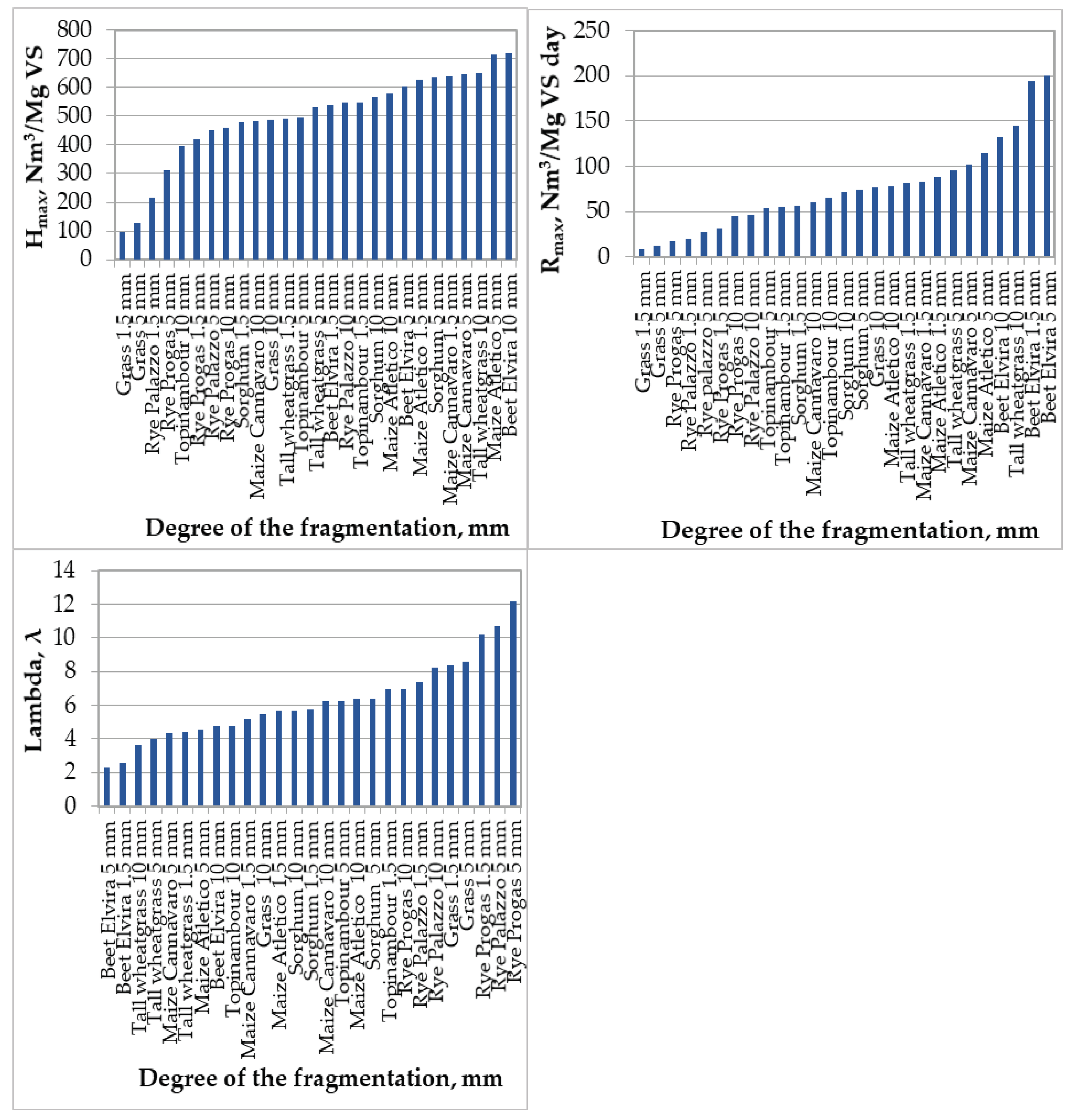
- QB is the cumulative biogas production (Nm3/Mg VS) at time t (day), t is the time (day) over the digestion period, Hmax is biogas production potential (Nm3/Mg VS), Rmax is maximum rate of biogas production (Nm3/Mg VS day) while λ is the lag phase (day) or minimum time between the inoculation and biogas appearance). Kinetic constants Hmax, Rmax, λ and R2 were determined using the non-linear regression and the root mean square error (RMSE) error was estimated. The use of the Gompertz model to describe the test results gave good results at work [15], where the model was characterized by a good fit to the test results. First, the use of the model was dictated by the need to demonstrate the effect of the degree of fragmentation of substrates on fermentation kinetics.
