On the Sustainability and Progress of Energy Neutral Mineral Processing
Abstract
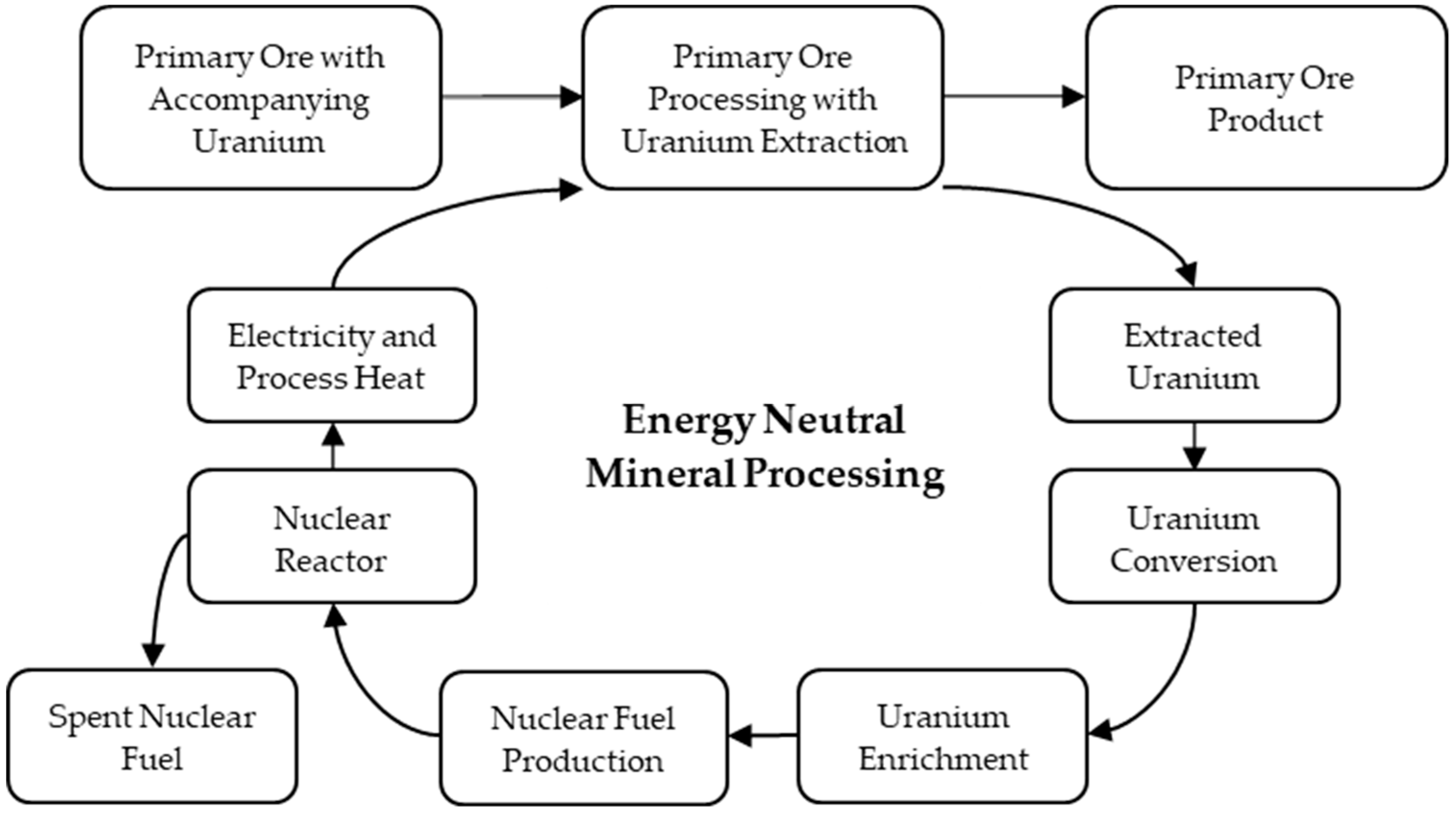
:1. Introduction to Energy Neutral Mineral Processing
2. Motivation Behind Energy Neutral Mineral Processing
3. Mineral Processes Currently Considered
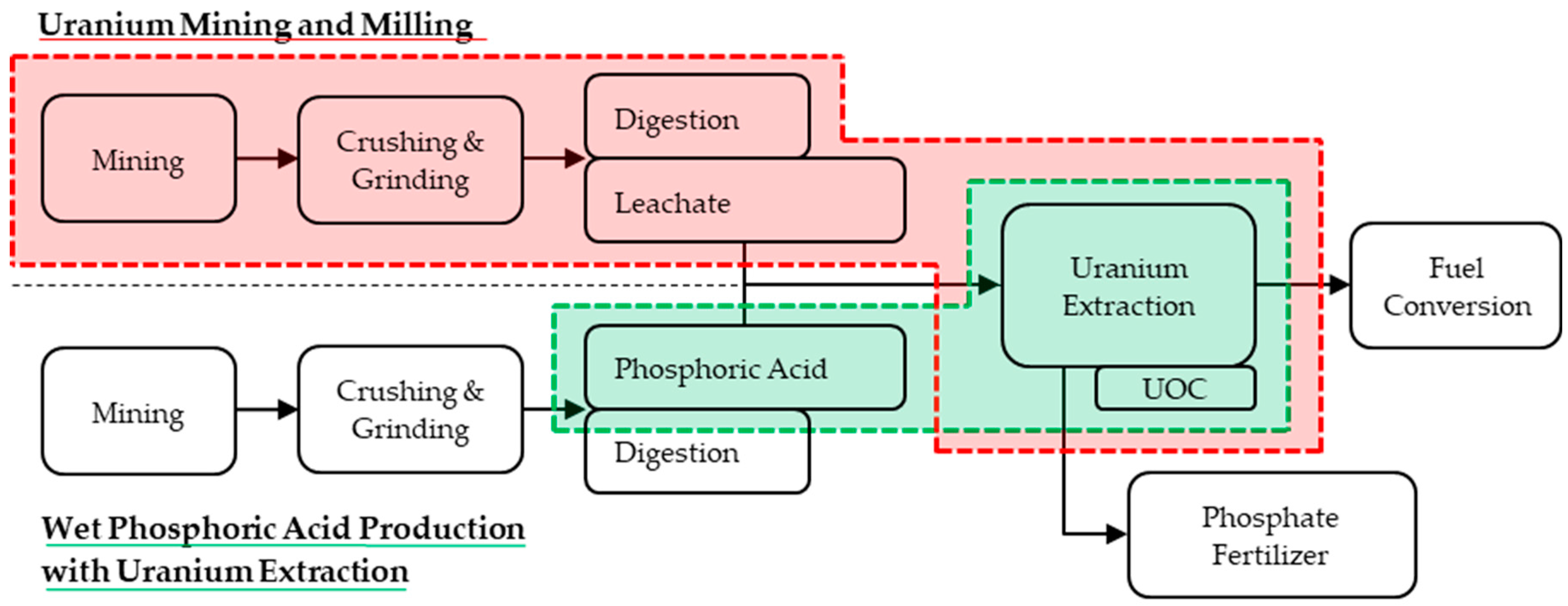
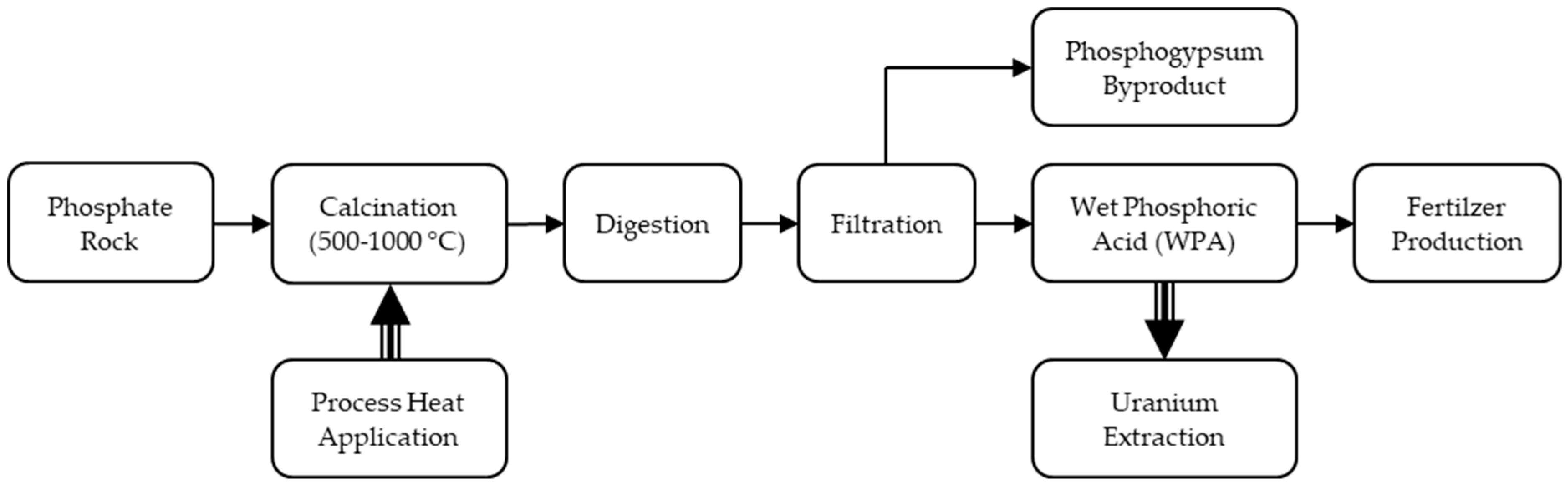
3.1. Phosphate Rock
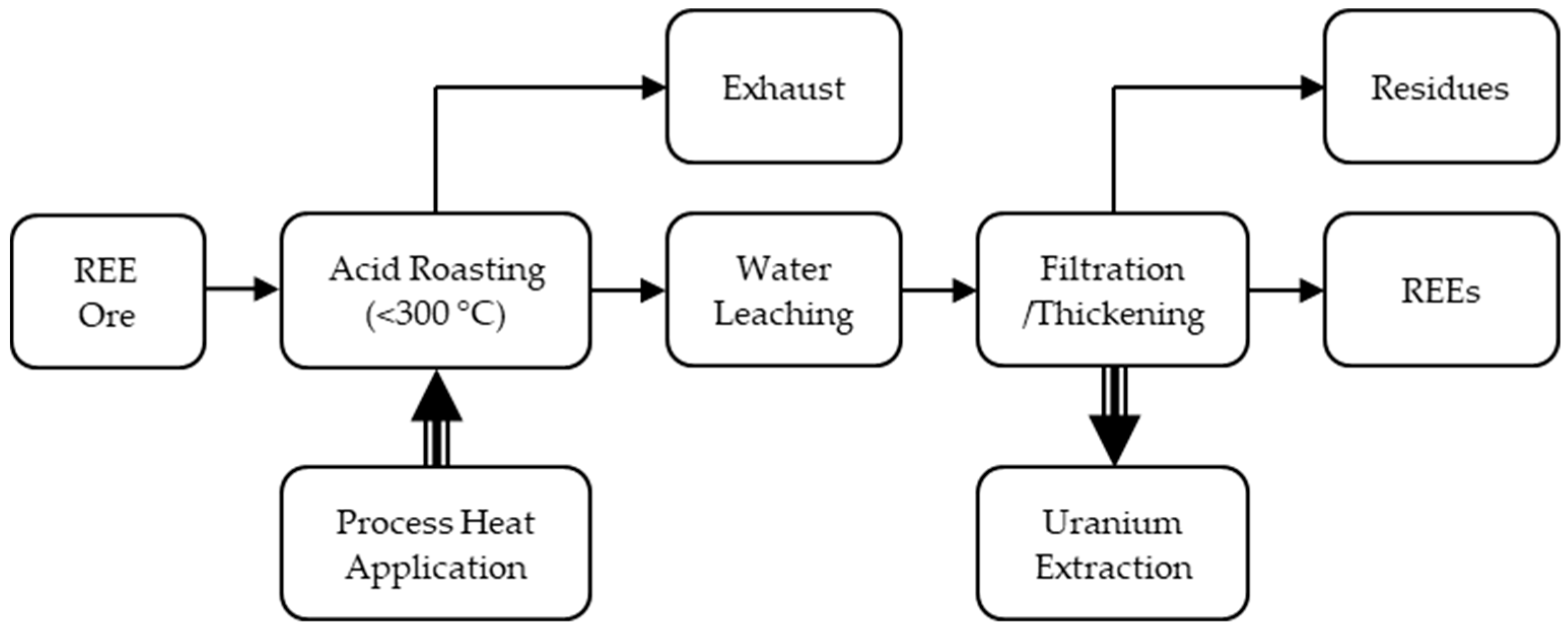
3.2. Rare Earth Element Ore
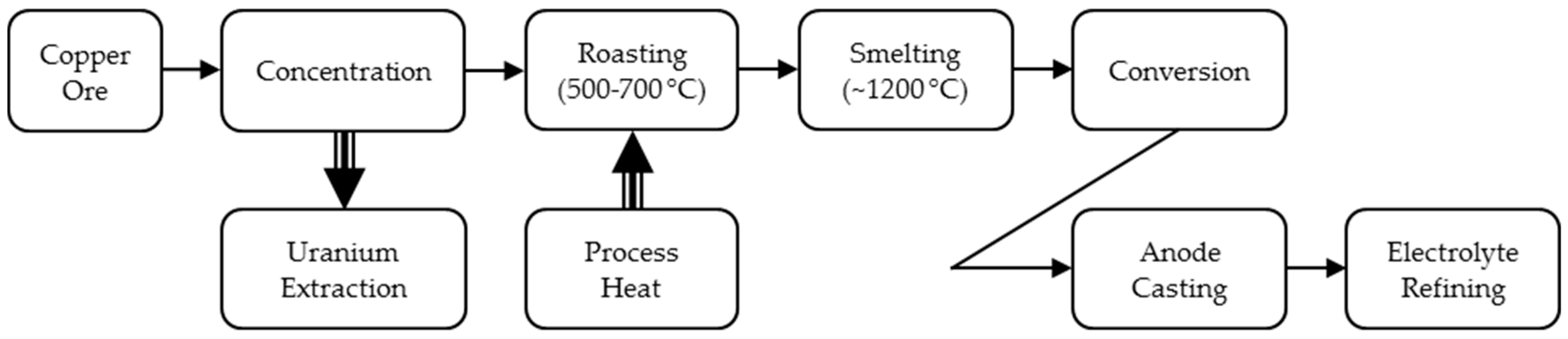
3.3. Copper Ore
3.4. Tin Slag
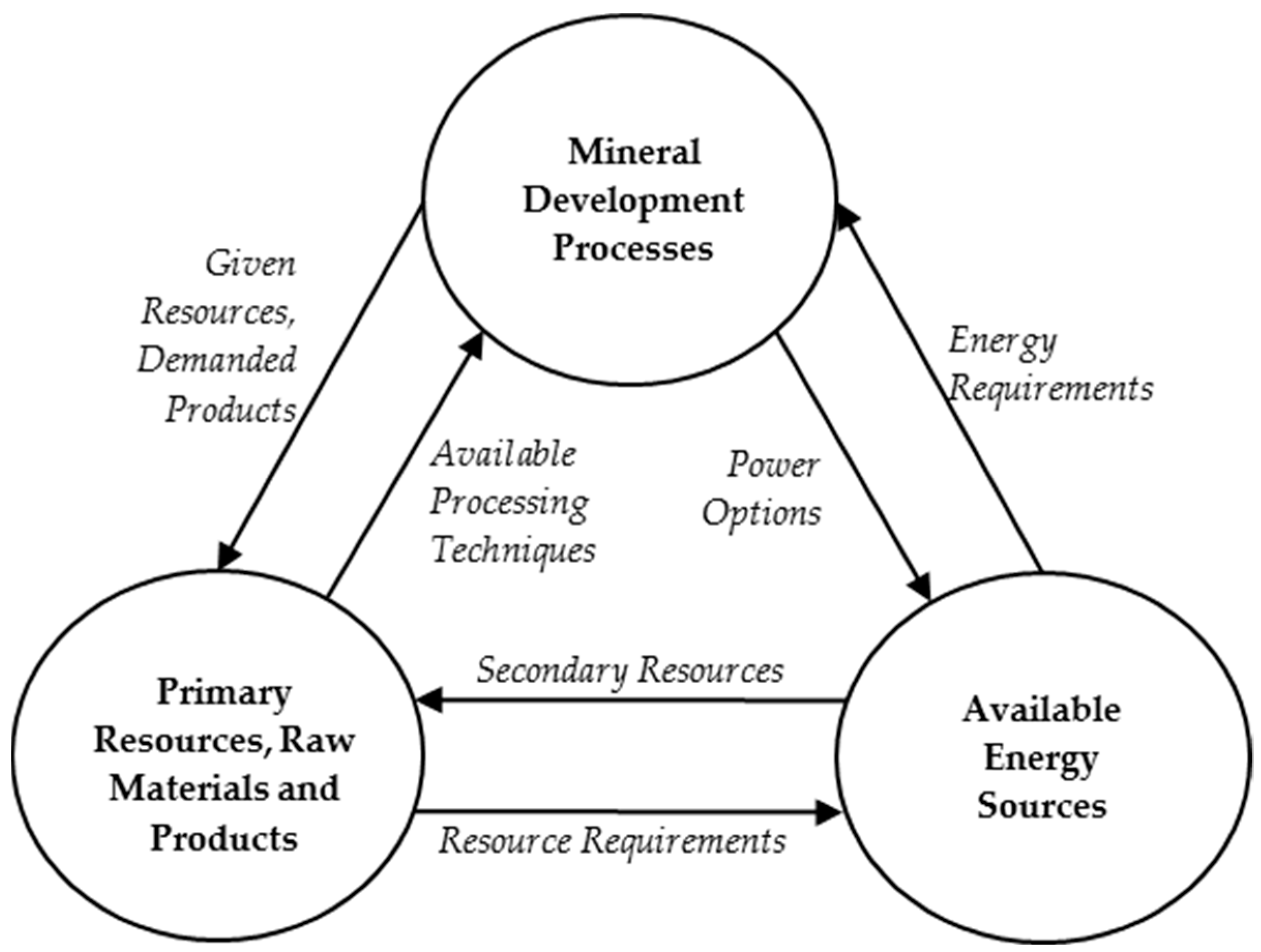
4. Modeling of the Coupled System
5. Identified Challenges
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Haneklaus, N.; Reitsma, F.; Tulsidas, H.; Schnug, E.; Lottermoser, B.G.; Allelein, H.J. Energy Neutral Mineral Development Processes—An Overview. In Proceedings of the AIMS 2016, Aachen, Germany, 18–19 May 2016. [Google Scholar]
