Effects of Quartz Powder on the Microstructure and Key Properties of Cement Paste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Paste Constituents
2.2. Mixture Proportions
2.3. Test Methods
2.3.1. Compressive Strength Testing
2.3.2. Mercury Intrusion Porosimetry (MIP)
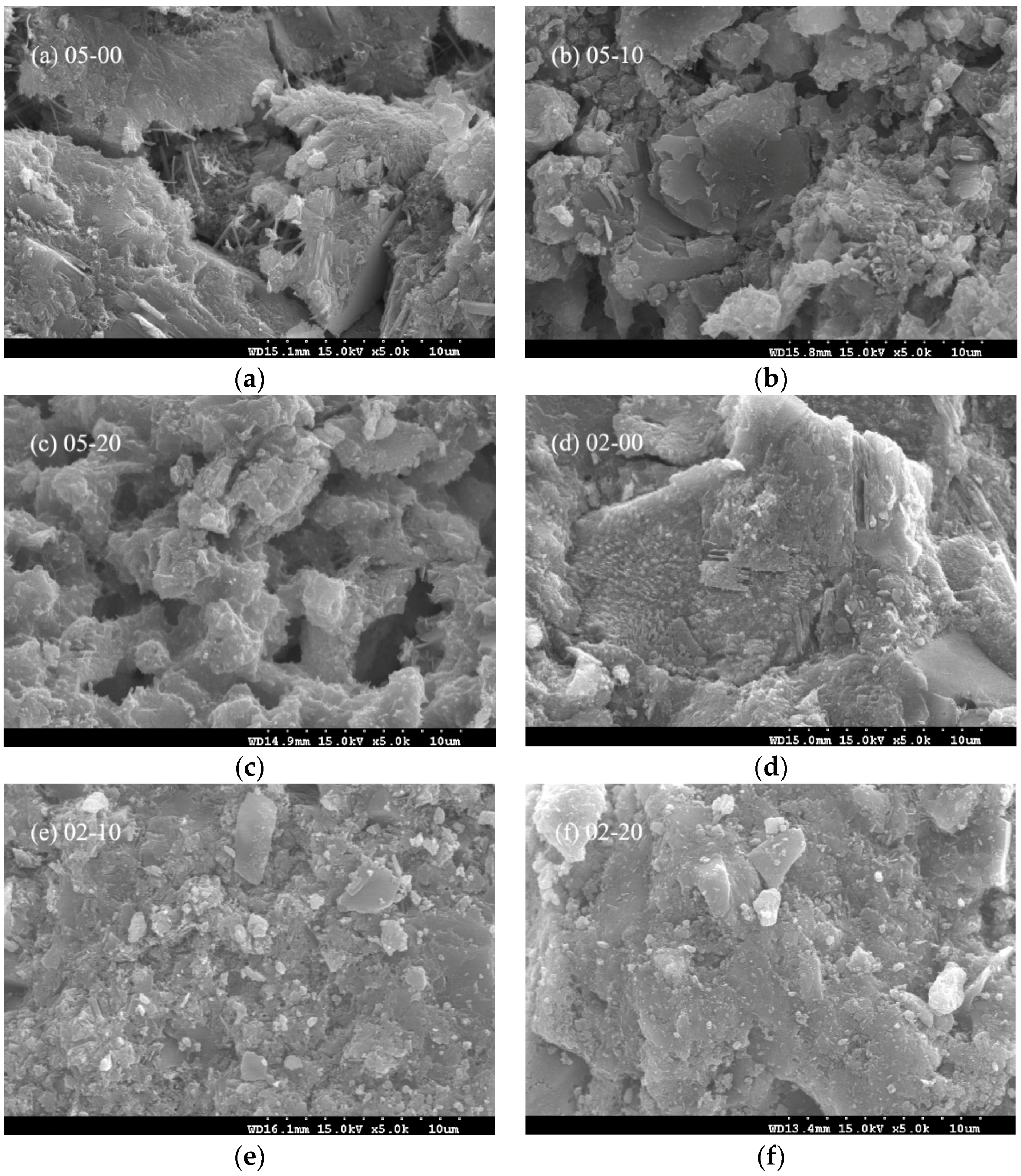
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. Isothermal Calorimetry
2.3.5. X-ray Powder Diffraction (XRD)
2.3.6. Thermogravimetric Analysis (TGA)
3. Results and Discussion
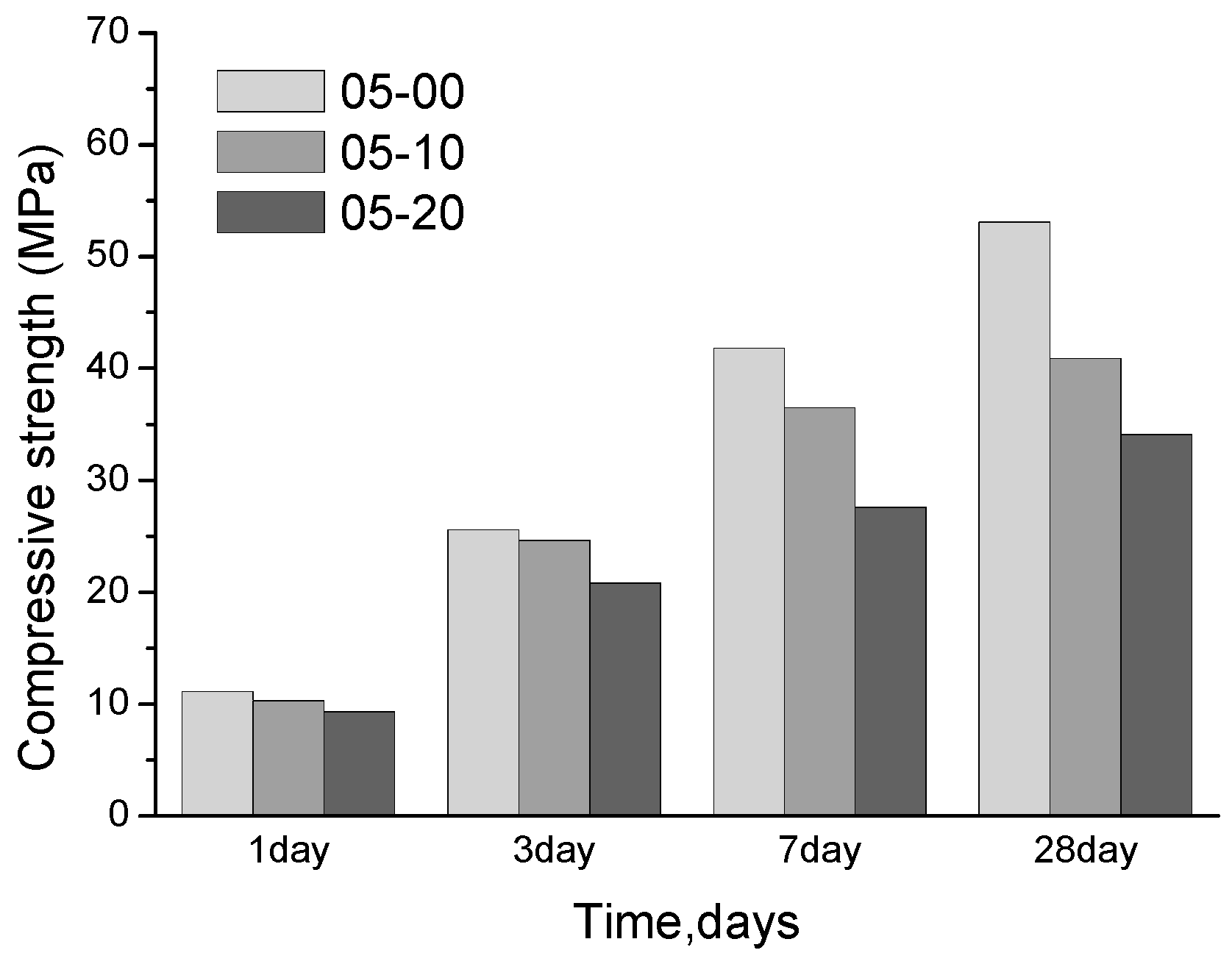
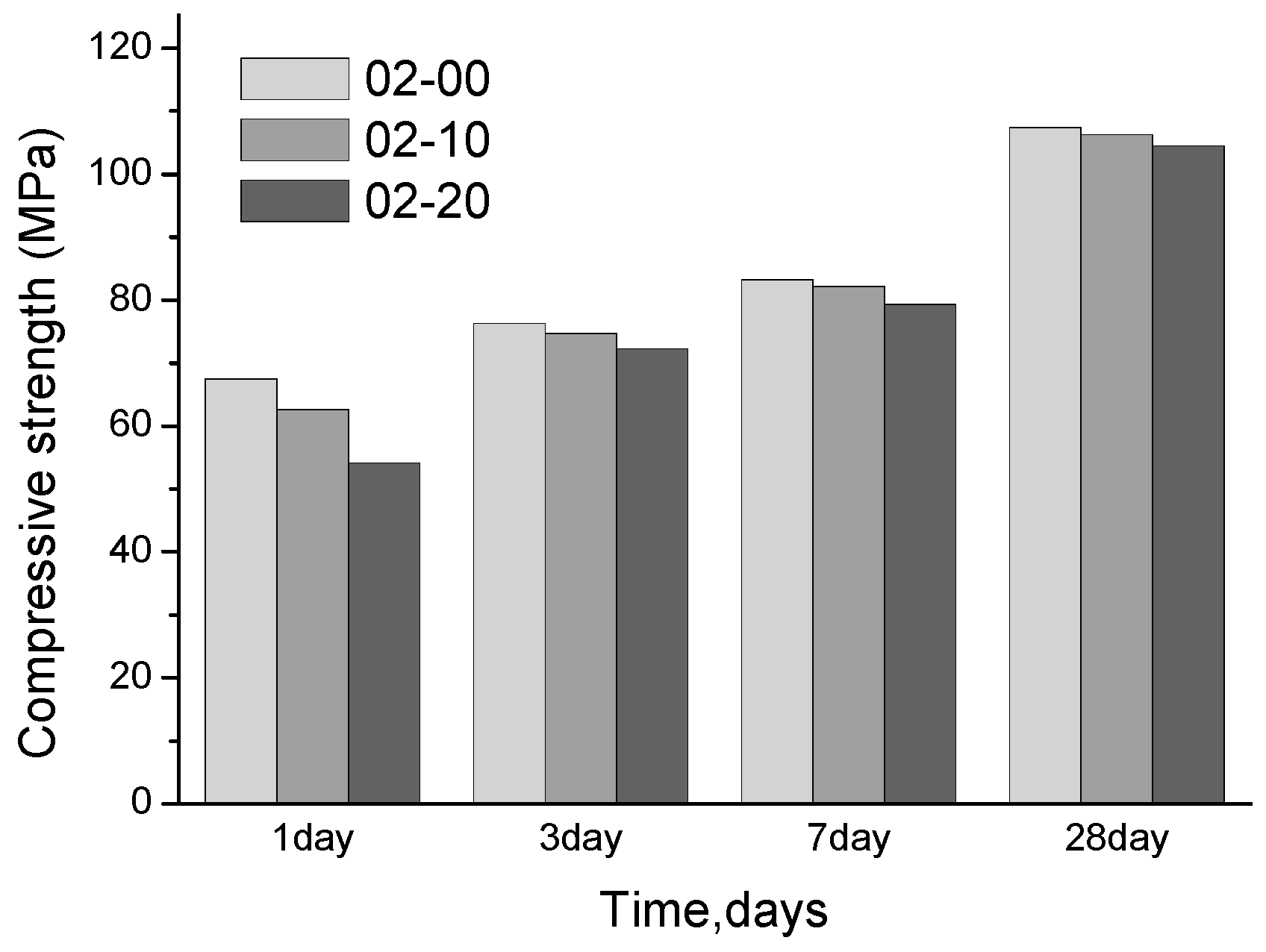
3.1. Compressive Strength
3.2. Pore Structure
3.3. Paste Microstructure
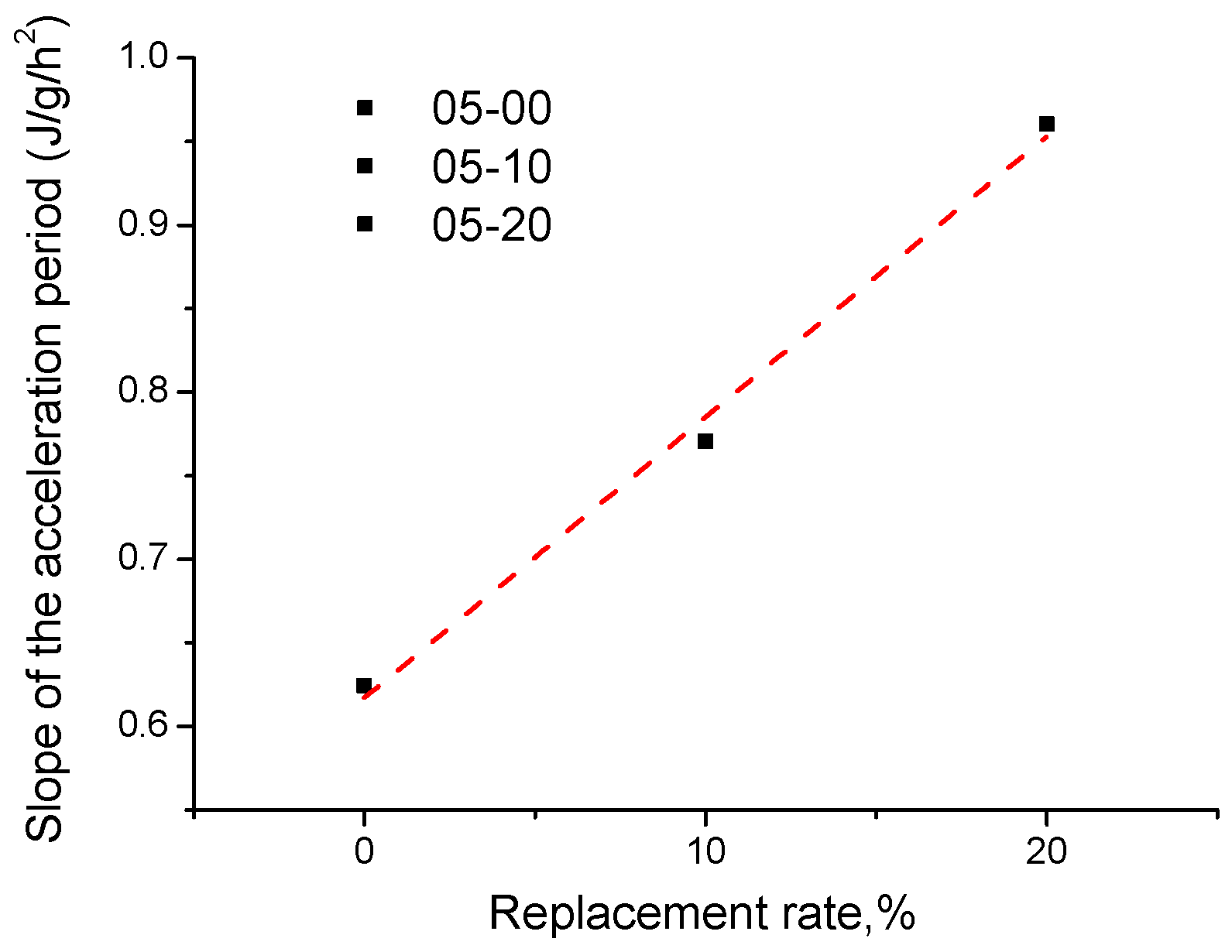
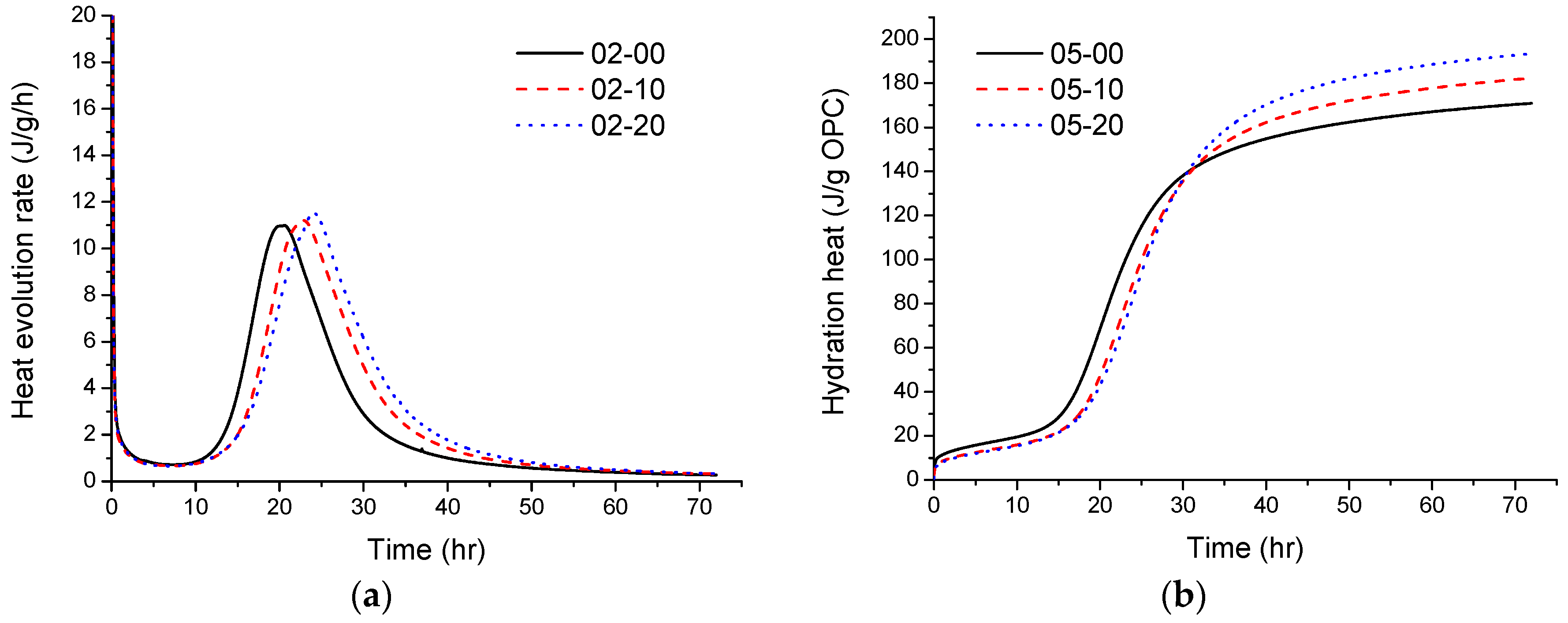
3.4. Heat of Hydration
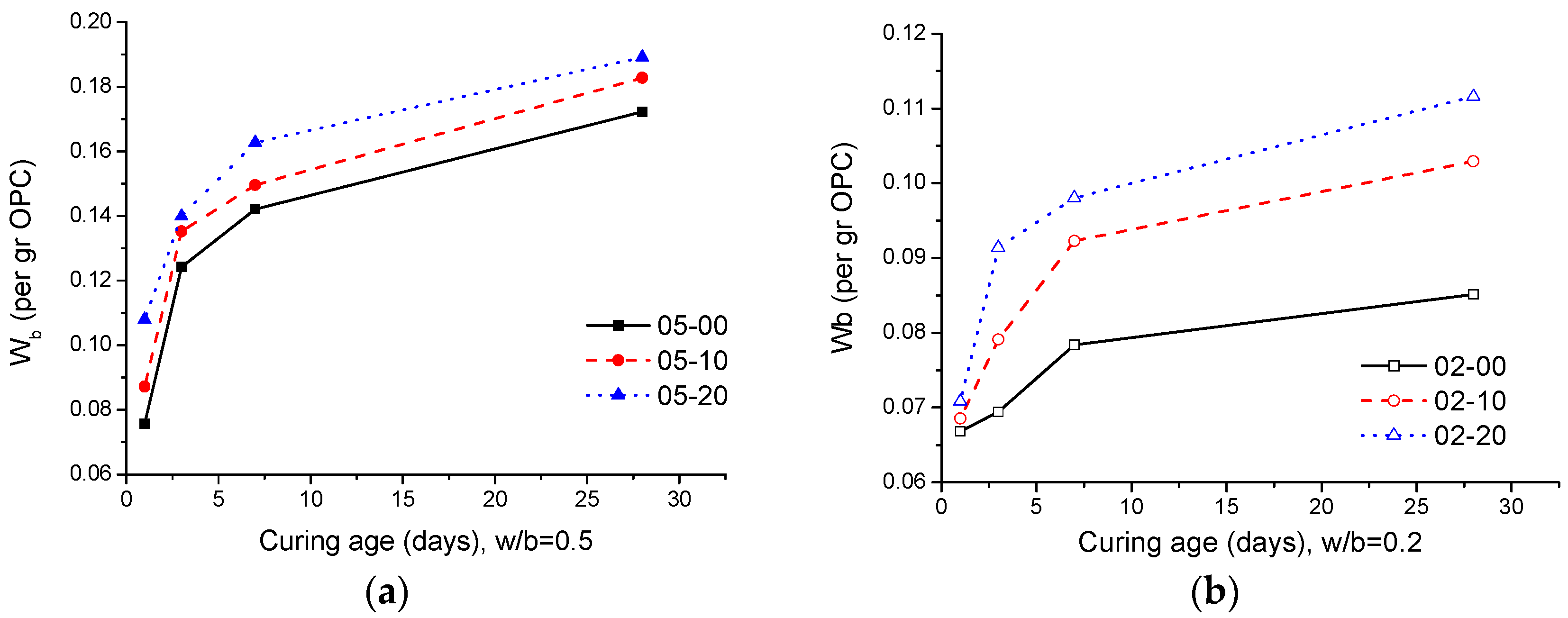
3.5. Thermogravimetric Analysis
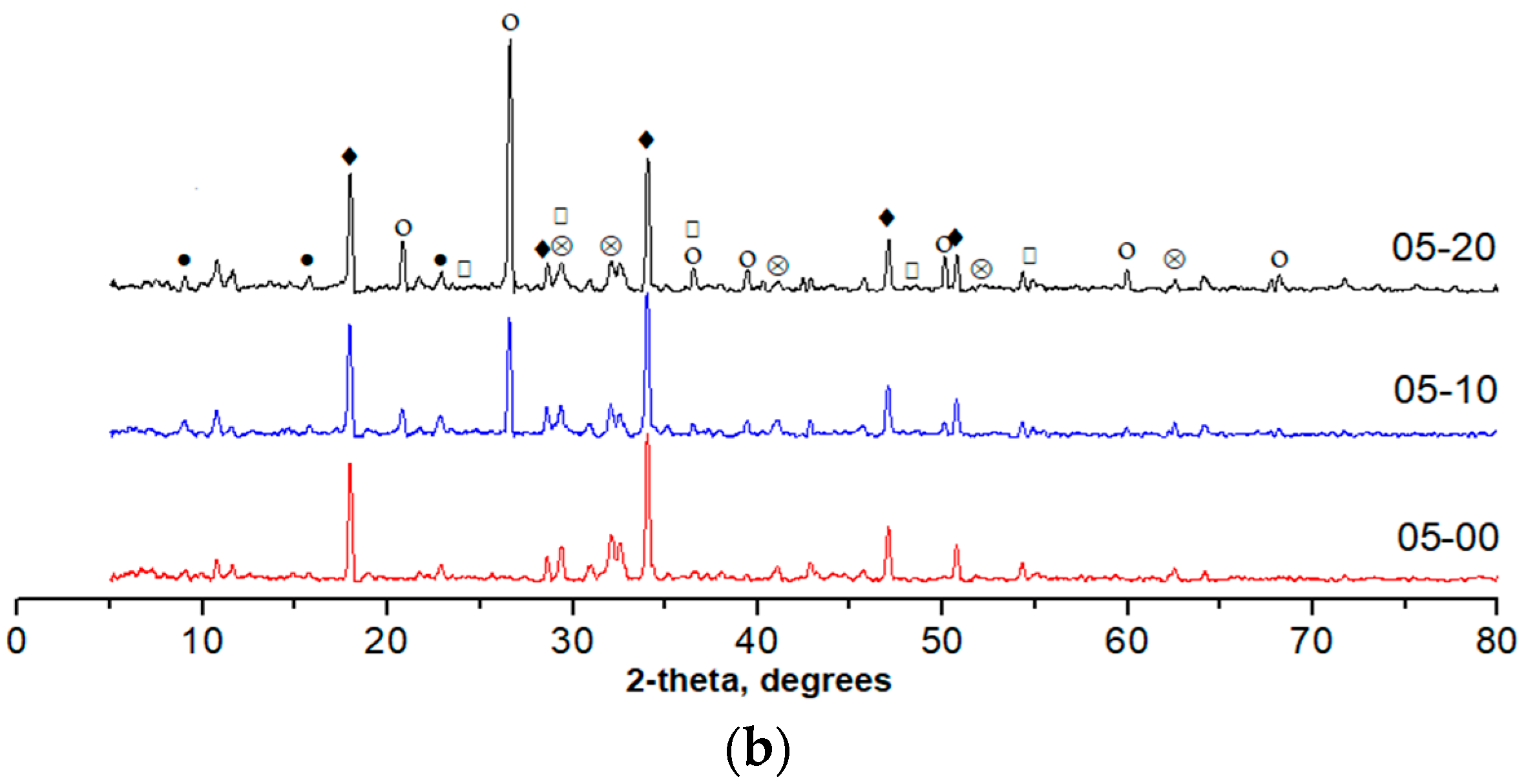
3.6. X-ray Powder Diffraction
3.7. Comparison between Cumulative Heat of Hydration and Chemically Bound Water
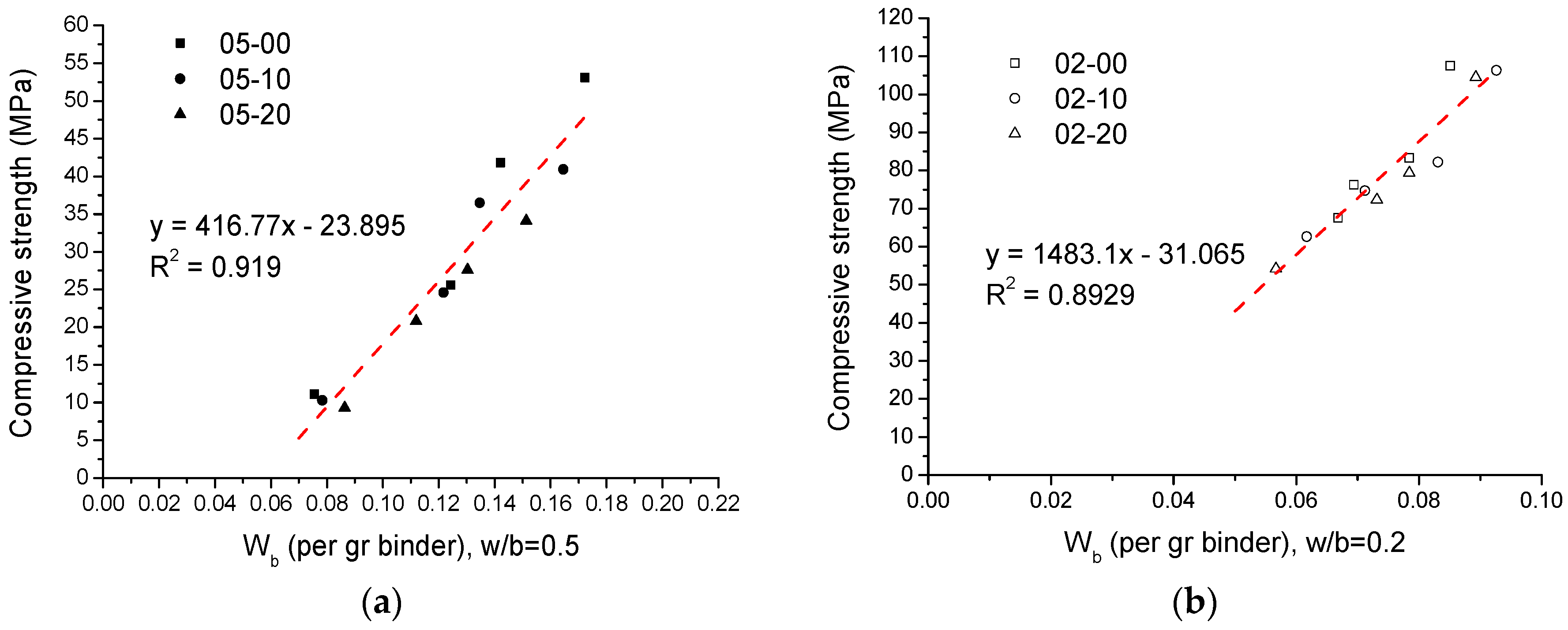
3.8. Comparison between Compressive Strength and Chemically Bound Water
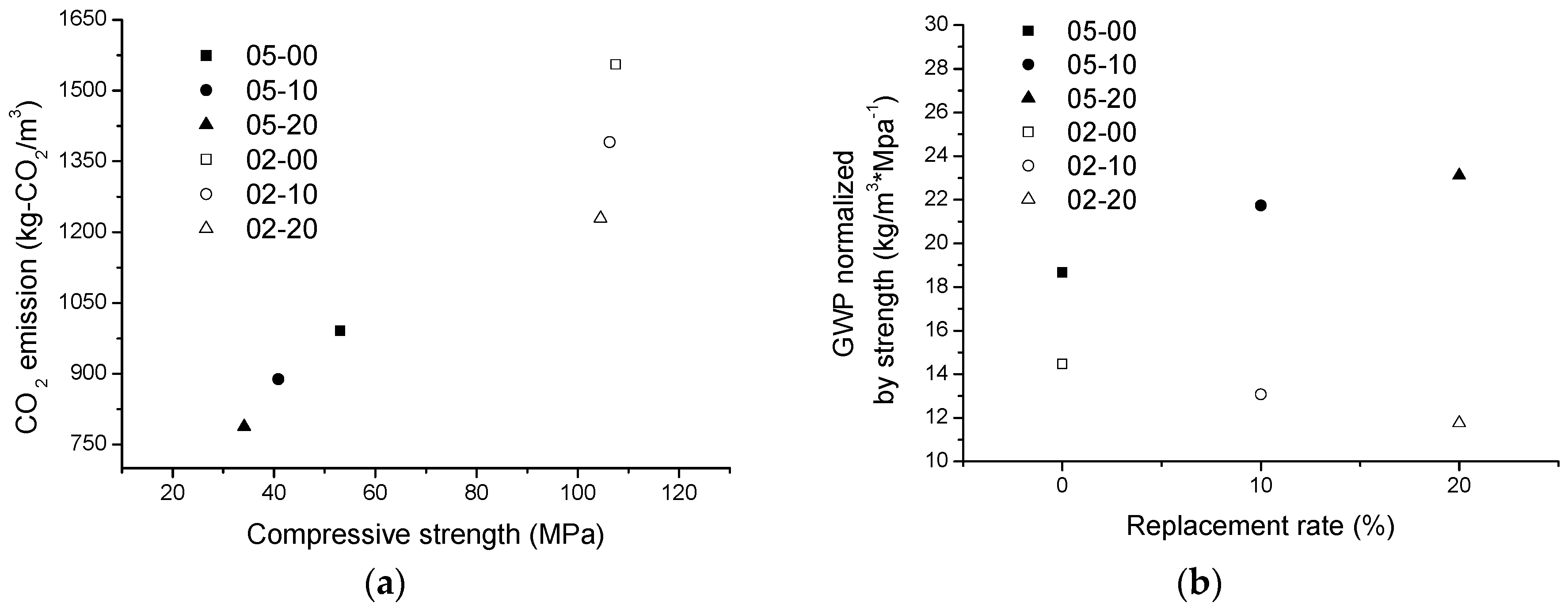
3.9. Sustainability
