A Robust Liquid Chromatographic Method for Confirmation of Drug Stability of Azithromycin in Bulk Samples, Tablets and Suspensions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. HPLC Instruments and Analytical Conditions
2.3. Preparation of Solutions
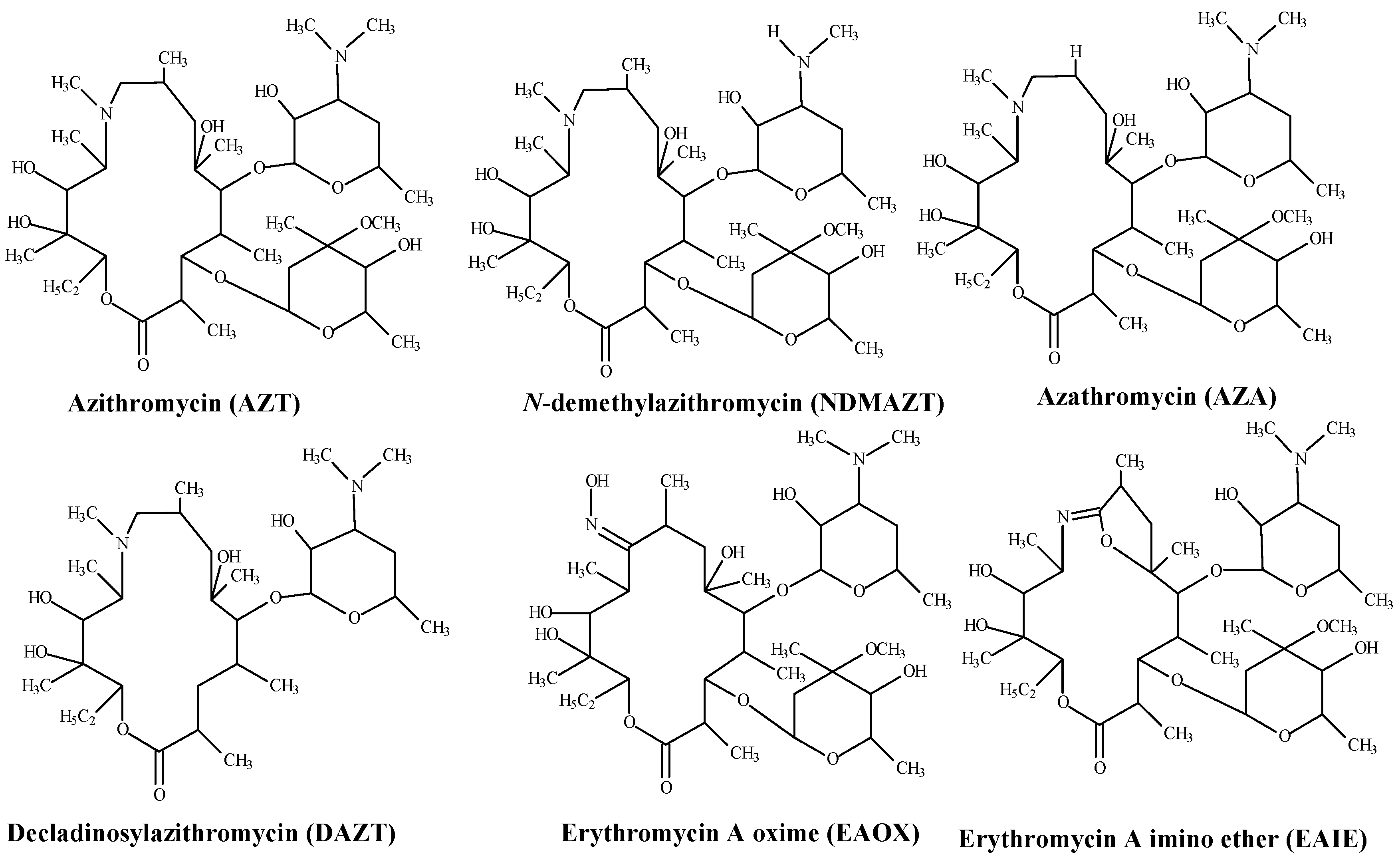
2.3.1. Working Standard Mixture
2.3.2. Mobile Phases
2.4. Peak Purity Analysis
2.5. Method Validation
2.5.1. Linearity
2.5.2. Sensitivity
2.5.3. Accuracy
2.5.4. Robustness
2.5.5. Precision
2.5.6. Specificity
2.6. Assay of Azithromycin in Bulk Samples, Tablets and Suspensions
2.7. Forced Degradation and Stability-Indicating Study
2.7.1. Oxidative Degradation of Azithromycin
2.7.2. Degradation of Azithromycin in Acid
3. Results and Discussion
3.1. Method Development and Optimization
3.2. Method Validation
3.2.1. Linearity
3.2.2. Sensitivity
3.2.3. Accuracy
3.2.4. Robustness
3.2.5. Precision
3.2.6. Specificity
3.3. Forced Degradation of Azithromycin in Acidic and Oxidative Environments
3.4. Assay of Azithromycin Tablets and Suspensions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Piscitelli, S.C.; Danziger, L.H.; Rodvold, K.A. Clarithromycin and azithromycin: New macrolide antibiotics. Clin. Pharm. 1992, 11, 137–152. [Google Scholar] [PubMed]
- Stott, G.A. New macrolide antibiotics: Clarithromycin and azithromycin. Am. Fam. Physician 1992, 46, 863–869. [Google Scholar] [PubMed]
- World Health Organization (WHO). WHO Model Essential Medicines List 2015; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Ministry of Health. Kenya Essential Medicines List 2016; Ministry of Health: Nairobi, Kenya, 2016.