- Robertson, A.; Grant, D.; Liebezeit, V.; Ehrig, K.; Badenhorst, C.; Durandt, G. Olympic Dam Mine, BHP Biliton. Australasian Mining Metallurgical Operating Practices. 2013. Available online: https://www.ausimm.com.au/publications/publication.aspx?ID=15461 (accessed on 17 January 2018).
- Yan, X.; Noguchi, H.; Sato, H.; Tachibana, Y.; Kunitomi, K.; Hino, R. A hybrid HTGR system producing electricity, hydrogen and such other products as water demanded in the Middle East. Nucl. Eng. Des. 2014, 271, 20–29. [Google Scholar] [CrossRef]
- Dardour, S.; Nisan, S.; Charbit, F. Utilisation of waste heat from GT-MHR and PBMR reactors for nuclear desalination. Desalination 2007, 205, 254–268. [Google Scholar] [CrossRef]
- Nisan, S.; Benzarti, N. A comprehensive economic evaluation of integrated desalination systems using fossil fuelled and nuclear energies and including their environmental costs. Desalination 2008, 229, 125–146. [Google Scholar] [CrossRef]
- Nisan, S.; Dardour, S. Economic evaluation of nuclear desalination systems. Desalination 2007, 205, 231–242. [Google Scholar] [CrossRef]
- Peterson, P.F. Spent Nuclear Fuel is not the Problem. Proc. IEEE 2017, 105, 411–414. [Google Scholar] [CrossRef]
- IEA. World Energy Outlook 2016 (Executive Summary). World Energy Outlook. 2016, pp. 1–8. Available online: http://www.iea.org/publications/freepublications/publication/WEB_WorldEnergyOutlook2015ExecutiveSummaryEnglishFinal.pdf (accessed on 12 January 2018).