2.6. Measurement Error Analysis and Statistical Analysis
2.7. Data Analysis
3. Results and Discussion
3.1. Substrate Characteristics
3.2. Batch Anaerobic Digestion
3.3. Kinetics of Anaerobic Digestion
4. Discusion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- El Achkar, J.H.; Lendormi, T.; Hobaika, Z.; Salameh, D.; Louka, N.; Maroun, R.G.; Lanoisellé, J.L. Anaerobic digestion of nine varieties of grape pomace: Correlation between biochemical composition and methane production. Biomass Bioener. 2017, 107, 335–344. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Fdez-Güelfo, L.A.; Zhou, Y.; Álvarez-Gallego, C.J.; Garcia, L.I.R.; Ng, W.J. Anaerobic co-digestion of organic fraction of municipal solid waste (OFMSW): Progress and challenges. Renew Sustain. Energy Rev. 2018, 93, 380–399. [Google Scholar] [CrossRef]
- Kalambura, S.; Krička, T.; Kiš, D.; Marić, S.; Guberac, S.; Kozak, D.; Stoić, A.; Racz, A. Anaerobic digestion of specific biodegradable waste and final disposal. Tehnicki Vjesnik 2016, 23, 1601–1607. [Google Scholar]
- Ministry of Economy. Energy policy of Poland until 2030 — Annex to Resolution No. 202/2009 of the Council of Ministers; Ministry of Economy: Warsaw, Poland, 2009.
- Gonzalez-Fernandez, C.; Sialve, B.; Molinuevo-Salces, B. Anaerobic digestion of microalgal biomass: Challenges, opportunities and research needs. Bioresour. Technol. 2015, 198, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Lindmark, J.; Thorin, E.; Bel Fdhila, R.; Dahlquist, E. Effects of mixing on the result of anaerobic digestion: Review. Renew Sustain. Energy Rev. 2014, 40, 1030–1047. [Google Scholar] [CrossRef]
- Rada, E.C.; Ragazzi, M.; Torretta, V. Laboratory-scale anaerobic sequencing batch reactor for treatment of stillage from fruit distillation. Water Sci. Technol. 2013, 67, 1068–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nizami, A.S.; Orozco, A.; Groom, E.; Dieterich, B.; Murphy, J.D. How much gas can we get from grass? Appl. Energy 2012, 92, 783–790. [Google Scholar] [CrossRef]
- Chiumenti, A.; Boscaro, D.; da Borso, F.; Sartori, L.; Pezzuolo, A. Biogas from Fresh Spring and Summer Grass: Effect of the Harvesting Period. Energies 2018, 11, 1466. [Google Scholar] [CrossRef]
- Boscaro, D.; Pezzuolo, A.; Sartori, L.; Marinello, F.; Mattioli, A.; Bolzonella, D.; Grigolato, S. Evaluation of the energy and greenhouse gases impacts of grass harvested on riverbanks for feeding anaerobic digestion plants. J. Clean. Prod. 2018, 172, 4099–4109. [Google Scholar] [CrossRef]
- Fugol, M.; Prask, H. Porównanie uzysku biogazu z trzech rodzajów kiszonek; z kukurydzy, lucerny i trawy. Comparison of biogas yield from three types of silage; from corn, alfalfa and grass. Inż. Rol. 2011, 9, 31–39. [Google Scholar]
- Szlachta, J. Expertise on Obtaining Agricultural Biogas as a Renewable Energy Source. Available online: http://www.agengpol.pl/LinkClick.aspx?fileticket=O67VGkyovAE%3d&tabid=144 (accessed on 22 October 2009).
- Szlachta, J.; Fugol, M.; Prask, H. The impact of the raw material composition on fermentation kinetics and the yield of biogas and methane. Przem. Chem. 2016, 95, 1805–1810. [Google Scholar]
- Kozyra, A.; Production of biomass and GMOs. Renewable Energy Sources a New Challenge for Rural Areas in Poland. Available online: https://docplayer.pl/2898851-Odnawialne-zrodla-energii-nowym-wyzwaniem-dla-obszarow-wiejskich-w-polsce.html (accessed on 22 October 2009).
- Podkówka, W.; Podkówka, Z.; Kowalczyk-Juśko, A.; Pasyniuk, P. Agricultural biogas renewable energy source. Theory and practical application. Wydawnictwo PWRiL 2012, 147–152. [Google Scholar]
- Budzichowska, J. Will Sorghum Replace Maize. Warszawa. Available online: http://www.modr.mazowsze.pl/index.php/porady-dla-rolnikow/produkcja-roslinna/115-czy-sorgo-zastapi-kukurydze (accessed on 14 October 2009).