- Breier, A.R.; Garcia, C.V.; Oppe, T.P.; Steppe, M.; Schapoval, E.E.S. Microbiological assay for azithromycin in pharmaceutical formulations. J. Pharm. Biomed. Anal. 2002, 29, 957–961. [Google Scholar] [CrossRef]
- Nigović, B.; Šimunić, B. Voltammetric assay of azithromycin in pharmaceutical dosage forms. J. Pharm. Biomed. Anal. 2003, 32, 197–202. [Google Scholar] [CrossRef]
- Walash, M.I.; Rizk, M.S.; Eid, M.I.; Fathy, M.E. Spectrophotometric determination of four macrolide antibiotics in pharmaceutical formulations and biological fluids via binary complex formation with eosin and spectrophotometry. J. AOAC Int. 2007, 90, 1579–1587. [Google Scholar] [PubMed]
- Zhang, S.; Huang, X.; Yao, N.; Horváth, C. Preparation of monodisperse porous polymethacrylate microspheres and their application in the capillary electrochromatography of macrolide antibiotics. J. Chromatogr. A 2002, 948, 193–201. [Google Scholar] [CrossRef]
- Khedr, A.; Sheha, M. Quantitative thin-Layer chromatographic method of analysis of azithromycin in pure and capsule forms. J. Chromatogr. Sci. 2003, 41, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Kamau, F.N.; Chepkwony, H.K.; Ngugi, J.K.; Debremaeker, D.; Roets, E.; Hoogmartens, J. Isocratic liquid chromatographic method for the analysis of azithromycin and its structurally related substances in bulk samples. J. Chromatogr. Sci. 2002, 40, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Debremaeker, D.; Visky, D.; Chepkwony, H.K.; van Schepdael, A.; Roets, E.; Hoogmartens, J. Analysis of unknown compounds in azithromycin bulk samples with liquid chromatography coupled to ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 342–350. [Google Scholar] [CrossRef] [PubMed]
- British Pharmacopoeia Commission. British Pharmacopoeia 2015; The Stationery Office Ltd.: Norwich, UK, 2014. [Google Scholar]
- United States Pharmacopeial. USP 38-NF 33; United States Pharmacopeial Convention: Rockville, MD, USA, 2015. [Google Scholar]
- Sharma, K.; Mullangi, R. A concise review of HPLC, LC-MS and LC-MS/MS methods for determination of azithromycin in various biological matrices. Biomed. Chromatogr. 2013, 27, 1243–1258. [Google Scholar] [CrossRef] [PubMed]
- El-Gindy, A.; Attia, K.A.; Nassar, M.W.; Al Abasawi, N.M.; Al-Shabrawi, M. Optimization and validation of a stability-indicating RP-HPLC method for determination of azithromycin and its related compounds. J. AOAC Int. 2011, 94, 513–522. [Google Scholar] [PubMed]
- Sachin, K.S.; Prakash, D.; Tamizh, M.T. Development and validation of stability indicating UV spectrophotometric method for the estimation of azithromycin suspension. Int. J. ChemTech Res. 2010, 2, 1939–1944. [Google Scholar]
- Miguel, L.; Barbas, C. LC determination of impurities in azithromycin tablets. J. Pharm. Biomed. Anal. 2003, 33, 211–217. [Google Scholar] [CrossRef]
- Stahl, M. Peak Purity Analysis in HPLC and CE Using Diode-Array Technology. Available online: http://www.agilent.com/cs/library/applications/5988-8647EN.pdf (accessed on 24 April 2016).
- Wiberg, K.; Andersson, M.; Hagman, A.; Jacobsson, S.P. Peak purity determination with principal component analysis of high-performance liquid chromatography-diode array detection data. J. Chromatogr. A 2004, 1029, 13–20. [Google Scholar] [CrossRef] [PubMed]
- ICH Q2 (R1) Validation of Analytical Procedures: Text and Methodology. Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf (accessed on 10 April 2016).
- Abuga, K.O.; Chepkwony, H.K.; Roets, E.; Hoogmartens, J. A Stability-indicating HPLC method for the separation of clarithromycin and its related substances in bulk Samples. J. Sep. Sci. 2001, 24, 849–855. [Google Scholar] [CrossRef]
- ICH Q6A Specifications: Test Procedures and Acceptance Criteria for New Drug Substance and New Drug Products: Chemical Substances. Available online: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm134966.htm (accessed on 10 April 2016).
