1. Introduction
Water is an essential element for the life on the Earth, playing a major role in climate regulation and in bio-geological cycles [
1]. This resource generates development in socio-economical issues crucial to society in general and more specifically for industries, agricultural activities and for the public use [
2]. Indeed the demand of water for human consumption and for agricultural and industrial purposes is drastically increasing, so that the most easily available resources became insufficient.
According to the formulation reported in the ‘‘Brundtland Report’’, the goal of sustainability is to “meet the needs of the present generation without compromising the ability of future generations to meet their own needs”. The meaning of sustainability in the context of water resources management has been progressively changed in a quite perceptibly mode during the human history [
3,
4].
It is expected that if today wars are fought over oil, in a not very distant future these wars will be fought for water. Thus water is become one of the pivot of sustainable development.
Many industries, such as the pulp and paper industries [
5], are increasingly interested in reducing the fresh water consumption, mainly because of stricter environmental legislation. Indeed, a lot of water, after industrial use, is discharged in rivers, lakes or sea. When the natural self-purification processes in receiving water bodies became insufficient, they need to be coupled with advanced engineered solutions with the purpose to close the hydrologic cycle in a sustainable way [
6]. Thus the wastewater becomes a valuable resource, and not a waste to be disposed of. The treated water can be used for the irrigation, but also for ground or surface water recharge, because in most cases the wastewater treatment does not produce water for direct potable applications.
Water contamination by heavy metals constitutes a large health hazard [
7,
8]. E.g., Cu
2+ ions are essential nutrients, but long-term exposure to Cu
2+ leads to kidney and liver damage, producing DNA mutation, evidence of its carcinogenic character. Traditional separation processes for metal ions removal include precipitation, inorganic and polymeric adsorption, reverse osmosis. These techniques produce water within international health standards, but has some important drawbacks: i) production of big amounts of sludge [
9,
10,
11] containing residues of the reagent(s) used, which results in a pollution problem; ii) treated water may contain residues if the process is not correctly controlled or operated; iii) the ion exchange process is not a continuous process, because of the need for regeneration; iv) the reverse osmosis process requires high operative pressures, which results in high operation costs.
In the last years, the interest of the scientific community in the presence of pharmaceutical active compounds (PhACs) and their metabolites in our waterways has increased significantly [
12,
13]. A large number of PhAC products originating from human therapy are not completely eliminated into sewage treatment plants (STPs), resulting in discharge to the aquatic environment [
14,
15,
16,
17,
18]. E.g., gemfibrozil (GEM) is a persistent and highly stable pharmaceutical under normal environmental condition present in STPs [
19].
Innovative chemical processes, characterized by high performances and low environmental impact, are crucial for a sustainable development. With such a perspective membrane technology can offer new opportunities in process design, rationalization and optimization.
A membrane is a selective barrier between two phases controlling mass and energy exchange in a very specific way. Membrane operations can give a meaningful contribution in Process Intensification, that is the implementation of new chemical and technological processes to reduce costs of production, energy and environmental pollution caused by the emission of wastes and/or cumbersome equipments with equal productivity.
Membrane processes are widely used in the food, pharmaceutical and biotechnological industries, improving the energetic efficiency and raising the level of sustainability in comparison to traditional processes.
In the case of wastewater treatment, membrane processes can represent an efficient alternative to traditional techniques [
20], guaranteeing a greater process sustainability, since they do not produce by-products of difficult disposal (the sludge typical of precipitation based processes) and they are continuous. In particular, two water treatment processes, known in literature as Supported Liquid Membrane (SLM) and Complexation Ultrafiltration (CP-UF), are based on the synergy between membrane processes and chemical techniques. Both techniques use the complexing properties of some functional groups chemically bound to chemical substrates soluble in aqueous phase in the case of CP-UF and in organic phase in the case of LM. The similitude of these two techniques can be clarified by considering the use of the water soluble polymer polyethylenimine (PEI) [
21] and of the carrier Amberlite La-2® [
22]. These two complexing agents represent the possibility of using the same functional group, that is a secondary amino group, bound to a different substrate, soluble in aqueous and organic phases, respectively.
Development of membrane technologies that can be integrated in existing chemical processes, contributes significantly in meeting the sustainability criteria. This is the challenge to which the present generations have been called: satisfy the demand by respecting the ecosystem, considering that the comfort of all living organisms also depends on ecosystem health.
In the present work some tests on the SSwLM systems as an improvement of the traditional SLM system configuration are reported employing as permeating species GEM (organic) and copper(II) (inorganic). In particular, SSwLM fluxes and stabilities are compared with the SLM ones. The influence of permeating module position on system performances has been also considered, and a SSwLM destabilization mechanism has been hypothesized to clarify the experimental results.
Furthermore, the CP-UF process has been described in the removal of metal chelates from waters (e.g., washing water from contaminated soils), by introducing an integrated membrane process where the CP-UF step is combined to a diafiltration step, for recovering and recycling the complexing agent, and a photocatalytic step, where the free metal ions were recovered. The selectivity of the CP-UF process is also considered in the selective removal of copper(II) and nickel(II) both contained in a synthetic solution and in a real aqueous effluent.
2. Basics and Generality
The CP-UF and the SLM techniques have been largely tested for the removal of inorganic pollutants (essentially metal ions) contained in wastewater, obtaining not only a liquid effluent to be recycled in the hydrologic cycle, but also the selective recovery of the pollutants. The SLM systems have been also tested in the recovery of organic substances, essentially molecules of biological interest (drugs, amino acids and proteins) from aqueous matrixes.