- Węglarzy, K.; Podkówka, W. Agrobiogazownia; Instytut Zootechniki: Grodziec Śląski, Poland, 2010. [Google Scholar]
- Angelidaki, I.; Ahring, B.K. Methods for increasing the biogas potential from the recalcitrant organic matter contained in manure. Water Sci. Techno. 2000, 41, 189–194. [Google Scholar] [CrossRef]
- Saulnier, L.; Marot, C.; Chanliaud, E.; Thibauld, J.F. Cell wall Polysaccharide interactions in maize bran. Carbohydr. Polym. 1995, 26, 279–287. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomas. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Antognoni, S.; Ragazzi, M.; Rada, E.C.; Plank, R.; Aichinger, P.; Kuprian, M.; Ebner, C. Potential Effects of Mechanical Pre-treatments on Methane Yield from Solid Waste Anaerobically Digested. Int. J. Environ. Bioremed. Biodegrad. 2013, 1, 20–25. [Google Scholar]
- Jędrczak, A.; Królik, D. Influence of paper particle size on the efficiency of digestion process. Environ. Prot. Eng. 2007, 33, 145–155. [Google Scholar]
- Hartmann, H.; Angelidaki, I.; Ahring, B.K. Increase of anaerobic degradation of particulate organic matter in full-scale biogas plants by mechanical maceration. Water Sci. Technol. 2004, 41, 145–153. [Google Scholar] [CrossRef]
- Lindmark, J.; Leksell, N.; Schnürer, A.; Thorin, E. Effects of mechanical pre-treatment on the biogas yield from ley crop silage. Appl. Energy 2012, 97, 498–502. [Google Scholar] [CrossRef]
- Tsapekos, P.; Kougias, P.G.; Angelidaki, I. Biogas production from ensiled meadow grass; effect of mechanical pretreatments and rapid determination of substrate biodegradability via physicochemical methods. Bioresour. Technol. 2015, 182, 329–335. [Google Scholar] [CrossRef] [PubMed]
- DIN 38414 S 8. German Standard Methods for the Examination of Water, Waste Water and Sludge; Sludge and Sediments (Group S); Determination of the Amenability to Anaerobic Digestion (S 8); DIN Deutches Institut für Normung e. V.: Berlin, Germany, 2012. [Google Scholar]
- Polski Komitet Normalizacyjny. Oznaczanie Suchej Masy Osadu i Substancji Organicznych. Woda i ścieki. Badania Specjalne Osadów PN-75/C-04616/01; PKN: Płock, Poland, 2004. [Google Scholar]
- Polski Komitet Normalizacyjny. Charakterystyka Osadów ściekowych—Oznaczanie Strat Przy Prażeniu Suchej Masy Osadu PN-EN 12879; PKN: Płock, Poland, 2004. [Google Scholar]