- Harrison, J.D.; Jones, J.A.; Morris, D.L. Azithromycin levels in plasma and gastric tissue, juice and mucus. Eur. J. Clin. Microbiol. Infect. Dis. 1991, 10, 862–864. [Google Scholar] [CrossRef] [PubMed]
- FDA Guidance for Industry Bioavailability and Bioequivalence Studies for Orally Administered Drug Products—General Guidance for Industry Bioavailability and Bioequivalence. Available online: http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/AbbreviatedNewDrugApplicationANDAGenerics/UCM154838.pdf (accessed on 12 April 2016).



| Factor | Low Level (−1) | Central Level (0) | High Level (+1) |
|---|---|---|---|
| pH | 6.0 | 6.5 | 7.0 |
| Acetonitrile (% vol.) | 22 | 25 | 28 |
| Temperature (°C) | 41 | 43 | 45 |
| Target Concentration (%) | Azithromycin | Mean Recovery (%) | |||
|---|---|---|---|---|---|
| Amount Added (mg/mL) | Amount Recovered (mg/mL) | Recovery (Absolute %) | Recovery (CV %) | ||
| 80 | 4.98 | 4.975 | 99.9 | 0.16 | 100.7 |
| 100 | 5.08 | 5.171 | 101.8 | 0.20 | |
| 120 | 5.13 | 5.152 | 100.4 | 0.50 | |
| Product Code | Formulation | AZT Content (%) | Remarks |
|---|---|---|---|
| I | (a) Tablet | 93.6 (1.6) | Complied |
| (b) Tablet | 94.8 (1.0) | Complied | |
| II | (a) Tablet | 110.3 (0.2) | Complied |
| (b) Tablet | 109.1 (0.1) | Complied | |
| III | (a) Tablet | 98.5 (0.5) | Complied |
| (b) Tablet | 99.2 (0.3) | Complied | |
| IV | (a) Tablet | 97.2 (0.2) | Complied |
| (b) Tablet | 97.6 (0.2) | Complied | |
| V | Tablet | 102.0 (1.5) | Complied |
| VI | (a) Tablet | 109.9 (0.3) | Complied |
| (b) Tablet | 107.3 (0.3) | Complied | |
| VII | (a) Tablet | 105.1 (1.6) | Complied |
| (b) Tablet | 104.3 (1.3) | Complied | |
| VIII | (a) Suspension (M) | 110.0 (0.6) | Complied |
| (b) Suspension (M) | 109.3 (0.5) | Complied | |
| IX | (a) Suspension (M) | 96.4 (0.7) | Complied |
| (b) Suspension (M) | 96.1 (0.2) | Complied | |
| X | Suspension (M) | 69.3 (1.7) | Did not comply |
| XI | Suspension (M) | 89.3 (0.4) | Did not comply |
| XII | Tablet | 81.4 (0.5) | Did not comply |
| XIII | Tablet | 99.8 (0.3) | Complied |
| XIV | Tablet | 83.3 (0.2) | Did not comply |
| XV | Tablet | 87.0 (1.1) | Did not comply |
| XVI | Tablet | 103.3 (1.3) | Complied |
| XVII | Suspension (P) | 88.6 (0.2) | Did not comply |
| XVIII | Suspension (P) | 105.5 (0.4) | Complied |
| XIX | Suspension (M) | 87.8 (1.3) | Did not comply |
| XX | Suspension (P) | 98.7 (0.7) | Complied |
| Bulk sample | Powder | 94.7 (0.1) | Complied |
| Zithromax® | Suspension (P) | 99.6 (0.7) | Complied |
| Zithromax® | Tablet | 96.4 (1.5) | Complied |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okaru, A.O.; Abuga, K.O.; Kamau, F.N.; Ndwigah, S.N.; Lachenmeier, D.W. A Robust Liquid Chromatographic Method for Confirmation of Drug Stability of Azithromycin in Bulk Samples, Tablets and Suspensions. Pharmaceutics 2017, 9, 11. https://doi.org/10.3390/pharmaceutics9010011
Okaru AO, Abuga KO, Kamau FN, Ndwigah SN, Lachenmeier DW. A Robust Liquid Chromatographic Method for Confirmation of Drug Stability of Azithromycin in Bulk Samples, Tablets and Suspensions. Pharmaceutics. 2017; 9(1):11. https://doi.org/10.3390/pharmaceutics9010011
Chicago/Turabian StyleOkaru, Alex O., Kennedy O. Abuga, Franco N. Kamau, Stanley N. Ndwigah, and Dirk W. Lachenmeier. 2017. "A Robust Liquid Chromatographic Method for Confirmation of Drug Stability of Azithromycin in Bulk Samples, Tablets and Suspensions" Pharmaceutics 9, no. 1: 11. https://doi.org/10.3390/pharmaceutics9010011







