Development and Validation of a Successful Microbiological Agar Assay for Determination of Ceftriaxone Sodium in Powder for Injectable Solution
Abstract
:1. Introduction
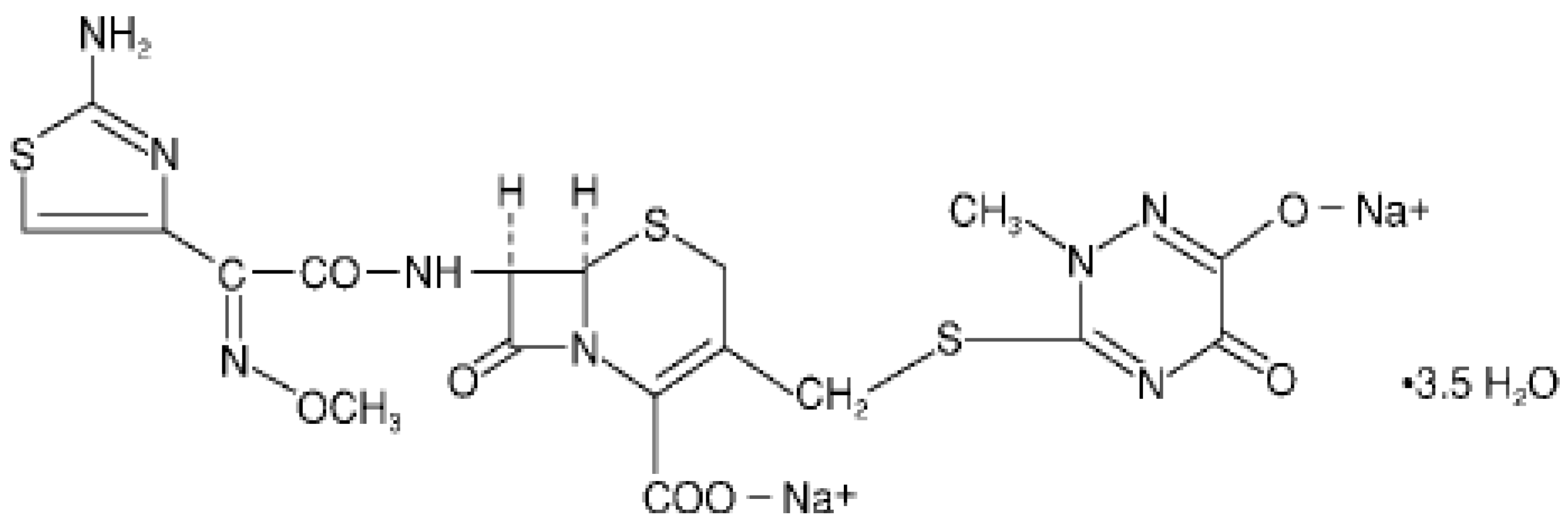
2. Experimental Section
2.1. Chemicals
2.2. Ceftriaxone Sodium Reference Solutions
2.3. Preparation of the Sample Solutions
2.4. Microorganism and Inoculum Standardization
2.5. Agar Diffusion Bioassay

2.6. Calculation of Activity and Method Validation
2.6.1. Linearity
2.6.2. Precision
2.6.3. Accuracy
2.6.4. Robustness
3. Results and Discussion
| Diameter of inhibition zones (mm) a | ||||||
|---|---|---|---|---|---|---|
| P1 | P2 | P3 | A1 | A2 | A3 | |
| (15 µg/mL) | (30 µg/mL) | (60 µg/mL) | (15 µg/mL) | (30 µg/mL) | (60 µg/mL) | |
| Day 1 | 16.41 | 18.45 | 20.80 | 16.31 | 18.45 | 20.70 |
| Day 2 | 16.19 | 18.45 | 20.85 | 16.23 | 18.35 | 20.85 |
| Day 3 | 16.20 | 18.30 | 20.75 | 16.01 | 18.45 | 20.90 |
| Diameter medium | 16.27 | 18.40 | 20.80 | 16.18 | 18.42 | 20.82 |
| RSD% b | 0.76 | 0.47 | 0.24 | 0.96 | 0.31 | 0.50 |
| Ceftriaxone sodium SQR added (µg/mL) | Ceftriaxone sodium SQR found a (µg/mL) | Recovery (%) | Recovery average (%) | RSD b(%) | |
|---|---|---|---|---|---|
| R1 | 4.0 | 4.00 | 100.00 | 100.46 | |
| R2 | 10.0 | 10.05 | 100.50 | 0.20 | |
| R3 | 16.0 | 16.14 | 100.88 |
| Variable | Range investigated | Ceftriaxone sodium a (g/vial) | Ceftriaxone sodium a (%) | RSD b(%) |
|---|---|---|---|---|
| Incubation time (time) | 18 | 0.974 | 97.40 | 1.01 |
| 24 | 0.992 | 99.23 | ||
| Incubation temperature (°C) | 35 | 0.967 | 96.72 | 1.28 |
| 30 | 0.962 | 96.29 | ||
| Inoculum concentration (%) | 1.0 | 0.980 | 98.03 | 1.12 |
| 1.2 | 0.996 | 99.68 |
4. Conclusions
Acknowledgments
Conflict of Interest
References
- El-Shaboury, S.R.; Saleh, G.A.; Mohamed, F.A.; Rageh, A.H. Analysis of cephalosporin antibiotics. J. Pharm. Biomed. Anal. 2007, 45, 1–19. [Google Scholar] [CrossRef]
- Christian, S.S.; Christian, J.S. The cephalosporins antibiotics. Prim. Care Update OB/GYNS 1997, 4, 168–174. [Google Scholar] [CrossRef]
- Pasha, C.; Narayana, B. A simple method for the spectrophometric determination of cephalosporins in pharmaceuticals using variamine blue. Eclet. Quim. 2008, 33, 41–46. [Google Scholar] [CrossRef]
- Hardman, J.G.; Limbird, L.E.; Gilman, A.G. Goodman e Gilman: As bases farmacológicas da terapêutica, 10th ed; McGraw-Hill: Rio de Janeiro, Brazil, 2003; p. 1647. [Google Scholar]
- Akl, M.A.; Ahmed, M.A.; Ahmed, R. Validation of an HPLC-UV method for the determination of ceftriaxone sodiumresidues on stainless steel surface of pharmaceutical manufacturing equipments. J. Pharm. Biomed. Anal. 2011, 55, 247–252. [Google Scholar] [CrossRef]
- Saleh, G.A.; El-Shaboury, S.R.; Mohamed, F.A.; Rageh, A.H. Kinetic spectrophotometric determination of certain cephalosporins using oxidized quercetin reagent. Spectrochim. Acta Part A 2009, 73, 946–954. [Google Scholar] [CrossRef]
- Mohamed, G.G. Spectrophotometric determination of ampicillin, dicluxacillin, flucoxacillin and amoxicillin antibiotic drugs: Ion-pair formation with molybdenum and thiocyanate. J. Pharm. Biomed. Anal. 2001, 24, 561–567. [Google Scholar] [CrossRef]