4. Conclusions
- When the w/b is 0.5, the compressive strength of the pastes with quartz powder significantly decreases. From the MIP and SEM tests, the porosity of the paste is increased significantly due to the incorporation of quartz powder. However, when the w/b ratio is 0.2, the strength of the pastes mixed with quartz powder shows no significant difference from that of the control paste and the porosity is almost the same;
- The heat of the hydration of the pastes was tested: in the acceleration period, when the w/b is 0.5, the addition of quartz powder can promote cement hydration. At a low w/b, the cumulative heat release of the quartz powder pastes is higher than the control, which also indicates that when the w/b ratio of the paste used is low, the quartz powder can also promote the early hydration of cement;
- According to the results obtained from TGA, when the w/b ratio is low, the addition of quartz powder can promote the hydration of cement significantly compared to a high w/b ratio. Similar results are also obtained from XRD analysis;
- The compressive strength and cumulative hydration heat of binder pastes are well-correlated with their chemically bound water; the cumulative hydration heat is proportional to the total chemically bound water. Also, regardless of the w/b ratio, the compressive strength of binder pastes shows a good linear relation with the ratio of chemically bound water to all water.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Worrell, E.; Price, L.; Martin, N.; Hendriks, C.; Meida, L.O. Carbon dioxide emissions from the global cement industry1. Annu. Rev. Energy Environ. 2001, 26, 303–329. [Google Scholar] [CrossRef]
- Bentz, D.P. Best Practices Guide for High-Volume Fly Ash Concretes: Assuring Properties and Performance; Technical Note (NIST TN)-1812; National Institute of Standards and Technology, US department of Commerce: Gaithersburg, MD, USA, 2013. [CrossRef]
- Howard, I.L.; Shannon, J.; Cost, V.T.; Stovall, M. Davis Wade Stadium Expansion and Renovation: Performance of Concrete Produced with Portland-Limestone Cement, Fly Ash, and Slag Cement. J. Mater. Civ. Eng. 2015, 27, 04015044. [Google Scholar] [CrossRef]
- Menéndez, G.; Bonavetti, V.; Irassar, E.F. Strength development of ternary blended cement with limestone filler and blast-furnace slag. Cem. Concr. Compos. 2003, 25, 61–67. [Google Scholar] [CrossRef]