- Lo, H.M.; Kurniawan, T.A.; Sillanpää, M.E.T.; Pai, T.Y.; Chiang, C.F.; Chao, K.P.; Liu, M.H.; Chuang, S.H.; Banks, C.J.; Wang, S.C.; et al. Modeling biogas production from organic fraction of MSW co-digested with MSWI ashes in anaerobic bioreactors. Bioresour. Technol. 2010, 101, 6329–6335. [Google Scholar] [CrossRef] [PubMed]
- Mulka, R.; Szulczewski, W.; Szlachta, J. Estimation of methane production for batch technology—A new approach. Renew. Energy 2016, 90, 440–449. [Google Scholar] [CrossRef]
- Budiyono, B.; Widiasa, I.N.; Johari, S.; Sunarso, S. The kinetic of biogas production rate from cattle manure in batch mode. Int. J. Chem. Biol. Eng. 2010, 3, 39–44. [Google Scholar]
- Jabłoński, S.; Rodowicz, P.; Łukaszewicz, M. Methanogenic archaea database containing physiological and biochemical characteristics. Int. J. Syst. Evol. Microbiol. 2015, 65, 1360–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daisy, A.; Kamaraj, S. The impact and treatment of night soil in anaerobic digester: A review. J. Microbial Biochem. Technol. 2011, 3, 43–50. [Google Scholar]
- Staubmann, R.; Foidl, G.; Foidl, N.; Gübitz, G.M.; Lafferty, R.M.; Arbizu, V.M.; Steiner, W. Biogas production from Jatropha curcas press-cake. Appl. Biochem. Biotechnol. 1997, 63, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Ziemiński, K.; Kowalska-Wentel, M. Effect of Different Sugar Beet Pulp Pretreatments on Biogas Production Efficiency. Appl. Biochem. Biotechnol. 2017, 181, 1211–1227. [Google Scholar] [CrossRef] [PubMed]
- Tsapekos, P.; Egelund, P.G.; Larsen, U.; Pedersenc, J.; Trénelc, P.; Angelidakia, I. Mechanical pretreatment at harvesting increases the bioenergy output from marginal land grasses. Renew. Energy 2017, 111, 914–921. [Google Scholar] [CrossRef]
- Ziemiński, K.; Frąc, M. Methane fermentation process as anaerobic digestion of biomass Transformations, stages and microorganisms. Afr. J. Biotechnol. 2012, 11, 4127–4139. [Google Scholar]




| Parameter | Inoculum | Maize Cannavaro Silage | Maize Atletico Silage | Sorghum Silage | Beet Silage | Topinambur Silage | Rye Progas Silage | Rye Palazzo Silage | Grass Silage | Tall Wheatgrass Silage |
|---|---|---|---|---|---|---|---|---|---|---|
| Total solid (TS, %) | 5.9 ± 0.1 | 40.63 ± 4.20 | 37.32 ± 3.51 | 25.81 ± 3.32 | 18.74 ± 2.32 | 21.84 ± 3.33 | 58.20 ± 4.12 | 54.54 ± 4.21 | 17.92 ± 2.34 | 22.25 ± 0.23 |
| Volatile solid (VS, % TS) | 76.9 ± 0.12 | 96.92 ± 2.25 | 96.22 ± 2.23 | 93.53 ± 2.43 | 91.41 ± 3.21 | 81.94 ± 2.20 | 86.75 ± 4.21 | 82.11 ± 4.12 | 74.36 ± 2.11 | 87.41 ± 4.21 |