Advantages of SLMs and CP-UF in comparison to the convectional separation techniques, that make these processes interesting for a sustainable future, are: i) high selectivity, achievable when a proper selective complexing agent is chosen, thus obtaining a recovery and not a simple separation, avoiding the costs of the successive separations and reducing the land surface needed to locate the plant; ii) lower energy consumption with respect to traditional techniques; iii) an expensive complexing agent can be used thank to its cyclical use, because it does not pass in the treated water, but it is recovered and recycled, thus producing high quality water able to be directly reintroduced in the hydrologic cycle and avoiding the costs due to its removal from purified water; iv) minimization of chemical additives use, thus reducing the costs and the ecological impact of the treatment; v) high volume reduction achieving separation and concentration in a single step, reducing land request with respect to traditional plants; vi) ability to separate low concentration metal ions from very dilute solutions, because of the effective binding, making SLMs suitable for waste treatments and recovery operations; vii) optimal quality of the treated water.
2.1. Supported and Stagnant Sandwich Liquid Membrane
Transport of molecules across a liquid membrane (LM) is a powerful technology, which combines extraction and stripping into a one step process, thus having great potential for reducing costs significantly [
23,
24]. SLMs usually consist of an organic solution immobilised in the pores of a hydrophobic microfiltration membrane. This liquid membrane phase contains a carrier which binds one or a class of components in the feed phase transporting it (or they) in the receiving phase (strip) across the membrane.
SLMs have been studied in the transport of various inorganic pollutants (i.e., metal ions) [
25,
26], as well as different organic molecules [
20,
27,
28]. In the case of copper(II) transport the complexation reaction can be written as:
where the subscripts ‘f’ and ‘org’ mean feed and organic phases, respectively, RH is the carrier molecule and CuR
2 is the copper-carrier complex.
Despite the above mentioned advantages, the low system stability does not yet allow large scale application of SLMs [
29]. Many studies have shown that one of the principal causes of the poor SLM stability is the loss of the LM phase (carrier and/or solvent) from the pores of the support followed by its substitution with the aqueous solutions, thus influencing both the flux and the selectivity [
30,
31]. Several research efforts have been carried out to enhance the SLM stability, by testing membrane materials or by developing new liquid membrane configurations.
In 2002 we proposed the use of a Stagnant Sandwich Liquid Membrane (SSwLM) for copper(II) removal from aqueous media [
28,
32]. This system, starting from the idea of Zhu
et al. [
33], was made by sandwiching the LM phase between two thin hydrophilic membrane supports. This configuration was conceived with the intention to: i) increase system stability, with the two hydrophilic membrane supports acting as a barrier to LM phase loss; ii) increase the permeation flux, by minimising the total transport resistance that in many cases is caused by the presence of pores and their tortuosity. In 2006 the functioning of the SSwLM system was studied in the separation of an organic species [
31], such as the drug diclofenac. In the next years we tried to increase SSwLM stability and flux by using different microfiltration membranes. They were characterized by a wide pore size distribution and a hydrophilic-lypophilic character. As direct consequence, the organic phase (the LM) stays in the chamber between the two supports, but it is also adsorbed by the supports.
2.2. Complexation–Ultrafiltration
CP-UF [also named Polymer Assisted Ultrafiltration (PAUF)] is a relatively new process in water and wastewater purification and it is the subject of an increasing amount of publications in the field of polymer and membrane science [
1,
21,
34]. CP-UF combines the ion exchange or chelating properties of a water soluble complexing agent and the sieving power of an UF membrane. The process is based on the complexation of the metallic ion to remove which reacts with a water soluble complexing agent. Then, in the ultrafiltration step, the metal-complex is retained by the UF membrane while the non-complexed species permeate through the membrane [
35] producing a low metal concentration solution.
3. Materials and Methods
Copper sulphate pentahydrate (MW = 249.08 g·mol–1, purity > 99%) from Fluka and nickel(II) sulphate hexa-hydrate NiSO4·6H2O (MW = 262.9 g mol–1, purity > 99%) from Sigma were used as copper and nickel source, respectively. Gemfibrozil [GEM, 5-(2,5-dimethylphenoxy)-2,2- dimethylpentanoic acid, C15H22O3, M 250.3 g·mol–1, melting point 58–61 °C, pKa 4.7] was purchased from Sigma.
D2EHPA (di-(2-ethylhexyl) phosphoric acid, MW = 322.4 g·mol–1, purity = 95%, d20 = 0.9602 g·mL–1) from Sigma and tributylphosphate (TBP, MW = 266.32 g·mol–1, purity = 97%) from Aldrich were used as carrier for copper and GEM transport, respectively. n-Decane (MW = 142.15 g·mol–1, purity = 99%), also from Sigma, was used as the organic solvent.
The chelating agent citric acid monohydrate (MW = 210.1 g·mol–1) and the water soluble complexing polymeric agent polyethylenimine (50% wt. solution in water, MW = 60 kDa, branched) were purchased from Sigma-Aldrich.
Sulphuric acid H2SO4 (MW = 98.07 g·mol–1, purity = 96%) from Carlo Erba Reagenti and sodium hydroxide NaOH in pellets (MW = 40 g·mol–1, purity = 98%, from Sigma Aldrich) were used to correct the pH of the aqueous phases.