- Shannon, J.; Howard, I.L.; Tim Cost, V. Potential of Portland-Limestone Cement to Improve Performance of Concrete Made with High Slag Cement and Fly Ash Replacement Rates. J. Test. Eval. 2017, 45, 873–889. [Google Scholar] [CrossRef]
- Suraneni, P.; Weiss, J. Examining the pozzolanicity of supplementary cementitious materials using isothermal calorimetry and thermogravimetric analysis. Cem. Concr. Compos. 2017, 83, 273–278. [Google Scholar] [CrossRef]
- Mehta, P.K. Pozzolanic and Cementitious By-Products as Mineral Admixtures for Concrete—A critical Review. Spec. Publ. 1983, 79, 1–46. [Google Scholar]
- Rahhal, V.; Talero, R. Early hydration of portland cement with crystalline mineral additions. Cem. Concr. Compos. 2005, 35, 1285–1291. [Google Scholar] [CrossRef]
- Kadri, E.H.; Aggoun, S.; De Schutter, G.; Ezziane, K. Combined effect of chemical nature and fineness of mineral powders on Portland cement hydration. Mater. Struct. 2010, 43, 665–673. [Google Scholar] [CrossRef]
- Berodier, E.; Scrivener, K. Understanding the filler effect on the nucleation and growth of C-S-H. J. Am. Ceram. Soc. 2014, 97, 3764–3773. [Google Scholar] [CrossRef]