| Ash (%) | 1.37 ± 0.10 | 1.28 ± 0.13 | 1.41 ± 0.23 | 1.69 ± 016 | 1.61 ± 052 | 3.94 ± 1.12 | 7.86 ± 0.87 | 9.70 ± 0.32 | 4.59 ± 0.38 | 2.79 ± 0.43 |
| pH | 8,8 | 4.4 | 4.3 | 4.4 | 4.0 | 5.3 | 5.2 | 4.9 | 5.6 | 4.8 |
| N (% TS) | 7,84 ± 0.11 | 1.59 ± 0.12 | 1.55 ± 0.11 | 1.54 ± 0.09 | 0.98 ± 0.07 | 1.51 ± 0.14 | 1.15 ± 015 | 1.32 ± 0.19 | 2.92 ± 0.21 | 2.14 ± 0.23 |
| C (% TS) | 46.3 ± 014 | 45.66 ± 3.21 | 46.25 ± 2.18 | 44.70 ± 1.98 | 40.95 ± 1.98 | 41.00 ± 2.08 | 36.92 ± 2.12 | 29.63 ± 2.17 | 40.46 ± 2.17 | 45.66 ± 2.19 |
| C/N | 5.9 | 28.7 | 29.8 | 29.0 | 41.7 | 27.2 | 32.1 | 22.4 | 13.8 | 21.3 |
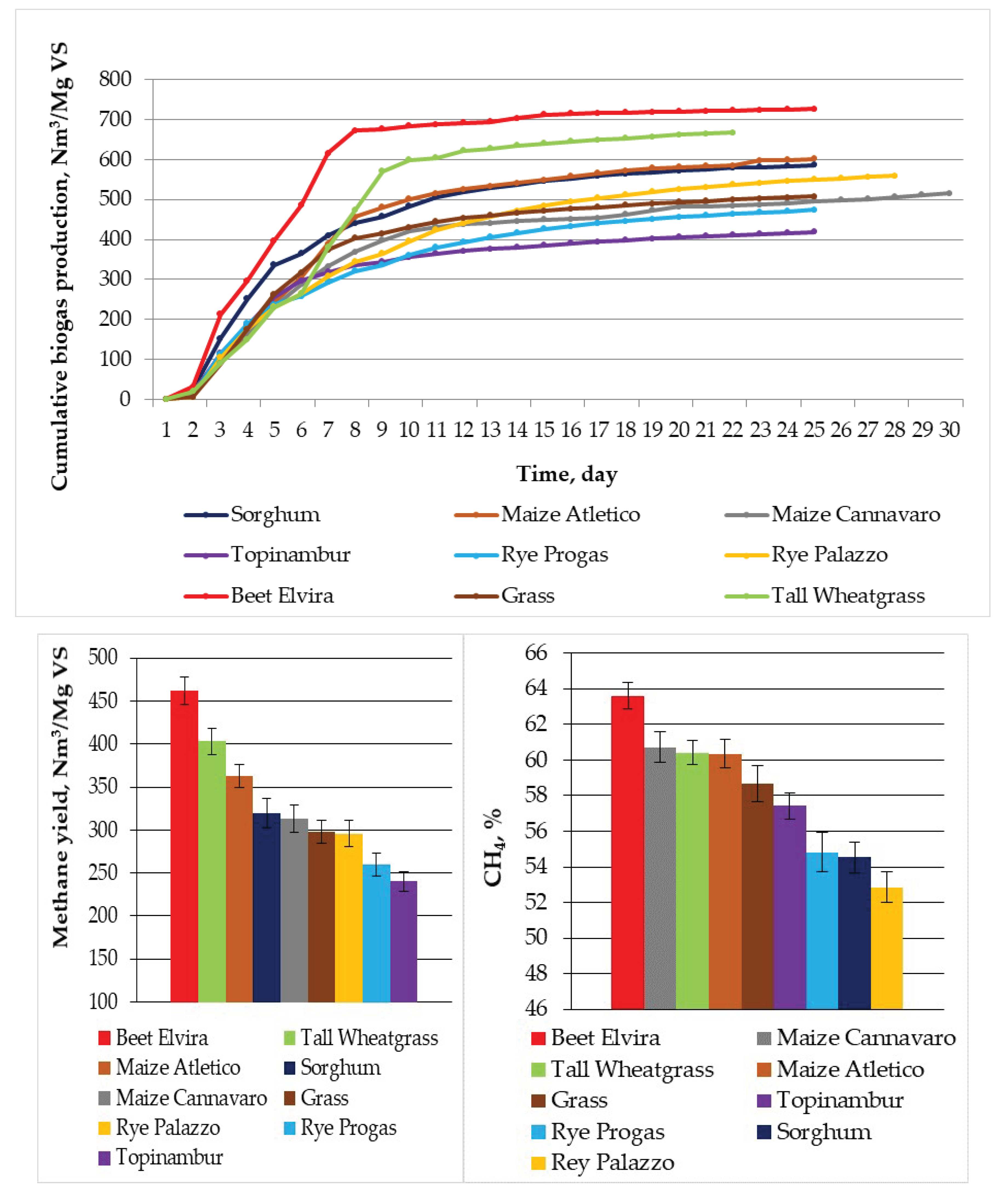
| QB (Nm3/Mg VS) | 212.84 ± 15.2 | 515.61 ± 12.36 | 601.20 ± 22.50 | 586.62 ± 21.14 | 726.14 ± 33.31 | 418.70 ± 25.54 | 473.87 ± 23.16 | 559.18 ± 33.17 | 508.32 ± 32.87 | 667.47 ± 43.12 |
| P (g/kg TS) | 9.3 | 3.21 | 1.46 | 1.84 | 1.29 | 2.37 | 1.27 | 1.06 | 2.27 | 1.8 |
| P-PO4 (g/kg TS) | 1.8 | 1.34 | 1.24 | 1.28 | 1.26 | 1.35 | 0.37 | 0.51 | 1.29 | 1.67 |
| K (g/kg TS) | 94.6 | 7.69 | 6.49 | 12.17 | 11.73 | 25.9 | 8.05 | 8.15 | 31.63 | 20.96 |
| Ca (g/kg TS) | 93.6 | 1.56 | 1.76 | 5.26 | 3.94 | 27.69 | 1.62 | 15.8 | 29.95 | 8.44 |
| Na (g/kg TS) | 9.9 | 0.4 | 0.55 | 0.91 | 1.71 | 1.26 | 0.87 | 0.75 | 1.23 | 1.14 |
| N-NH4 (g/kg TS) | 40.4 | 1.01 | 1.28 | 1.49 | 0.42 | 0.05 | 1.03 | 1.47 | 0.24 | 2.19 |
| Hg (mg/kg TS) | 0.0130 | 0.0048 | 0.0050 | 0.0136 | 0.0175 | 0.0528 | 0.0248 | 0.0250 | 0.0580 | 0.0253 |
| Zn (mg/kg TS) | 832 | 29.0 | 35.0 | 50.3 | 25.0 | 42.7 | 41.5 | 47.2 | 51.0 | 36.0 |
| Pb (mg/kg TS) | 5.54 | 1.38 | 2.09 | 2.12 | 2.43 | 8.26 | 6.94 | 6.74 | 11.6 | 5.26 |
| Cd (mg/kg TS) | <0.900 | <0.100 | <0.100 | 0.479 | 0.178 | 0.391 | 0.283 | 0.277 | 0.416 | 0.119 |
| Cr (mg/kg TS) | 21.7 | 15.6 | 9.10 | 20.2 | 21.7 | 7.95 | 12.41 | 9.24 | 7.76 | 26.31 |
| Cu (mg/kg TS) | 295 | 5.37 | 6.04 | 7.72 | 9.79 | 16.82 | 13.54 | 13.81 | 22.83 | 10.32 |
| Ni (mg/kg TS) | 18.5 | 10.70 | 6.45 | 7.11 | 18.6 | 5.01 | 52.3 | 45.92 | 4.74 | 11.90 |
| S (g/kg TS) | 5.98 | 1.10 | 1.04 | 1.40 | 1.18 | 1.77 | 1.47 | 1.60 | 2.47 | 2.63 |
| Mg (g/kg TS) | 3.46 | 1.39 | 1.53 | 2.05 | 2.24 | 4.86 | 0.801 | 0.825 | 6.42 | 4.24 |
| Fe (g/kg TS) | 2.58 | 0.213 | 0.167 | 0.266 | 2.56 | 0.394 | 2.82 | 3.52 | 0.541 | 0.781 |
| P (g/kg TS) | 11.4 | 2.68 | 2.66 | 2.25 | 2.23 | 1.61 | 1.24 | 1.47 | 2.35 | 3.26 |
| K (g/kg TS) | 45.2 | 8.24 | 7.84 | 17.0 | 16.1 | 39.0 | 10.7 | 10.5 | 3.62 | 29.1 |
| Co (g/kg TS) | <0.250 | <0.100 | <0.100 | <0.100 | <0.100 | <0.100 | 0.269 | 0.280 | <0.100 | <0.100 |