- Norgate, T.; Jahanshahi, S. Low grade ores—Smelt, leach or concentrate? Miner. Eng. 2010, 23, 65–73. [Google Scholar] [CrossRef]
- Ragnarsdóttir, K.V. Rare metals getting rarer. Nat. Geosci. 2008, 1, 720–721. [Google Scholar] [CrossRef]
- West, J. Decreasing Metal Ore Grades: Are They Really Being Driven by the Depletion of High-Grade Deposits? J. Ind. Ecol. 2011, 15, 165–168. [Google Scholar] [CrossRef]
- Dittmar, M. The end of cheap uranium. Sci. Total Environ. 2013, 461–462, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Prior, T.; Giurco, D.; Mudd, G.; Mason, L.; Behrisch, J. Resource depletion, peak minerals and the implications for sustainable resource management. Glob. Environ. Chang. 2012, 22, 577–587. [Google Scholar] [CrossRef]
- Norgate, T.; Haque, N. Energy and greenhouse gas impacts of mining and mineral processing operations. J. Clean. Prod. 2010, 18, 266–274. [Google Scholar] [CrossRef]
- Northey, S.; Mohr, S.; Mudd, G.M.; Weng, Z.; Giurco, D. Modelling future copper ore grade decline based on a detailed assessment of copper resources and mining. Resour. Conserv. Recycl. 2014, 83, 190–201. [Google Scholar] [CrossRef]
- Elshkaki, A.; Graedel, T.E.; Ciacci, L.; Reck, B. Copper demand, supply, and associated energy use to 2050. Glob. Environ. Chang. 2016, 39, 305–315. [Google Scholar] [CrossRef]
- Calvo, G.; Mudd, G.; Valero, A.; Valero, A. Decreasing Ore Grades in Global Metallic Mining: A Theoretical Issue or a Global Reality? Resources 2016, 5, 36. [Google Scholar] [CrossRef]
- Mudd, G.M. The Environmental sustainability of mining in Australia: Key mega-trends and looming constraints. Resour. Policy 2010, 35, 98–115. [Google Scholar] [CrossRef]
- Hilson, G. Corporate Social Responsibility in the extractive industries: Experiences from developing countries. Resour. Policy 2012, 37, 131–137. [Google Scholar] [CrossRef]
- Owen, J.R.; Kemp, D. Social licence and mining: A critical perspective. Resour. Policy 2013, 38, 29–35. [Google Scholar] [CrossRef]
- Vesborg, P.C.K.; Jaramillo, T.F. Addressing the terawatt challenge: Scalability in the supply of chemical elements for renewable energy. RSC Adv. 2012, 2, 7933–7947. [Google Scholar] [CrossRef] [Green Version]
- Bradshaw, A.M.; Reuter, B.; Hamacher, T. The potential scarcity of rare elements for the Energiewende. Green 2013, 3, 93–111. [Google Scholar] [CrossRef]
- Habib, K.; Wenzel, H. Exploring rare earths supply constraints for the emerging clean energy technologies and the role of recycling. J. Clean. Prod. 2014, 84, 348–359. [Google Scholar] [CrossRef]
- Arent, D.; Pless, J.; Mai, T.; Wiser, R.; Hand, M.; Baldwin, S.; Heath, G.; Macknick, J.; Bazilian, M.; Schlosser, A.; et al. Implications of high renewable electricity penetration in the U.S. for water use, greenhouse gas emissions, land-use, and materials supply. Appl. Energy 2014, 123, 368–377. [Google Scholar] [CrossRef]
- Moss, R.L.; Tzimas, E.; Kara, H.; Willis, P.; Kooroshy, J. The potential risks from metals bottlenecks to the deployment of Strategic Energy Technologies. Energy Policy 2013, 55, 556–564. [Google Scholar] [CrossRef]
- Baldi, L.; Peri, M.; Vandone, D. Clean energy industries and rare earth materials: Economic and financial issues. Energy Policy 2014, 66, 53–61. [Google Scholar] [CrossRef]
- Stegen, K.S. Heavy rare earths, permanent magnets, and renewable energies: An imminent crisis. Energy Policy 2015, 79, 1–8. [Google Scholar] [CrossRef]
- Kawatra, S.K.; Carlson, J.T. Beneficiation of Phosphate Ore; SME: Englewood, CO, USA, 2013; p. 168. [Google Scholar]
- Abouzeid, A. Upgrading of Phosphate Ores—A Review. J. ORE Dress. 2007, 9, 15–20. [Google Scholar]
- Zhang, P. Comprehensive recovery and sustainable development of phosphate resources. Procedia Eng. 2014, 83, 37–51. [Google Scholar] [CrossRef]
- El-Shall, H.; Zhang, P.; Abdel-Khalek, N.; El-Mofty, S. Beneficiation technology of phosphates: Challenges and solutions. Miner. Metall. Process. 2004, 21, 17–26. [Google Scholar]
- Ulrich, A.E.; Frossard, E. On the history of a reoccurring concept: Phosphorus scarcity. Sci. Total Environ. 2014, 490, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Cordell, D.; White, S. Peak phosphorus: Clarifying the key issues of a vigorous debate about long-term phosphorus security. Sustainability 2011, 3, 2027–2049. [Google Scholar] [CrossRef]
- Smil, V. Phousphorus in the environment: Natural Flows and Human Interferences. Annu. Rev. Energy Environ. 2000, 25, 53–88. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Van Vuuren, D.P.; Bouwman, A.F.; Beusen, A.H.W. Phosphorus demand for the 1970–2100 period: A scenario analysis of resource depletion. Glob. Environ. Chang. 2010, 20, 428–439. [Google Scholar] [CrossRef]
- Carvalho, F.P.; Madruga, M.J.; Reis, M.C.; Alves, J.G.; Oliveira, J.M.; Gouveia, J.; Silva, L. Radioactivity in the environment around past radium and uranium mining sites of Portugal. J. Environ. Radioact. 2007, 96, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.M.; Sahoo, S.K.; Jha, V.N.; Khan, A.H.; Puranik, V.D. Assessment of environmental radioactivity at uranium mining, processing and tailings management facility at Jaduguda, India. Appl. Radiat. Isot. 2008, 66, 1666–1670. [Google Scholar] [CrossRef] [PubMed]
- Abdelouas, A. Uranium mill tailings: Geochemistry, mineralogy, and environmental impact. Elements 2006, 2, 335–341. [Google Scholar] [CrossRef]
- Norgate, T.; Haque, N.; Koltun, P. The impact of uranium ore grade on the greenhouse gas footprint of nuclear power. J. Clean. Prod. 2014, 84, 360–367. [Google Scholar] [CrossRef]
- Lenzen, M. Life cycle energy and greenhouse gas emissions of nuclear energy: A review. Energy Convers. Manag. 2008, 49, 2178–2199. [Google Scholar] [CrossRef]
- Poinssot, C.; Bourg, S.; Ouvrier, N.; Combernoux, N.; Rostaing, C.; Vargas-Gonzalez, M.; Bruno, J. Assessment of the environmental footprint of nuclear energy systems. Comparison between closed and open fuel cycles. Energy 2014, 69, 199–211. [Google Scholar] [CrossRef]
- Beltrami, D.; Cote, G.; Mokhtari, H.; Courtaud, B.; Moyer, B.A.; Chagnes, A. Recovery of Uranium from Wet Process Phosphoric Acid by Solvent Extraction. Chem. Rev. 2014, 114, 12002–12023. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Mondal, S.; Chakravartty, J.K. Recovery of Uranium from Phosphoric Acid: A Review. Solvent Extr. Ion Exch. 2016, 34, 201–225. [Google Scholar] [CrossRef]
- Bunus, F.T. Uranium and Rare Earth Recovery from Phosphate Fertilizer Industry by Solvent Extraction. Miner. Process. Extr. Metall. Rev. 2000, 21, 381–478. [Google Scholar] [CrossRef]
- Astley, V.; Stana, R. There and Back Again 2.5Again Who did What in Solvent Extraction? A Demonstrated & Proven Technology for Uranium Recovery from Phosphoric Acid. Procedia Eng. 2014, 83, 270–278. [Google Scholar] [CrossRef]
- Kim, H.; Eggert, R.G.; Carlsen, B.W.; Dixon, B.W. Potential uranium supply from phosphoric acid: A U.S. analysis comparing solvent extraction and Ion exchange recovery. Resour. Policy 2016, 49, 222–231. [Google Scholar] [CrossRef]
- Haneklaus, N.; Sun, Y.; Bol, R.; Lottermoser, B.; Schnug, E. To Extract, or not to Extract Uranium from Phosphate Rock, that is the Question. Environ. Sci. Technol. 2016, 753–754. [Google Scholar] [CrossRef] [PubMed]
- Hore-Lacy, I. 9-Production of byproduct uranium and uranium from unconventional resources. Uranium Nucl. Power 2016, 239–251. [Google Scholar] [CrossRef]