Titanium dioxide TiO2 DEGUSSA P25 (composition: 80% anatase and 20% rutile, specific surface area ≈ 50 m2·g–1, size of the primary particles ≈ 30 nm, band gap 3.2 eV) was the catalyst employed in the photocatalytic tests.
Flat sheet microfiltration hydrophobic membranes, made of polypropylene (Accurel, manufactured by Membrana, thickness 142 ± 5 μm; pore size 0.2 μm; porosity 70%) were used in the SLM tests. Flat sheet microfiltration supports, of polyethersulphone (Supor®200, thickness 145 μm; pore size 0.2 μm; typical water flux 26 mL·min–1·cm–2 at 70 kPa) were tested for SSwLM assembling.
All the analytical and experimental methods, as the transport mechanism for copper and GEM, were described in our previous works [
1,
28,
35,
36].
The stability tests were performed by doing sequential runs on the LM systems by changing feed and strip phases with ‘fresh’ ones when the quite complete transport of the target compound was achieved, provided the system did not show signs of destabilization. The stability was measured as the time when a significant change of the volumes of the two aqueous phases was observed. This was caused by a release of the LM phase from the pores of the support followed by its substitution with the aqueous phase.
The fluxes obtained during the stability tests were compared by considering the exponential average counter transport flux J
EAV(0-CTT). CTT (Counter Transport Time) is the time needed to reach transport against the concentration gradient that is when concentration in the strip becomes greater than the feed (C
Strip > C
Feed). The use of J
EAV(0-CTT) with respect to J
AV(0-CTT) used in our previous works [
33,
36] has the advantage to minimize the effects of the scattering of the experimental data.
In particular, the J
EAV(0-CTT) was determined as follows: a) writing the mass balance equation of the pollutant in the feed; b) considering that experimental data of pollutant concentration in the feed vs. the time C
F(t) show an exponential decay; c) correcting feed concentration in order to take into account only the transport contribute, and not the phenomenon of LM phase saturation. Thus the punctual flux J(t) can be evaluated on the basis of the following equation [
31]:
where V
F is the feed phase volume, S
exp is the membrane surface exposed to feed and strip phases, C’
F is the corrected feed concentration, and a’ and b’ are the two parameters of the exponential model. In particular a’ and b’ parameters were evaluated by fitting the experimental data in the time interval 0-CTT with the exponential model. All the fittings showed a good regression factor (R
2 at least 95%).
On the basis of equation (2), the JEAV(0-CTT) was calculated by mediating the punctual fluxes calculated with a time interval of 15 minutes in the time range 0-CTT. The optimal time interval of 15 minutes was fixed by considering that the affordability of the JEAV(0-CTT) parameter increases by decreasing the time interval, till a convergence found when a time step of 15 minutes was fixed.3.2. Complexation Ultrafiltration .
4. Results and Discussion