- Gutteridge, W.A.; Dalziel, J.A. Filler cement: The effect of the secondary component on the hydration of Portland cement. Part 2: Fine hydraulic binders. Cem. Concr. Res. 1990, 20, 853–861. [Google Scholar] [CrossRef]
- Oey, T.; Kumar, A.; Bullard, J.W.; Neithalath, N.; Sant, G. The filler effect: The influence of filler content and surface area on cementitious reaction rates. J. Am. Ceram. Soc. 2013, 96, 1978–1990. [Google Scholar] [CrossRef]
- Garrault-Gauffinet, S.; Nonat, A. Experimental investigation of calcium silicate hydrate (C-S-H) nucleation. J. Cryst. Growth 1999, 200, 565–574. [Google Scholar] [CrossRef]
- Stark, J.; Moser, B.; Bellmann, F. Nucleation and Growth of C-S-H Phases on Mineral Admixtures; Springer: Berlin, Germany, 2007; ISBN 9783540724476. [Google Scholar]
- Bentz, D.P.; Ferraris, C.F.; Jones, S.Z.; Lootens, D.; Zunino, F. Limestone and silica powder replacements for cement: Early-age performance. Cem. Concr. Compos. 2017, 78, 43–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Mao, R.; Lü, L.; Hu, S. Hydration products of cement-silica fume-quartz powder mixture under different curing regimes. J. Wuhan Univ. Tech. Mater. Sci. Ed. 2017, 32, 598–602. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials Edited; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-4987-3865-1. [Google Scholar]
- Scherer, G.W.; Zhang, J.; Thomas, J.J. Nucleation and growth models for hydration of cement. Cem. Concr. Res. 2012, 42, 982–993. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials; McGraw-Hill: New York, NY, USA, 2006; ISBN 0071589198. [Google Scholar]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford: London, UK, 1997; ISBN 0727725920. [Google Scholar]
- Krstulović, R.; Dabić, P. A conceptual model of the cement hydration process. Cem. Concr. Res. 2000, 30, 693–698. [Google Scholar] [CrossRef]
- Van Tuan, N.; Ye, G.; Van Breugel, K.; Copuroglu, O. Hydration and microstructure of ultra high performance concrete incorporating rice husk ash. Cem. Concr. Res. 2011, 41, 1104–1111. [Google Scholar] [CrossRef]
- Neville, A.M. Properties of Concrete, 4th and final eds.; Hohn Wiley Sons: Hoboken, NJ, USA, 1997; ISBN 0-582-23070-5. [Google Scholar]
- Pane, I.; Hansen, W. Investigation of blended cement hydration by isothermal calorimetry and thermal analysis. Cem. Concr. Res. 2005, 35, 1155–1164. [Google Scholar] [CrossRef]
- Rupasinghe, M.; San Nicolas, R.; Mendis, P.; Sofi, M.; Ngo, T. Investigation of strength and hydration characteristics in nano-silica incorporated cement paste. Cem. Concr. Compos. 2017, 80, 17–30. [Google Scholar] [CrossRef]
- Hendriks, C.A.; Worrell, E.; de Jager, D.; Blok, K.; Riemer, P. Emission Reduction of Greenhouse Gases from the Cement Industry; Ieaghg: Cheltenham, UK, 2004; pp. 1–11. [Google Scholar] [CrossRef]
- Müller, H.S.; Haist, M.; Vogel, M. Assessment of the sustainability potential of concrete and concrete structures considering their environmental impact, performance and lifetime. Constr. Build. Mater. 2014, 67, 321–337. [Google Scholar] [CrossRef]
- Yang, K.-H.; Jung, Y.-B.; Cho, M.-S.; Tae, S.-H. Effect of supplementary cementitious materials on reduction of CO2 emissions from concrete. J. Clean. Prod. 2015, 103, 774–783. [Google Scholar] [CrossRef]
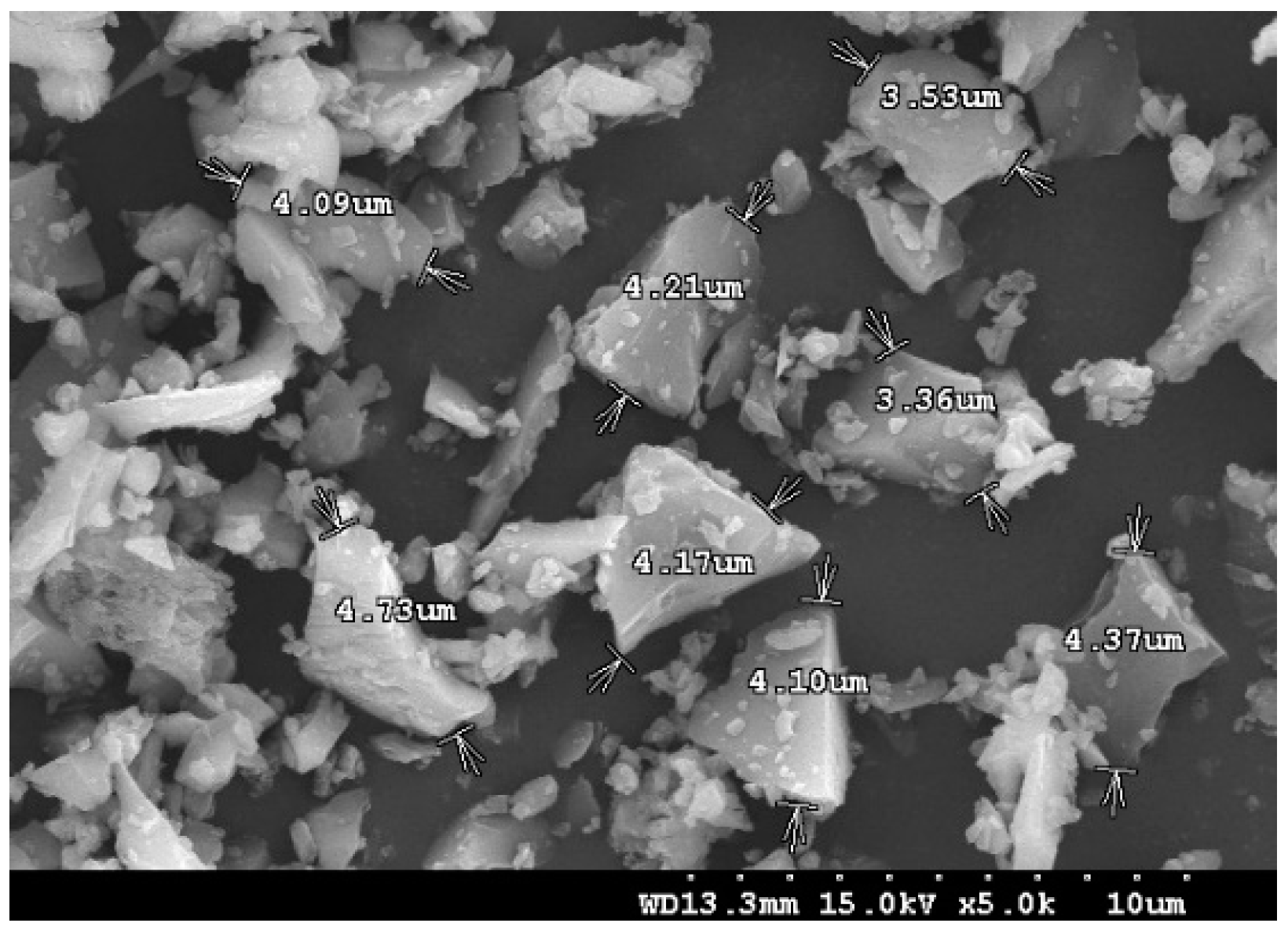