| Mo (g/kg TS) | <0.480 | 0.705 | 0.557 | 0.579 | 0.958 | 0.629 | 2.01 | 1.12 | 0.801 | 1.93 |
| Mn (g/kg TS) | 15.6 | 16.6 | 19.6 | 44.8 | 42.9 | 71.4 | 160 | 164 | 90.3 | 45.3 |
| Silages from Substrates | Degree of Fragmenta -tion (mm) | TS (%) | VS (% TS) | Cumulative Biogas Yield QB (Nm3/Mg VS) | Methane Yield (Nm3/Mg VS) | CO2 (%) | CH4/CO2 | VS Removal (%) | pH Initial | pH Final |
|---|---|---|---|---|---|---|---|---|---|---|
| 1.5 | 18.00 | 74.36 | 98.02 ± 32.17 | 46.86 ± 11.2 | 34.02 | 1.68 | 51.2 | 7.12 | 6.06 | |
| Grass | 5 | 17.92 | 74.36 | 131.23 ± 31.19 | 63.99 ± 10.02 | 25.30 | 1.87 | 51.7 | 7.21 | 6.12 |
| 10 | 17.92 | 74.36 | 508.34 ± 32.87 | 298.18 ± 7.02 | 24.68 | 1.92 | 51.9 | 7.27 | 6.13 | |
| 1.5 | 58.23 | 86.75 | 414.82 ± 21.87 | 206.38 ± 11.73 | 36.48 | 1.52 | 51.5 | 7.18 | 6.14 | |
| Rye Progas | 5 | 58.20 | 86.75 | 287.65 ± 22.25 | 148.65 ± 6.24 | 43.75 | 1.14 | 51.9 | 7.25 | 6.19 |
| 10 | 58.20 | 86.75 | 473.78 ± 23.16 | 259.77 ± 6.75 | 34.30 | 1.67 | 51.9 | 7.22 | 6.12 | |
| 1.5 | 54.54 | 82.11 | 213.46 ± 31.19 | 111.51 ± 6.78 | 38.84 | 1.32 | 51.3 | 7.14 | 6.12 | |
| Rye Palazzo | 5 | 54.54 | 82.11 | 421.49 ± 32.19 | 209.86 ± 11.01 | 37.83 | 1.36 | 51.3 | 7.23 | 6.13 |
| 10 | 54.54 | 82.11 | 559.11 ± 33.17 | 295.51 ± 9.51 | 35.31 | 1.48 | 51.8 | 7.32 | 6.14 | |
| 1.5 | 25.81 | 93.53 | 482.96 ± 22.09 | 284.85 ± 6.90 | 39.72 | 1.29 | 51.5 | 7.13 | 6.12 | |
| Sorghum | 5 | 25.81 | 93.53 | 649.80 ± 20.98 | 334.22 ± 8.67 | 34.99 | 1.36 | 51.8 | 7.22 | 6.12 |
| 10 | 25.81 | 93.53 | 586.59 ± 21.14 | 319.88 ± 6.42 | 37.62 | 1.23 | 51.2 | 7.22 | 6.12 | |
| 1.5 | 22.25 | 87.41 | 501.54 ± 40.25 | 287.03 ± 12.48 | 36.04 | 1.59 | 51.1 | 7.23 | 6.13 | |
| Tall Wheatgrass | 5 | 22.25 | 87.41 | 544.06 ± 39.18 | 297.95 ± 12.99 | 35.03 | 1.52 | 51.3 | 7.19 | 6.13 |
| 10 | 22.25 | 87.41 | 667.36 ± 43.12 | 403.15 ± 13.20 | 34.49 | 1.75 | 51.9 | 7.13 | 6.14 | |
| 1.5 | 18.74 | 91.41 | 549.66 ± 32.87 | 349.88 ± 9.63 | 35.02 | 1.82 | 52.4 | 7.23 | 6.93 | |
| Beet | 5 | 18.74 | 91.41 | 612.37 ± 32.98 | 388.57 ± 10.53 | 34.87 | 1.99 | 52.7 | 7.32 | 6.95 |
| 10 | 18.74 | 91.41 | 726.12 ± 33.31 | 461.80 ± 9.93 | 34.12 | 2.08 | 52.9 | 7.13 | 6.98 | |
| 1.5 | 37.32 | 96.22 | 629.35 ± 21.98 | 383.30 ± 6.06 | 36.57 | 1.67 | 51.9 | 7.22 | 6.72 | |
| Maize Atletico | 5 | 37.32 | 96.22 | 735.59 ± 21.87 | 413.93 ± 6.75 | 35.97 | 1.76 | 52.7 | 7.22 | 6.83 |
| 10 | 37.32 | 96.22 | 601.20 ± 22.50 | 362.80 ± 7.50 | 36.54 | 1.61 | 51.8 | 7.23 | 6.72 | |