4.1. Supported and Stagnant Sandwich Liquid Membranes
GEM permeation tests were carried out by using the SSwLM made with Supor 200 supports placing the module horizontally as reported in our previous works [
28,
31]. The results are reported in
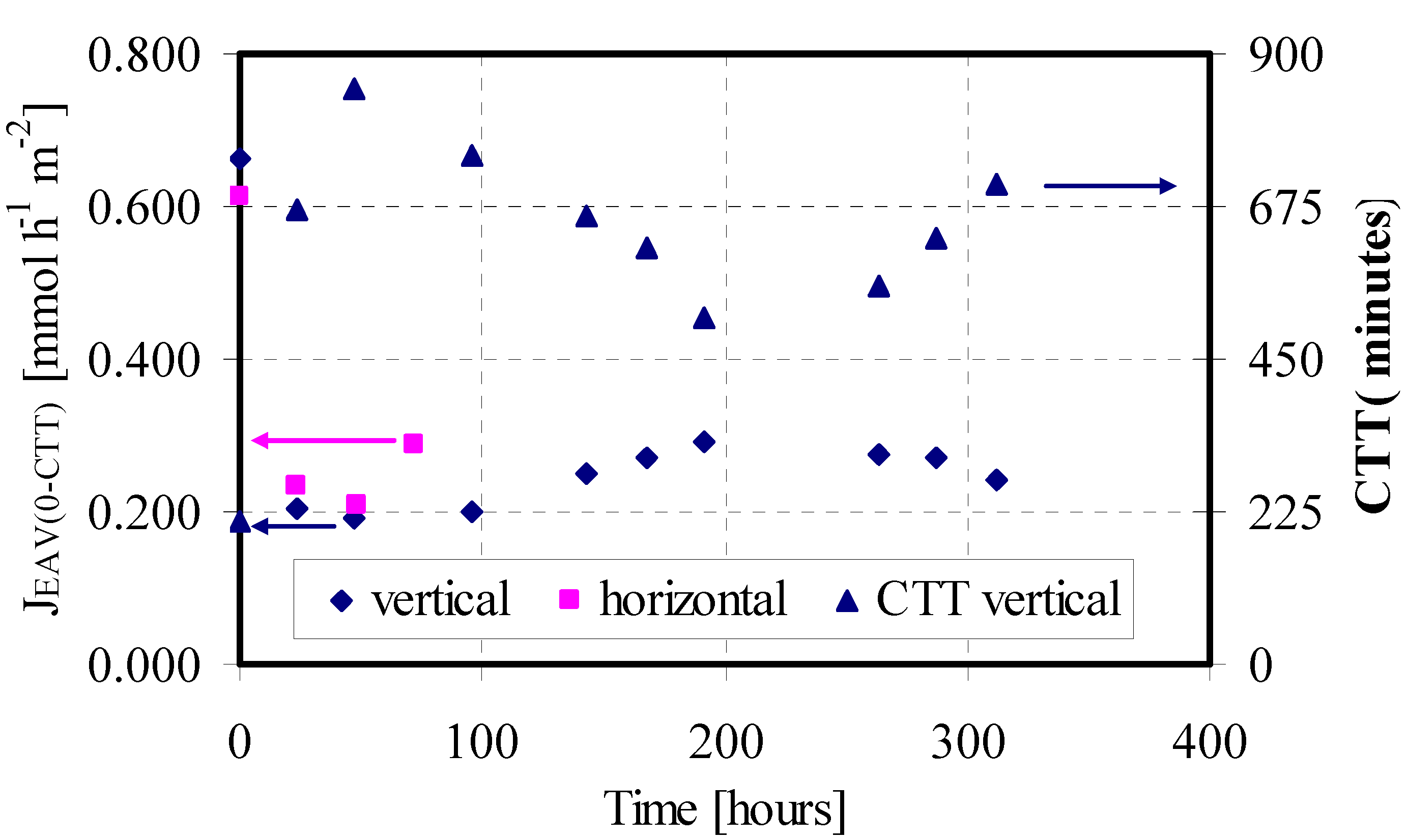
Figure 1 in terms of obtained J
EAV(
0-CTT) for the four feed/strip couples progressively injected in the LM system. The time in the x-axis indicates when a feed/strip couple is changed. The trend of J
EAV(
0-CTT) vs. the time shows a sharp decrease from the first to the second couple (0.611 vs 0.234 mmol·h
–1·m
–2), and then drug transport rate remains practically constant until system destabilization. At the time 119.5 hours the permeation test was stopped because the SSwLM system became instable, with passage of the aqueous phase across the membrane.
Figure 1.
JEAV(0-CTT) of GEM versus time for the feed/strip couples progressively injected in the SSwLM system operated with different orientations of the permeation module and CTT values for the vertical orientation (SSwLM assembled with the Supor 200 supports; Feed phase: CIN = 10 mg·L–1; V = 75 mL; pH 7.5; Strip phase: CIN = 0 mg·L–1; V = 75 mL; pH 11.5; Organic phase: TBP 30% v/v in n-decane; Q = 75 mL·min–1, T = 25 °C).
Figure 1.
JEAV(0-CTT) of GEM versus time for the feed/strip couples progressively injected in the SSwLM system operated with different orientations of the permeation module and CTT values for the vertical orientation (SSwLM assembled with the Supor 200 supports; Feed phase: CIN = 10 mg·L–1; V = 75 mL; pH 7.5; Strip phase: CIN = 0 mg·L–1; V = 75 mL; pH 11.5; Organic phase: TBP 30% v/v in n-decane; Q = 75 mL·min–1, T = 25 °C).
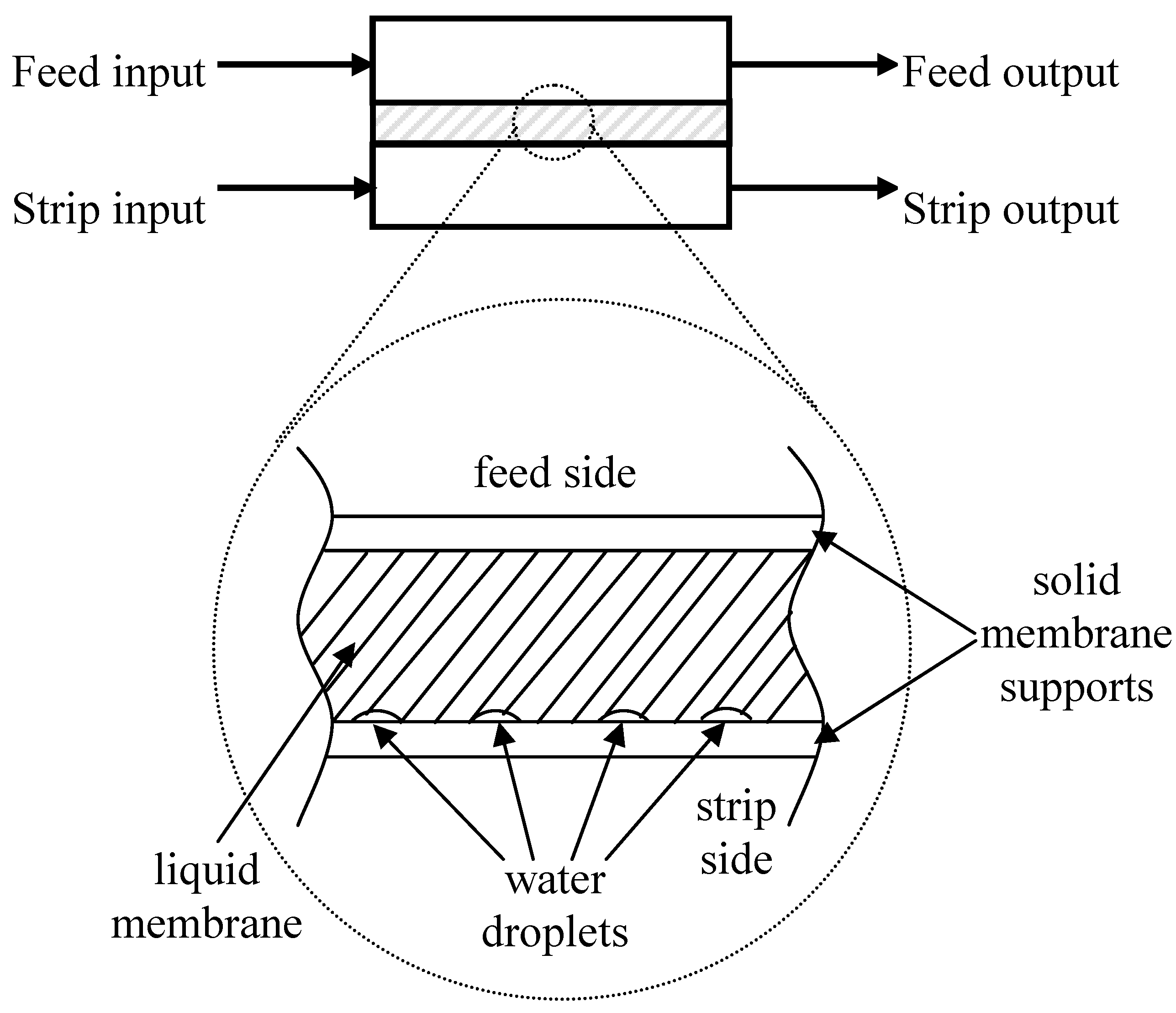
The mass balance between total initial drug mass and final one evidenced that 7.29% (0.232 mg) of the drug remained in the SSwLM system chamber. Then, a discrete amount of GEM, extracted from the feed phase, was not released in the strip phase. A possible explanation on this GEM entrapment in the LM phase chamber between the two hydrophilic/lypophilic supports was found in the permeation module orientation. In particular, in the module horizontally positioned, placing the feed permeation element on top of the strip one (
Figure 2) water droplets entering into the chamber probably stratified on bottom because of the higher density with respect to the LM phase (see
Figure 2). Thus, the contact area between the LM and the strip phase was reduced, and GEM release in the strip was hindered.
On the basis of this hypothesis, other permeation tests were carried out by positioning the module vertically. Obtained results are reported in
Figure 1 in terms of J
EAV(0-CTT) and Counter Transport Time (CTT). As expected, operating in this way no GEM entrapment in the LM phase was observed. Besides, system stability drastically increased (335.5 vs. 119.5 hours), and in that larger time interval ten feed/strip couples were progressively injected in the LM system, obtaining a quite complete GEM transport from feed to strip phase. The J