| Chemical Component (% wt) | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | MnO | TiO2 | SO3 | ZnO | P2O5 | K2O | LOI 1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cement | 20.4 | 4.55 | 2.54 | 63.1 | 3.15 | 0.07 | 0.23 | 2.28 | 0.06 | 0.14 | 1.4 | 0.68 |
| Quartz powder | 99.0 | 0.25 | - | - | - | - | 0.11 | - | - | - | - | 0.62 |
| Mix No. | %OPC | %Quartz | w/b | %HRWRA |
|---|---|---|---|---|
| 05-00 | 100 | 0 | 0.5 | - |
| 05-10 | 90 | 10 | 0.5 | - |
| 05-20 | 80 | 20 | 0.5 | - |
| 02-00 | 100 | 0 | 0.2 | 1 |
| 02-10 | 90 | 10 | 0.2 | 1 |
| 02-20 | 80 | 20 | 0.2 | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, R.-S.; Wang, X.-Y.; Zhang, G.-Y. Effects of Quartz Powder on the Microstructure and Key Properties of Cement Paste. Sustainability 2018, 10, 3369. https://doi.org/10.3390/su10103369
Lin R-S, Wang X-Y, Zhang G-Y. Effects of Quartz Powder on the Microstructure and Key Properties of Cement Paste. Sustainability. 2018; 10(10):3369. https://doi.org/10.3390/su10103369
Chicago/Turabian StyleLin, Run-Sheng, Xiao-Yong Wang, and Gui-Yu Zhang. 2018. "Effects of Quartz Powder on the Microstructure and Key Properties of Cement Paste" Sustainability 10, no. 10: 3369. https://doi.org/10.3390/su10103369