- Gabriel, S.; Baschwitz, A.; Mathonnière, G.; Eleouet, T.; Fizaine, F. A critical assessment of global uranium resources, including uranium in phosphate rocks, and the possible impact of uranium shortages on nuclear power fleets. Ann. Nucl. Energy 2013, 58, 213–220. [Google Scholar] [CrossRef]
- Schnug, E.; Haneklaus, N. Uranium, the hidden treasure in phosphates. Procedia Eng. 2014, 83, 265–269. [Google Scholar] [CrossRef]
- Chen, M.; Graedel, T.E. The potential for mining trace elements from phosphate rock. J. Clean. Prod. 2015, 91, 337–346. [Google Scholar] [CrossRef]
- Ulrich, A.E.; Schnug, E.; Prasser, H.M.; Frossard, E. Uranium endowments in phosphate rock. Sci. Total Environ. 2014, 478, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Schnug, E.; Haneklaus, N. Energetic and economic significance of uranium in mineral phosphorous fertilizers. In The New Uranium Mining Boom; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Rutherford, P.M.; Dudas, M.J.; Samek, R.A. Environmental impacts of phosphogypsum. Sci. Total Environ. 1994, 149, 1–38. [Google Scholar] [CrossRef]
- Sabiha-Javied; Mehmood, T.; Chaudhry, M.M.; Tufail, M.; Irfan, N. Heavy metal pollution from phosphate rock used for the production of fertilizer in Pakistan. Micorchem. J. 2009, 91, 94–99. [Google Scholar]
- Schnug, E.; Lottermoser, B.G. Fertilizer-Derived Uranium and its Threat to Human Health. Environ. Sci. Technol. 2013, 47, 2433–2434. [Google Scholar] [CrossRef] [PubMed]
- Haneklaus, N.; Bayok, A.; Fedchenko, V. Phosphate Rocks and Nuclear Proliferation. Sci. Glob. Secur. 2017, 25, 143–158. [Google Scholar] [CrossRef]
- Haneklaus, N.; Reitsma, F.; Tulsidas, H. High Temperature Reactors for a new IAEA Coordinated Research Project on energy neutral mineral development processes. Nucl. Eng. Des. 2016, 306, 198–202. [Google Scholar] [CrossRef]
- Haneklaus, N.; Reitsma, F.; Tulsidas, H.; Tyobeka, B.; Schnug, E.; Allelein, H.J.; Birky, B.; Peterson, P.F.; Dyck, G.; Koshy, T. Using high temperature reactors for energy neutral mineral development processes a proposed IAEA Coordinated Research Project. In Proceedings of the International Symposium on Uranium Raw Material for the Nuclear Fuel Cycle, Vienna, Austria, 23–27 June 2014. [Google Scholar]
- Emsbo, P.; McLaughlin, P.I.; Breit, G.N.; du Bray, E.A.; Koenig, A.E. Rare earth elements in sedimentary phosphate deposits: Solution to the global REE crisis? Gondwana Res. 2015, 27, 776–785. [Google Scholar] [CrossRef]
- Haneklaus, N.; Zheng, Y.; Allelein, H.J. Stop Smoking—Tube-In-Tube Helical System for Flameless Calcination of Minerals. Processes 2017, 5, 67. [Google Scholar] [CrossRef]
- Haneklaus, N.; Schnug, E.; Tulsidas, H.; Tyobeka, B. Annals of Nuclear Energy Using high temperature gas-cooled reactors for greenhouse gas reduction and energy neutral production of phosphate fertilizers. Ann. Nucl. Energy 2015, 75, 275–282. [Google Scholar] [CrossRef]
- Haneklaus, N.; Reyes, R.; Lim, W.G.; Tabora, E.U.; Palattao, B.L.; Petrache, C.; Vargas, E.P.; Kunitomi, K.; Ohashi, H.; Sakaba, N.; et al. Energy Neutral Phosphate Fertilizer Production Using High Temperature Reactors: A Philippine Case Study. Philipp. J. Sci. 2015, 144, 69–79. [Google Scholar]
- Haneklaus, N.; Schnug, E. Energy Neutral Phosphate Fertilizer Production Using High Temperature Reactors. In Phosphorus Agriculture: 100% Zero; Springer: Dordrecht, The Netherlands, 2016; pp. 309–316. [Google Scholar]
- Flamant, G.; Hernandez, D.; Bonet, C.; Traverse, J.P. Experimental aspects of the thermochemical conversion of solar energy; Decarbonation of CaCO3. Sol. Energy 1980, 24, 385–395. [Google Scholar] [CrossRef]
- Flamant, G.; Gauthier, D.; Boudhari, C.; Flitris, Y. A 50 kW fluidized bed high temperature solar receiver: Heat transfer analysis. J. Sol. Energy Eng. 1988, 110, 313–320. [Google Scholar] [CrossRef]
- Licht, S.; Wu, H.; Hettige, C.; Wang, B.; Asercion, J.; Lau, J.; Stuart, J. STEP cement: Solar Thermal Electrochemical Production of CaO without CO2 emission. Chem. Commun. 2012, 48, 6019. [Google Scholar] [CrossRef] [PubMed]
- Meier, A.; Bonaldi, E.; Cella, G.M.; Lipinski, W. Multitube Rotary Kiln for the Industrial Solar Production of Lime. J. Sol. Energy Eng. 2005, 127, 386–395. [Google Scholar] [CrossRef]
- Meier, A.; Gremaud, N.; Steinfeld, A. Economic evaluation of the industrial solar production of lime. Energy Convers. Manag. 2005, 46, 905–926. [Google Scholar] [CrossRef]
- Meier, A.; Bonaldi, E.; Cella, G.M.; Lipinski, W.; Wuillemin, D.; Palumbo, R. Design and experimental investigation of a horizontal rotary reactor for the solar thermal production of lime. Energy 2004, 29, 811–821. [Google Scholar] [CrossRef]
- Meier, A.; Bonaldi, E.; Cella, G.M.; Lipinski, W.; Wuillemin, D. Solar chemical reactor technology for industrial production of lime. Sol. Energy 2006, 80, 1355–1362. [Google Scholar] [CrossRef]
- Salman, O.A.; Kraishi, N. Thermal decomposition of limestone and gypsum by solar energy. Sol. Energy 1988, 41, 305–308. [Google Scholar] [CrossRef]
- Cooper, J.; Lombardi, R.; Boardman, D.; Carliell-Marquet, C. The future distribution and production of global phosphate rock reserves. Resour. Conserv. Recycl. 2011, 57, 78–86. [Google Scholar] [CrossRef]
- Edixhoven, J.D.; Gupta, J.; Savenije, H.H.G. Recent revisions of phosphate rock reserves and resources: A critique. Earth Syst. Dyn. 2014, 5, 491–507. [Google Scholar] [CrossRef]
- Haneklaus, N.; Schröders, S.; Zheng, Y.; Allelein, H.-J. Economic evaluation of flameless phosphate rock calcination with concentrated solar power and high temperature reactors. Energy 2017, 140, 1148–1157. [Google Scholar] [CrossRef]
- Haneklaus, N.; Tulsidas, H.; Reitsma, F.; Schnug, E. Using high temperature reactors for energy neutral phosphate fertilizer and phosphogypsum processing. In Uranium-Past and Future Challenges; Merkel, B., Arab, A., Eds.; Springer: Cham, Switzerland, 2014; pp. 785–792. [Google Scholar]
- Tayibi, H.; Choura, M.; López, F.A.; Alguacil, F.J.; López-Delgado, A. Environmental impact and management of phosphogypsum. J. Environ. Manag. 2009, 90, 2377–2386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahu, S.K.; Ajmal, P.Y.; Bhangare, R.C.; Tiwari, M.; Pandit, G.G. Natural radioactivity assessment of a phosphate fertilizer plant area. J. Radiat. Res. Appl. Sci. 2014, 7, 123–128. [Google Scholar] [CrossRef]
- Zhang, J.; Edwards, C. Mineral decomposition and leaching processes for treating rare earth ore concentrates. Can. Metall. Q. 2013, 52, 243–248. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Phosphate Rock; Mineral Commodity Summaries; U.S. Geological Survey: Reston, VA, USA, 2017; pp. 124–125. [CrossRef]
- Kumari, A.; Panda, R.; Jha, M.K.; Lee, J.Y.; Kumar, J.R.; Kumar, V. Thermal treatment for the separation of phosphate and recovery of rare earth metals (REMs) from Korean monazite. J. Ind. Eng. Chem. 2015, 21, 696–703. [Google Scholar] [CrossRef]
- Habashi, F. Extractive metallurgy of rare earths. Can. Metall. Q. 2013, 52, 224–233. [Google Scholar] [CrossRef]
- Amaral, J.C.B.S.; Morais, C.A. Thorium and uranium extraction from rare earth elements in monazite sulfuric acid liquor through solvent extraction. Miner. Eng. 2010, 23, 498–503. [Google Scholar] [CrossRef]
- Aarden, H.M.; Arozena, J.M.I.; Moticska, P.; Navarro, J.; Pasquali, J.; Sifontes, R.S. El Complejo Geológico del area de Impacto, Distrito Cedeño, Estado Bolivar, Venezuela; Ministerio de Minas e Hidrocarburos, Dirección de Geología: Caracas, Venezuela, 1973.