EAV(0-CTT) obtained with the first couple was a little higher than that one obtained with the horizontal module orientation (0.662 vs. 0.611 mmol·h
–1·m
–2), which is another advantage of the different module orientation. Also in this case the trend of the flux in the time showed a first sharp decrease by changing the first feed/strip couple (from 0.662 to 0.206 mmol·h
–1·m
–2). A further flux decrease was observed with the third feed/strip couple. With the fourth phase change this trend changed, with an increase of permeation flux till to the maximum value obtained with the 7th couple (0.292 mmol·h
–1·m
–2). This trend was also confirmed by the CTT values (see
Figure 1), meaning probably a dynamic equilibrium of the water droplets into the LM chamber. Indeed, during the sequential runs the dimension of these droplets probably increased as consequence of the continue water infiltration, causing a diminution of contact area between the feed and the LM phase and then the flux decreased. This behaviour continued till to a critical value of water droplets volume, where a creeping phenomenon took place, the surface between feed and LM phase was cleaned and then the flux increased. From the 8th couple the flux progressively decreased till to system destabilization, evidenced by a little passage of the aqueous phase from feed to strip in the membrane system.
Figure 2.
Permeation module schematization and zoom on the SSwLM evidencing the proposed mechanism of Liquid Membrane phase stratification.
Figure 2.
Permeation module schematization and zoom on the SSwLM evidencing the proposed mechanism of Liquid Membrane phase stratification.
The SSwLM was compared with the traditional SLM configuration. The experimental data, reported in
Table 1 in terms of flux CTT and stability , evidence the advantages of the SSwLM with respect to the SLM for what concerns both J
EAV(0-CTT) and system stability.
Table 1.
Comparison between the SLM and the SSwLM systems in terms of J
EAV(0-CTT), CTT and stability for GEM separation (same operating conditions of
Figure 1).
Table 1.
Comparison between the SLM and the SSwLM systems in terms of JEAV(0-CTT), CTT and stability for GEM separation (same operating conditions of Figure 1).
| | JEAV(0-CTT) (mmoli h–1 m–2) | CTT (minutes) | Stability (hours) |
|---|
| SLM Accurel | 0.302 | 420 | 23.5 |
| SSwLM Supor 200 | 0.662 | 210 | 335.5 |
The SSwLM system was also tested and compared to the traditional SLM in the removal of inorganic contaminants contained in aqueous media. Copper(II) transport tests in the SSwLM system, assembled with the Supor 200 supports and the permeation module vertically positioned, were also carried out. Obtained results, reported in
Table 2, show that nine feed-strip couples were sequentially injected in the SSwLM system. A quite complete copper transport from feed to strip phase for every couple was observed before system destabilization. In particular also in this case the J
EAV(0-CTT) progressively decreased and at the same time the CTT increased. After a time of 182.7 hours the SSwLM system showed destabilization mark, with water passage through the membrane system (
Table 3).
Table 2.