| 1.5 | 40.63 | 96.92 | 643.79 ± 11.87 | 392.49 ± 9.60 | 34.98 | 1.60 | 51.9 | 7.13 | 6.62 | |
| Maize Cannavaro | 5 | 40.63 | 96.92 | 671.83 ± 13.02 | 356.80 ± 3.36 | 35.89 | 1.51 | 52.6 | 7.32 | 6.68 |
| 10 | 40.63 | 96.92 | 515.61 ± 12.36 | 313.05 ± 5.55 | 35.44 | 1.58 | 51.7 | 7.22 | 6.62 | |
| 1.5 | 21.84 | 81.94 | 553.67 ± 26.15 | 291.48 ± 8.97 | 38.07 | 1.38 | 51.8 | 7.23 | 6.13 | |
| Topinambur | 5 | 21.84 | 81.94 | 510.43 ± 24.18 | 260.63 ± 7.86 | 35.27 | 1.44 | 51.3 | 7.13 | 6.12 |
| 10 | 21.84 | 81.94 | 418.70 ± 25.54 | 240.39 ± 8.04 | 40.10 | 1.34 | 52.2 | 7.17 | 6.12 |
| Parameters | Correlation Significant for N = 28, p < 0.05 | ||||||
|---|---|---|---|---|---|---|---|
| Average | Standard Deviation | Degree of Fragmentation | Substrate | Volatile Solid | Biogas Yield | Methane Yield | |
| Degree of fragmentation | 8.9107 | 18.3818 | 1.000000 | 0.972036 | 0.326022 | -−0.374277 | 0.878026 |
| Substrate | 8.4286 | 18.3251 | 0.972036 | 1.000000 | 0.399170 | −0.349097 | 0.896007 |
| Volatile solid | 88.3196 | 7.4841 | 0.326022 | 0.399170 | 1.000000 | 0.462835 | 0.545469 |
| Biogas yield | 493.1985 | 179.4309 | −0.374277 | −0.349097 | 0.462835 | 1.000000 | −0.105449 |
| Methane yield | 57.4587 | 9.7675 | 0.878026 | 0.896007 | 0.545469 | −0.105449 | 1.000000 |
| Substrate | Biogas Prod. Potential Hmax (Nm3/Mg VS) | Max. Rate of Biogas Prod. Rmax (Nm3/Mg VS day) | The Lag Phase λ (day) | Coefficient of Determination R2 | Root Mean Squared Error RMSE |
|---|---|---|---|---|---|
| Topinambour silage 1.5 mm | 545.84 | 55.60 | 6.93 | 0.998 | 11.78 |
| Topinambour silage 5 mm | 497.13 | 54.55 | 6.28 | 0.999 | 12.41 |
| Topinambour silage 10 mm | 396.99 | 65.83 | 4.78 | 0.987 | 15.42 |
| Rye Progas silage 1.5 mm | 420.81 | 30.97 | 10.21 | 0.999 | 4.74 |
| Rye Progas silage 5 mm | 313.82 | 17.28 | 12.19 | 0.999 | 6.04 |
| Rye Progas silage 10 mm | 459.79 | 45.36 | 6.95 | 0.989 | 17.91 |
| Maize Cannavaro silage 1.5 mm | 639.41 | 83.66 | 5.21 | 0.999 | 7.82 |
| Maize Cannavaro silage 5 mm | 647.94 | 102.52 | 4.34 | 0.998 | 18.85 |
| Maize Cannavaro silage 10 mm | 485.21 | 59.90 | 6.26 | 0.997 | 15.85 |
| Beet silage 1.5 mm | 539.85 | 194.58 | 2.55 | 0.998 | 9.48 |
| Beet silage 5 mm | 601.15 | 200.72 | 2.33 | 0.998 | 11.81 |
| Beet silage 10 mm | 718.16 | 131.98 | 4.75 | 0.999 | 17.82 |
| Grass silage 1.5 mm | 96.70 | 9.26 | 8.38 | 0.999 | 1.18 |
| Grass silage 5 mm | 129.79 | 12.29 | 8.60 | 0.997 | 3.06 |