- Greaves, E.D.; Pasquali, J.; Sifontes, R.S.; Manrique, M. Cerro Impacto, Venezuela’s Thorium Deposit. Thorium Energy Conference. London, UK, 2010. Available online: http://www.nuclear.fis.usb.ve/fn/wp-content/uploads/2018/01/Greaves-IMPACTO-Venezuela%E2%80%99s-Thorium-Deposit.pdf (accessed on 17 January 2018).
- Eduardo, D.; Greaves, J.; Pasquali, R.S.S.; Impacto, M.M. Venezuela’s Thorium Deposit; World Thorium Resource; Thiruvantaphuran: Kerala, India, 2011. [Google Scholar]
- Manrique, M.; Greaves, E.D.; Garcia, C. Extraction of Rare Earths from low-grade lateritic ore by pressure leaching. In Proceedings of the First International Conference on Processing Materials for Properties, Honolulu, HI, USA, 7–10 November 1993; pp. 445–448. [Google Scholar]
- José, A.H. “Lixiviación de Niobio y Torio” Tesis de Ingeniería Metalúrgica; Facultad de Ingeniería Universidad Central de Venezuela: Caracas, Venezuela, 1974. [Google Scholar]
- Fordt, M.A. Uranium in South Africa. J. South. Afr. Inst. Min. Metall. 1993, 93, 37–58. [Google Scholar] [CrossRef]
- Gupta, C.; Singh, H. Uranium Resource Processing—Secondary Resources; Springer: Berlin, Germany, 2003. [Google Scholar]
- Chmielewski, A.G.; Wawszczak, D.; Brykała, M. Possibility of uranium and rare metal recovery in the Polish copper mining industry. Hydrometallurgy 2016, 159, 12–18. [Google Scholar] [CrossRef]
- Memary, R.; Giurco, D.; Mudd, G.; Mason, L. Life cycle assessment: A time-series analysis of copper. J. Clean. Prod. 2012, 33, 97–108. [Google Scholar] [CrossRef]
- Alvarado, S.; Maldonado, P.; Barrios, A.; Jaques, I. Long term energy-related enviromental issues of copper production. Energy 2002, 27, 183–196. [Google Scholar] [CrossRef]
- Kim, H.S.; No, H.C. Thermal coupling of HTGRs and MED desalination plants, and its performance and cost analysis for nuclear desalination. Desalination 2012, 303, 17–22. [Google Scholar] [CrossRef]
- Pena, C.A.; Huijbregts, M.A.J. The blue water footprint of primary copper production in Northern Chile. J. Ind. Ecol. 2014, 18, 49–58. [Google Scholar] [CrossRef]
- Moreno, P.A.; Aral, H.; Cuevas, J.; Monardes, A.; Adaro, M.; Norgate, T.; Bruckard, W. The use of seawater as process water at Las Luces copper-molybdenum beneficiation plant in Taltal (Chile). Miner. Eng. 2011, 24, 852–858. [Google Scholar] [CrossRef]
- Moreno-Leiva, S.; Díaz-Ferrán, G.; Haas, J.; Telsnig, T.; Díaz-Alvarado, F.A.; Palma-Behnke, R.; Kracht, W.; Román, R.; Chudinzow, D.; Eltrop, L. Towards solar power supply for copper production in Chile: Assessment of global warming potential using a life-cycle approach. J. Clean. Prod. 2017, 164, 242–249. [Google Scholar] [CrossRef]
- Gaballah, I.; Allain, E.; Meyer-Joly, M.C.; Malau, K. A Possible Method for the Characterization of Amorphous Slags Recovery of Refractory Metal Oxides from Tin Slags. Metall. Trans. 1992, 23, 249–259. [Google Scholar] [CrossRef]
- Subramanian, C.; Suri, A.K.; Atomic, B. Recovery of niobium and tantalum from low grade tin slag—A hydrometallurgical approach. In Environmental & Waste Management in NoN-Ferrous Metallurgical Industries; NML: Jamshedpur, India, 1998; pp. 100–107. [Google Scholar]
- Trinopiawan, K.; Mubarok, M.Z.; Mellawati, J.; Ani, B.Y. Pelindian Logam Tanah Jarang dari Terak Timah dengan Asam Klorida Setelah Proses Fusi Alkali. Buletin Pusat Teknologi Bahan Galian Nuklir 2016, 1, 37. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, Y.; Li, F.; Zhang, Z.; Wang, H.; Huang, X.; Li, H.; Liu, B.; Wu, X.; Wang, H.; et al. The Shandong Shidao Bay 200 MWe High-Temperature Gas-Cooled Reactor Pebble-Bed Module (HTR-PM) Demonstration Power Plant: An Engineering and Technological Innovation. Engineering 2016, 2, 112–118. [Google Scholar] [CrossRef]
- Reutler, H.; Lohnert, G.H. Advantages of going modular in HTRs. Nucl. Eng. 1984, 78, 129–136. [Google Scholar] [CrossRef]
- Carelli, M.D.; Garrone, P.; Locatelli, G.; Mancini, M.; Mycoff, C.; Trucco, P.; Ricotti, M.E. Economic features of integral, modular, small-to-medium size reactors. Prog. Nucl. Energy 2010, 52, 403–414. [Google Scholar] [CrossRef]
- Boarin, S.; Ricotti, M.E. An Evaluation of SMR Economic Attractiveness. Sci. Technol. Nucl. Install. 2014, 2014, 803698. [Google Scholar] [CrossRef]
- Carlsson, J.; Shropshire, D.E.; van Heek, A.; Fütterer, M.A. Economic viability of small nuclear reactors in future European cogeneration markets. Energy Policy 2012, 43, 396–406. [Google Scholar] [CrossRef]
- Kessides, I.N.; Kuznetsov, V. Small modular reactors for enhancing energy security in developing countries. Sustainability 2012, 4, 1806–1832. [Google Scholar] [CrossRef]
- Hampe, J.; Madlener, R. Economics of High-Temperature Nuclear Reactors for Industrial Cogeneration; Institute for Future Energy Consumer Needs and Behavior (FCN): Aachen, Germany, 2012. [Google Scholar]
- Vujić, J.; Bergmann, R.M.; Škoda, R.; Miletić, M. Small modular reactors: Simpler, safer, cheaper? Energy 2012, 45, 288–295. [Google Scholar] [CrossRef]
- Alonso, G.; Ramirez, R.; del Valle, E.; Castillo, R. Process heat cogeneration using a high temperature reactor. Nucl. Eng. Des. 2014, 280, 137–143. [Google Scholar] [CrossRef]
- Locatelli, G.; Bingham, C.; Mancini, M. Small modular reactors: A comprehensive overview of their economics and strategic aspects. Prog. Nucl. Energy 2014, 73, 75–85. [Google Scholar] [CrossRef]