JEAV(0-CTT) of copper(II) and Counter Transport Time for nine feed/strip couples progressively injected in the SSwLM system with vertical module orientation (SSwLM assembled with the Supor 200 supports; Feed phase: CIN = 50 mg·L-1, V = 75 mL, pH 3.5; Strip phase: CIN = 0 mg·L–1, V = 75 mL, pH 1.6; Organic phase: D2EHPA 30% v/v in n-decane; Q = 75 mL·min-1, T = 25 °C).
Table 2.
JEAV(0-CTT) of copper(II) and Counter Transport Time for nine feed/strip couples progressively injected in the SSwLM system with vertical module orientation (SSwLM assembled with the Supor 200 supports; Feed phase: CIN = 50 mg·L-1, V = 75 mL, pH 3.5; Strip phase: CIN = 0 mg·L–1, V = 75 mL, pH 1.6; Organic phase: D2EHPA 30% v/v in n-decane; Q = 75 mL·min-1, T = 25 °C).
| Time [hours] | Feed/strip couple | JEAV(0-CTT) [mmol·h–1·m–2] | CTT [min] |
|---|
| 0 | 1st | 43.3 | 60 |
| 4 | 2nd | 34.8 | 90 |
| 20.7 | 3rd | 38.0 | 60 |
| 24.2 | 4th | 22.3 | 120 |
| 92.7 | 5th | 21.7 | 120 |
| 114.4 | 6th | 14.9 | 210 |
| 120.4 | 7th | 12.5 | 240 |
| 137.2 | 8th | 6.46 | 420 |
| 160.2 | 9th | 6.19 | 450 |
The SSwLM system was compared in terms of flux and stability with the SLM assembled by using the polypropylene (Accurel) and the Supor 200 supports. Obtained results, summarized in
Table 3, confirm the advantages of using the SSwLM with respect to the SLM configuration: indeed, higher J
EAV(0-CTT) and higher stability were obtained.
Table 3.
Comparison between the SLM and the SSwLM systems in terms of J
EAV(0-CTT), CTT and stability for copper(II) separation (same operating conditions of
Table 2).
Table 3.
Comparison between the SLM and the SSwLM systems in terms of JEAV(0-CTT), CTT and stability for copper(II) separation (same operating conditions of Table 2).
| | JEAV(0-CTT) (mmol·h-1·m-2) | CTT (minutes) | Stability (hours) |
|---|
| SLM Accurel | 31.0 | 105 | 49.2 |
| SLM Supor 200 | 39.8 | 90 | 22.3 |
| SSwLM Supor 200 | 43.3 | 60 | 182.7 |
Thus, for both organic (GEM) and inorganic (Cu2+) pollutants, better results were obtained by using the SSwLM assembled with the Supor 200 membranes. In particular lower JEAV(0-CTT) (0.662 vs 43.285 mmol·h–1·m–2) and higher stability (335.3 vs 182.7 hours) were obtained for GEM compared to copper(II) permeation. The difference of permeation fluxes can be due to the different size of the permeating species and to the different viscosity of LM phases, where only the carrier was changed. Also, the different stability could be ascribed to the different carrier used for the organic and inorganic separations, i.e., different interaction of LM phases with the Supor 200 supports and different LM solubilities in water. Studies are in progress to extensively study these factors.
4.2. Complexation Ultrafiltration for Metal Chelates Removal from Aqueous Media
A potential application of the CP-UF process is the removal of metal chelates from aqueous media [
2]. These type of chelates are generally present in waters generated from soil washing which is a very diffuse approach for removing metallic ions from contaminated soils. Practically the metal contaminated soil is washed with an aqueous solution containing particular molecules (e.g., citric, humic, fulvic, ethylenediamine tetraacetic acid (EDTA), etc.) which chelate the metallic ions extracting them from the soil. The washing solution, containing metal-chelates, is then appropriately treated to reduce the volume obtaining recyclable water, recovering the chelating agent and recovering the free metal.
On this basis, we have extensively studied the application of the CP-UF for separation of copper(II)-citrate chelate from aqueous media. In particular, we used the water soluble polyethylenimine (PEI) polymer as the macromolecular complexing agent, citric acid as the chelant and copper(II) as target metal ion.