| Grass silage 10 mm | 486.63 | 77.08 | 5.46 | 0.998 | 14.05 |
| Sorghum silage 1.5 mm | 478.67 | 56.22 | 5.73 | 0.996 | 15.21 |
| Sorghum silage 5 mm | 636.28 | 73.79 | 6.42 | 0.999 | 13.52 |
| Sorghum silage 10 mm | 567.51 | 71.75 | 5.70 | 0.999 | 20.23 |
| Rye palazzo silage 1.5 mm | 216.10 | 20.38 | 7.37 | 0.999 | 4.13 |
| Rye palazzo silage 5 mm | 451.32 | 27.39 | 10.68 | 0.998 | 12.50 |
| Rye palazzo silage 10 mm | 545.44 | 47.02 | 8.22 | 0.998 | 16.93 |
| Tall wheatgrass silage 1.5 mm | 490.73 | 81.66 | 4.44 | 0.999 | 15.62 |
| Tall wheatgrass silage 5 mm | 533.11 | 95.25 | 3.97 | 0.998 | 10.62 |
| Tall wheatgrass silage 10 mm | 650.83 | 144.29 | 3.62 | 0.999 | 11.72 |
| Atletico maize silage 1.5 mm | 625.65 | 88.05 | 5.67 | 0.999 | 9.66 |
| Atletico maize silage 5 mm | 715.82 | 114.19 | 4.54 | 0.998 | 15.38 |
| Atletico maize silage 10 mm | 579.66 | 77.75 | 6.39 | 0.999 | 13.35 |
| Parameters | Kinetic Constants of Biogas Production, N = 28 Correlation Significant for p < 0.05 | ||||||
|---|---|---|---|---|---|---|---|
| Average | Standard Deviation | Substrate | Degree of Fragmen-tation | Hmax (Nm3/Mg VS) | Rmax (Nm3/Mg VS day) | Lambda, λ | |
| Substrate | 8.4286 | 18.3251 | 1.000000 | 0.972036 | −0.405666 | 0.117710 | 0.978675 |
| Degree of fragmentation | 8.9107 | 18.3818 | 0.972036 | 1.000000 | −0.385217 | 0.114477 | 0.971377 |
| Hmax (Nm3/Mg VS) | 484.6907 | 173.2062 | −0.405666 | −0.385217 | 1.000000 | 0.567290 | −0.497057 |
| Rmax (Nm3/Mg VS day) | 76.4400 | 48.5657 | 0.117710 | 0.114477 | 0.567290 | 1.000000 | −0.008957 |
| Lambda, λ | 9.6061 | 18.0606 | 0.978675 | 0.971377 | 0.497057 | −0.008957 | 1.000000 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szlachta, J.; Prask, H.; Fugol, M.; Luberański, A. Effect of Mechanical Pre-Treatment of the Agricultural Substrates on Yield of Biogas and Kinetics of Anaerobic Digestion. Sustainability 2018, 10, 3669. https://doi.org/10.3390/su10103669
Szlachta J, Prask H, Fugol M, Luberański A. Effect of Mechanical Pre-Treatment of the Agricultural Substrates on Yield of Biogas and Kinetics of Anaerobic Digestion. Sustainability. 2018; 10(10):3669. https://doi.org/10.3390/su10103669
Chicago/Turabian StyleSzlachta, Józef, Hubert Prask, Małgorzata Fugol, and Adam Luberański. 2018. "Effect of Mechanical Pre-Treatment of the Agricultural Substrates on Yield of Biogas and Kinetics of Anaerobic Digestion" Sustainability 10, no. 10: 3669. https://doi.org/10.3390/su10103669