- Hidayatullah, H.; Susyadi, S.; Subki, M.H. Design and technology development for small modular reactors —Safety expectations, prospects and impediments of their deployment. Prog. Nucl. Energy 2015, 79, 127–135. [Google Scholar] [CrossRef]
- Locatelli, G.; Fiordaliso, A.; Boarin, S.; Ricotti, M.E. Cogeneration: An option to facilitate load following in Small Modular Reactors. Prog. Nucl. Energy 2017, 97, 153–161. [Google Scholar] [CrossRef]
- Ingersoll, D.T. Deliberately small reactors and the second nuclear era. Prog. Nucl. Energy 2009, 51, 589–603. [Google Scholar] [CrossRef]
- Shropshire, D. Economic viability of small to medium-sized reactors deployed in future European energy markets. Prog. Nucl. Energy 2011, 53, 299–307. [Google Scholar] [CrossRef]
- Angulo, C.; Bogusch, E.; Bredimas, A.; Delannay, N.; Viala, C.; Ruer, J.; Muguerra, P.; Sibaud, E.; Chauvet, V.; Hittner, D.; et al. EUROPAIRS: The European project on coupling of High Temperature Reactors with industrial processes. Nucl. Eng. Des. 2012, 251, 30–37. [Google Scholar] [CrossRef]
- Sovacool, B.K.; Ramana, M.V. Back to the Future. Sci. Technol. Hum. Values 2015, 40, 96–125. [Google Scholar] [CrossRef]
- Thomas, S. The Pebble Bed Modular Reactor: An obituary. Energy Policy 2011, 39, 2431–2440. [Google Scholar] [CrossRef]
- Söderholm, K. Challenges of Smr Licensing Practices. CNL Nucl. Rev. 2012, 1, 19–31. [Google Scholar] [CrossRef]
- Sainati, T.; Locatelli, G.; Brookes, N. Small Modular Reactors: Licensing constraints and the way forward. Energy 2015, 82, 1092–1095. [Google Scholar] [CrossRef]
- Ingersoll, D.T. Integration of Nuscale Smr with Desalination Technologies. In Proceedings of the ASME 2014 Small Modular Reactors Symposium, Washington, DC, USA, 15–17 April 2014; pp. 1–3392. [Google Scholar] [CrossRef]
- Ingersoll, D.T.; Houghton, Z.J.; Bromm, R.; Desportes, C. NuScale small modular reactor for Co-generation of electricity and water. Desalination 2014, 340, 84–93. [Google Scholar] [CrossRef]
- Brinkmann, G.; Will, M. Concept licensing procedure for an htr-module nuclear power plant. Nucl. Eng. Des. 1990, 121, 293–298. [Google Scholar] [CrossRef]
- Delaney, M.J.; Apostolakis, G.E.; Driscoll, M.J. Risk-informed design guidance for future reactor systems. Nucl. Eng. Des. 2005, 235, 1537–1556. [Google Scholar] [CrossRef]
- Scarlat, R.O.; Laufer, M.R.; Blandford, E.D.; Zweibaum, N.; Krumwiede, D.L.; Cisneros, A.T.; Andreades, C.; Forsberg, C.W.; Greenspan, E.; Hu, L.W.; et al. Design and licensing strategies for the fluoride-salt-cooled, high-temperature reactor (FHR) technology. Prog. Nucl. Energy 2014, 77, 406–420. [Google Scholar] [CrossRef]
- Nickel, H.; Hofmann, K.; Wachholz, W.; Weisbrodt, I. The helium-cooled high-temperature reactor in the Federal Republic of Germany: Safety features, integrity concept, outlook for design codes and licensing procedures. Nucl. Eng. Des. 1991, 127, 181–190. [Google Scholar] [CrossRef]
- Trikouros, N.G. A Perspective on Small Reactor Licensing and Implementation. Nucl. Technol. 2012, 178, 233–239. [Google Scholar] [CrossRef]
- Silady, F.A.; Cunliffe, J.C.; Walker, L.P. The licensing experience of the Modular High-Temperature Gas-Cooled Reactor (MHTGR). Energy 1991, 16, 417–424. [Google Scholar] [CrossRef]
- Ramana, M.V.; Hopkins, L.B.; Glaser, A. Licensing small modular reactors. Energy 2013, 61, 555–564. [Google Scholar] [CrossRef]
- Ramana, M.V.; Ahmad, A. Wishful thinking and real problems: Small modular reactors, planning constraints, and nuclear power in Jordan. Energy Policy 2016, 93, 236–245. [Google Scholar] [CrossRef]
- Abram, T.; Ion, S. Generation-IV nuclear power: A review of the state of the science. Energy Policy 2008, 36, 4323–4330. [Google Scholar] [CrossRef]
- Locatelli, G.; Mancini, M.; Todeschini, N. Generation IV nuclear reactors: Current status and future prospects. Energy Policy 2013, 61, 1503–1520. [Google Scholar] [CrossRef]
- Magwood, W.D.; Paillere, H. Looking ahead at reactor development. Prog. Nucl. Energy 2017, 1–10. [Google Scholar] [CrossRef]
- Dulera, I.V.; Sinha, R.K. High temperature reactors. J. Nucl. Mater. 2008, 383, 183–188. [Google Scholar] [CrossRef]
- Sinha, R.K. Advanced nuclear reactor systems—An Indian perspective. Energy Procedia 2011, 7, 34–50. [Google Scholar] [CrossRef]
- Qualls, A.L.; Betzler, B.R.; Brown, N.R.; Carbajo, J.J.; Greenwood, M.S.; Hale, R.; Harrison, T.J.; Powers, J.J.; Robb, K.R.; Terrell, J.; et al. Preconceptual design of a fluoride high temperature salt-cooled engineering demonstration reactor: Motivation and overview. Ann. Nucl. Energy 2017, 107, 144–155. [Google Scholar] [CrossRef]
- Krumwiede, D.; Andreades, C.; Choi, J.K.; Cisneros, A.T.; Huddar, L.; Huff, K.D.; Laufer, M.R.; Munk, M.; Scarlat, R.O.; Seifried, J.E.; et al. Design of the Mark-1 Pebble-Bed, Fluoride-salt-cooled, High-Temperature Reactor Commercial Power Plant. In Proceedings of the ICAPP 2014, Charlotte, NC, USA, 6–9 April 2014. [Google Scholar]
- Andreades, C.; Cisneros, A.T.; Choi, J.K.; Chong, A.Y.K.; Fratoni, M.; Hong, S.; Huddar, L.R.; Huff, K.D.; Kendrick, J.; Krumwiede, D.L.; et al. Design Summary of the Mark-I Pebble-Bed, Fluoride Salt–Cooled, High-Temperature Reactor Commercial Power Plant. Nucl. Technol. 2016, 195, 223–238. [Google Scholar] [CrossRef]
- Forsberg, C.W.; Hu, L.W.; Peterson, P.F.; Fratoni, M.; Sridharan, K.; Blandford, E. Progress in Development of Fluoride-Salt-Cooled High-Temperature Reactors (FHRs). 2017. Available online: https://www.osti.gov/scitech/biblio/1183687 (accessed on 12 January 2018).