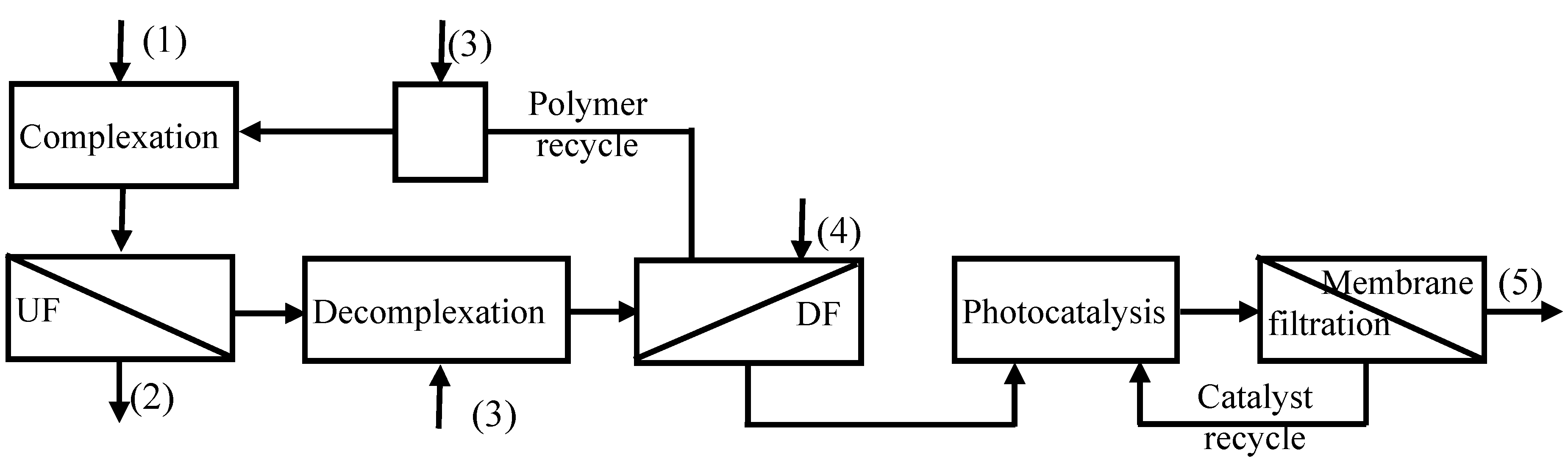
A modular integrated membrane process (
Figure 3) combining chemical processes with membrane separations was proposed, with the aim to meet the practical needs of obtaining recyclable water, recycling the polymer and recovering the free metals. In the proposed integrated process the CP-UF process was followed by a decomplexation step at pH = 3, and then was coupled with a Diafiltration (DF) step, where the polymer was recovered and recycled in the complexation step, and a photocatalytic step, which goal was to destroy the chelant (citrate) thus recovering free copper ions. The photocatalytic process was coupled with a membrane filtration for recovering and recycling the catalyst. The overall proposed system reduces significantly chemical reagents consumption thus avoiding their release in the produced effluent resulting in an economical and eco-friendly process.
Figure 3.
Integrated membrane process for water recycling and metal recovering. (1) Solution containing metal chalates to be treated; (2) treated recyclable water; (3) pH adjusting solutions; (4) acidic aqueous solution; (5) concentrated copper solution.
Figure 3.
Integrated membrane process for water recycling and metal recovering. (1) Solution containing metal chalates to be treated; (2) treated recyclable water; (3) pH adjusting solutions; (4) acidic aqueous solution; (5) concentrated copper solution.
The proposed system was tested by using two membrane of the Iris series in the UF steps, made by polyethersulphone manufactured by Tech-Sep, different in the molecular weight cut-off (10 and 30 kDa). Titanium dioxide (TiO2) was the photocatalyst in the photocatalytic step.
UF tests were carried out increasing feed concentration by withdrawing the permeate free of metal (the treated recyclable water of
Figure 3), thus simulating the increase in retentate concentration during the operation in an industrial plant. Goal was to determine the limiting concentration (i.e., maximum retentate concentration) for obtaining an acceptable permeate quality. Obtained results showed that the solution to be treated [(1) in
Figure 3] can be concentrated to a factor of 80 and 50 for the Iris 10 and the Iris 30 membranes, respectively, starting from a concentration of 5 mg·L
-1 which is the copper concentration in a real soil washing wastewater.
The DF tests were carried out at limiting feed concentration, thus simulating the treatment of concentrate coming from UF step. The results confirmed for both membranes the feasibility of recovering the polymer in the retentate, whilst copper-citrate was completely recovered in the diafiltrate.
Finally, the diafiltrate was submitted to photocatalysis, obtaining complete citrate destruction and complete copper recovery. The concentrated copper solution (5) can be treated by electrochemical [
37] or precipitation methods for recovering copper.
The overall results demonstrated the technical feasibility of the proposed integrated membrane process. Indeed, by means of a mass balance, water recoveries of 98.7% and 98% were obtained by using the Iris 10 and Iris 30 membranes, respectively. An interesting feature of the proposed integrated membrane process is the possibility to adapt it depending on the particular application, thanks to its modularity.
4.3. Selective Metal Separation by the CP-UF Process
An important feature of the CP-UF process is a selective separation, which can be obtained thanks to the chemical properties of the binding agent and/or to the operating conditions [
38,
39]. Indeed, the interaction of a metal ion with a binding polymer is affected by the operating pH and by the polymer/metal weight ratio. In particular, the pH can be used to obtain selective separation because the pH range of a polymer-metal complex formation varies significantly from metal to metal.
On this basis, we investigated the possibility to use the CP-UF process for the selective separation of copper(II) and nickel(II) both contained in the same solution. We used the water soluble PEI polymer as the complexing agent and Iris 10 and Iris 30 membranes as the separation medium. Two different aqueous media were tested: a laboratory aqueous solution and water from Emoli torrent (Rende (CS)), thus demonstrating the efficiency of the CP-UF process also by using a real effluent containing also dissolved substances.
The selective CP-UF tests were carried out: i) at pH = 6, that is the optimal pH for copper(II) complexation with PEI, but not for nickel(II) complexation (pH ≥ 8); ii) at PEI concentration of 150 mg·L–1 (polymer/metal weight ratio = 3), that is the polymer amount needed to complex only copper(II). Operating in this way followed by diafiltration mode, nickel(II) was washed in the permeate, whilst copper(II), selectively complexed by the polymer, was recovered in the retentate.
Recovery (REC) calculation was obtained by a mass balance on the two ions:
where m
i is the initial mass (mg) of the metal contained in the feed and m
p is the mass of metal in the permeate at time t.
Obtained results, reported in
Table 4, demonstrate the feasibility to use the CP-UF process to separate copper(II) and nickel(II) selectively, contained both in the considered aqueous media. In particular, by using the Iris 30 membrane the nickel(II) was completely recovered in the permeate. Copper(II) recovery in the retentate was not complete because of the poly-disperse nature of the commercial polymer, as confirmed by TOC analysis. A pre-filtration of the commercial polymer permitted to solve this problem [
1].
Table 4.
Copper and nickel recoveries (REC%) and membrane fouling for Iris 10 and Iris 30 membranes at different TMP ((Cu2+)IN = 50 mg·L–1; (Ni2+)IN = 50 mg·L–1; (PEI)IN = 150 mg·L–1; pH = 6.0; laboratory/real aqueous solution).
Table 4.
Copper and nickel recoveries (REC%) and membrane fouling for Iris 10 and Iris 30 membranes at different TMP ((Cu2+)IN = 50 mg·L–1; (Ni2+)IN = 50 mg·L–1; (PEI)IN = 150 mg·L–1; pH = 6.0; laboratory/real aqueous solution).
| Membrane | Iris 10 | Iris 30 |
|---|
| P (kPa) | 200 | 300 | 400 | 200 | 300 | 400 |
| REC% Cu | 97/96 | 96/96 | 95/95 | 94/93 | 93/92 | 92/92 |
| REC% Ni | 98/97 | 92/94 | 93/95 | 100/100 | 100/100 | 99/100 |
| JP/J0 | 0.78/0.78 | 0.71/0.65 | 0.63/0.46 | 0.67/0.61 | 0.69/0.50 | 0.64/0.42 |
By comparing the CP-UF performances using the two different aqueous media, it was clear that the difference by using the real effluent concerned the J
P/J
0 parameter, where J
P is the permeate flux measured during the CP-UF test, while J
0 is the pure water flux, measured during membrane characterization. This parameter is practically a measure of membrane fouling. Thus, by comparing the J
P/J
0 parameter, it is clear that the real medium gave higher membrane fouling, caused by natural organic matter rejection. In particular, by using the Iris 10 membrane at 200 kPa no differences were observed (see
Table 4). A flux decrease of 22% with respect to pure water for both aqueous media indicated that fouling was not severe in this condition. Fouling drastically increased till to a flux decrease of 54% by increasing the operating pressure till to 400 kPa. Regarding the Iris 30 membrane, characterized by higher permeate flux, the higher fouling with respect to the pure aqueous medium was evidenced just at 200 kPa, with a flux decrease of 39% vs. 33% obtained with the laboratory aqueous solution. The flux decrease grow till to 58% with the TMP. Despite this significant membrane fouling a 100% nickel recovery in the permeate was observed, indicating the formation of a very permeable dynamic membrane.
Then, the overall results showed that the CP-UF methodology can be efficiently used to obtain selective separation of metals by means of their binding to water soluble polymers.
5. Conclusions
The increasing scarcity of water in the World caused by the rapid population increase and the growing awareness of the impact of sewage contamination on rivers, lakes and sees, give reason for concern and the need for appropriate water managements practices. In particular, metal and drug contaminations are dangerous causes of water pollution and represent a big health hazard.
The use of membrane processes coupled with metal binding reaction could significantly contribute to the rational hydrologic resources management, thus closing the hydrologic cycle. Two processes based on complexation reactions correspond to this description: the Supported Liquid Membrane (SLM) and the Complexation Ultrafiltration (CP-UF) processes.
Stagnant Sandwich Liquid Membrane (SSwLM) was an implementation of the SLM system, responding to the need to obtain system stability suitable for industrial applications. The SSwLM system was tested in the removal of the inorganic ion copper(II) and of the organic drug Gemfibrozil. The results demonstrated in both cases that the SSwLM gave better fluxes and better stabilities with respect to the traditional SLM configuration. The influence of the permeation module orientation on system performances was also studied, and a mechanism for SSwLM destabilization was proposed. In particular, the results reported in the case of copper and GEM separation showed that vertical module orientation was the best one, giving higher flux (0.662 vs 0.611 mmol·h–1·m–2) and higher stability (335.5 vs. 119.5 hours) compared to the horizontal one.
The CP-UF process was tested both in the removal of metal chelates from water (e.g., washing water from contaminated soils) and in the selective separation of metal ions from wastewater.
An integrated membrane process, where the CP-UP technique was coupled with a diafiltration step for polymer recovery and recycle, followed by a photocatalytic step, where the citrate was degraded thus obtaining a free copper(II) solution, was proposed. The experimental results validated the technical sustainability of the overall process for treating waters containing metal chelates by obtaining water recyclable in the process or safely discharged, recycling the polymer and recovering the metal ions.
The selectivity of the CP-UF process was demonstrated by studying the selective separation of copper(II) and nickel(II) from a synthetic solution and from a real aqueous effluent.
The overall results showed that the synergic use of membrane separation and complexation reactions bring to eco-friendly processes for water decontamination from organic (drugs) and inorganic (metal ions) pollutants. In particular, the resulting separation techniques, guarantying the sustainability in water managements, generate more sustainable processes with respect to the traditional ones. Indeed, both processes are characterized by: i) high selectivity, permitting recovery and avoiding costs and need of land to locate wide traditional separation plants; ii) low energy consumption with respect to traditional techniques; iii) cyclic use of the complexing agents, thus avoiding their presence in the treated water; iv) minimization of chemical agents addition, thus reducing the costs and the ecological impact of the treatment.