- Van Rooijen, W.F.G. Gas-Cooled Fast Reactor: A Historical Overview and Future Outlook. Sci. Technol. Nucl. Install. 2009, 2009, 965757. [Google Scholar] [CrossRef]
- Stainsby, R.; Peers, K.; Mitchell, C.; Poette, C.; Mikityuk, K.; Somers, J. Gas cooled fast reactor research and development in the European Union. Sci. Technol. Nucl. Install. 2009, 2009. [Google Scholar] [CrossRef]
- Stainsby, R.; Peers, K.; Mitchell, C.; Poette, C.; Mikityuk, K.; Somers, J. Gas cooled fast reactor research in Europe. Nucl. Eng. Des. 2011, 241, 3481–3489. [Google Scholar] [CrossRef]
- Alemberti, A.; Smirnov, V.; Smith, C.F.; Takahashi, M. Overview of lead-cooled fast reactor activities. Prog. Nucl. Energy 2014, 77, 300–307. [Google Scholar] [CrossRef]
- Smith, C.F.; Halsey, W.G.; Brown, N.W.; Sienicki, J.J.; Moisseytsev, A.; Wade, D.C. SSTAR: The US lead-cooled fast reactor (LFR). J. Nucl. Mater. 2008, 376, 255–259. [Google Scholar] [CrossRef]
- Alemberti, A. The Lead Fast Reactor: An Opportunity for the Future? Engineering 2016, 2, 59–62. [Google Scholar] [CrossRef]
- Serp, J.; Allibert, M.; Beneš, O.; Delpech, S.; Feynberg, O.; Ghetta, V.; Heuer, D.; Holcomb, D.; Ignatiev, V.; Kloosterman, J.L.; et al. The molten salt reactor (MSR) in generation IV: Overview and perspectives. Prog. Nucl. Energy 2014, 77, 308–319. [Google Scholar] [CrossRef]
- Price, M.S.T. The Dragon Project origins, achievements and legacies. Nucl. Eng. Des. 2012, 251, 60–68. [Google Scholar] [CrossRef]
- Fang, C.; Min, Q.; Yang, Y.; Sun, Y. Process heat applications of HTR-PM600 in Chinese petrochemical industry: Preliminary study of adaptability and economy. Ann. Nucl. Energy 2017, 110, 73–78. [Google Scholar] [CrossRef]
- U.S. NRC. Research & Test Reactors 2017. Available online: https://www.nrc.gov/reactors/non-power.html. (accessed on 12 January 2018).
- World Nuclear Association. Research Reactors 2017. Available online: http://www.world-nuclear.org/information-library/non-power-nuclear-applications/radioisotopes-research/research-reactors.aspx (accessed on 17 November 2017).
- Elder, R.; Allen, R. Nuclear heat for hydrogen production: Coupling a very high/high temperature reactor to a hydrogen production plant. Prog. Nucl. Energy 2009, 51, 500–525. [Google Scholar] [CrossRef]
- Scarlat, R.O.; Cisneros, A.T.; Koutchesfahani, T.; Hong, R.; Peterson, P.F. Preliminary safety analysis of a PBMR supplying process heat to a co-located ethylene production plant. Nucl. Eng. Des. 2012, 251, 53–59. [Google Scholar] [CrossRef]
- Verfondern, K.; Yan, X.; Nishihara, T.; Allelein, H.J. Safety concept of nuclear cogeneration of hydrogen and electricity. Int. J. Hydrog. Energy 2017, 42, 7551–7559. [Google Scholar] [CrossRef]
- Sato, H.; Ohashi, H.; Nakagawa, S.; Tachibana, Y.; Kunitomi, K. Safety design consideration for HTGR coupling with hydrogen production plant. Prog. Nucl. Energy 2015, 82, 46–52. [Google Scholar] [CrossRef]




| Countries Participating | Ores and Other Forms Considered in the Study | Techniques and Equipment Used |
|---|---|---|
| Argentina, China, Egypt, Germany, India, Indonesia, Kuwait, Malaysia, Mexico, Morocco, Philippines, Poland, Tanzania, Tunisia, Venezuela | Carbonates, Columbite-Tantalite, Copper Tailings, Ilmenite, Monazite, Oil Sludge, Phosphate Rock, Phosphogypsum, Phosphoric Acid, Red Mud (Cerro Impacto Laterite), Tin Slag, Xenotime | Alpha Spectroscopy, Atomic Absorption, Electronic Microscope, Field Emission Scanning Electron Microscope, Gamma Spectroscopy, Gas Chromatography, Inductive Coupled Plasma—Optical Emission Spectroscopy, Inductive Coupled Plasma—Mass Spectroscopy, Ion Chromatography, Petrological Microscope, Pyrometallurgy Reduction, Nuclear Activation Analysis, X-ray Fluorescence |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reitsma, F.; Woods, P.; Fairclough, M.; Kim, Y.; Tulsidas, H.; Lopez, L.; Zheng, Y.; Hussein, A.; Brinkmann, G.; Haneklaus, N.; et al. On the Sustainability and Progress of Energy Neutral Mineral Processing. Sustainability 2018, 10, 235. https://doi.org/10.3390/su10010235
Reitsma F, Woods P, Fairclough M, Kim Y, Tulsidas H, Lopez L, Zheng Y, Hussein A, Brinkmann G, Haneklaus N, et al. On the Sustainability and Progress of Energy Neutral Mineral Processing. Sustainability. 2018; 10(1):235. https://doi.org/10.3390/su10010235
Chicago/Turabian StyleReitsma, Frederik, Peter Woods, Martin Fairclough, Yongjin Kim, Harikrishnan Tulsidas, Luis Lopez, Yanhua Zheng, Ahmed Hussein, Gerd Brinkmann, Nils Haneklaus, and et al. 2018. "On the Sustainability and Progress of Energy Neutral Mineral Processing" Sustainability 10, no. 1: 235. https://doi.org/10.3390/su10010235
APA StyleReitsma, F., Woods, P., Fairclough, M., Kim, Y., Tulsidas, H., Lopez, L., Zheng, Y., Hussein, A., Brinkmann, G., Haneklaus, N., Kacham, A. R., Sreenivas, T., Sumaryanto, A., Trinopiawan, K., Al Khaledi, N., Zahari, A., El Yahyaoui, A., Ahmad, J., Reyes, R., ... Greaves, E. D. (2018). On the Sustainability and Progress of Energy Neutral Mineral Processing. Sustainability, 10(1), 235. https://doi.org/10.3390/su10010235