- Ministério da Saúde, Relação Nacional de Medicamentos Essenciais—Rename; Ministério da Saúde: Brasília, Brizil, 2007.
- Chutipongtanate, S.; Thongboonkerd, V. Ceftriaxone crystallization and its potential role in kidney stone formation. Biochem. Biophy. Res. Commun. 2011, 406, 396–402. [Google Scholar] [CrossRef]
- Amin, A.S.; Ragab, G.H. Spectrophotometric determination of certain cephalosporins in pure form and in pharmaceutical formulations. Spectrochim. Acta A Mol Biomol Spectrosc. 2004, 60, 2831–2835. [Google Scholar] [CrossRef]
- Sweetman, S.C. Martindale–The Complete Drug Reference; Pharmaceutical Press: London, UK, 2002; p. 169. [Google Scholar]
- O’Neil, M.J. TheMerck Index: An Encyclopedia of Chemicals, Drugs and Biologicals, 14th ed; Merck: Whitehouse Station, NJ, USA, 2006; pp. 322–323. [Google Scholar]
- Hiremath, B.; Mruthyunjayaswamy, B.H.M. Development and validation of a high-performance liquid chromatographic determination of ceftriaxone sodium and its application to drug quality control. Anal. Lett. 2009, 42, 2180–2190. [Google Scholar] [CrossRef]
- Katzung, B.G. Farmacologia: Básica & Clínica; Guanabara Koogan: Rio de Janeiro, Brazil, 2006; pp. 623–624. [Google Scholar]
- Al-Momani, I.F. Spectrophotometric determination of selected cephalosporins in drug formulations using flow injection analysis. J. Pharm. Biomed. Anal. 2001, 25, 751–757. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, L.; Ke, Y.; Khang, F.; Liang, X. Determination of ceftriaxone sodium by HPLC. J. Pharm. Biomed. Anal. 2011, 54, 623–628. [Google Scholar] [CrossRef]
- Phattanawasin, P.; Sotanaphun, U.; Sriphong, L.; Kanchanaphibool, I. Stability-indication TLC-image analysis method for quantification of ceftriaxone sodium in pharmaceutical dosage forms. J. Planar Chromatogr. 2011, 24, 30–34. [Google Scholar] [CrossRef]
- Tariq, A.; Siddiqui, M.R.; Kumar, J.; Reddy, D.; Negi, P.S.; Chaudhary, M.; Srivastava, S.M.; Singh, R.M. Development and validation of high performance liquid chromatographic method for the simultaneous determination of ceftriaxone and vancomycin in pharmaceutical formulations and biological samples. Sci. Asia 2010, 36, 297–304. [Google Scholar]
- Fabre, H.; Blanchin, M.D.; Lerner, D.; Mandrou, B. Determination of cephalosporins utilising thin-layer chromatography with fluorescamine detection. Analyst 1985, 110, 775–779. [Google Scholar] [CrossRef]
- Aleksic, M.; Savic, V.; Popovic, G.; Buric, N.; Kapetanovic, V. Acidity constants of cefetamet, cefotaxime and ceftriaxone; the effect of the substituent at C3 position. J. Pharm. Biomed. Anal. 2005, 39, 752–756. [Google Scholar] [CrossRef]
- Martinez, G.L.; Falcó, C.P.; Cabeza, S.A. Comparison of several methods used for the determination of cephalosporins. Analysis of cephalexin in pharmaceutical samples. J. Pharm. Biomed. Anal. 2002, 29, 405–423. [Google Scholar] [CrossRef]
- Salem, H.; Saleh, G.A. Selective spectrophotometric determination of phenolic β-lactam antibiotics. J. Pharm. Biomed. Anal. 2002, 28, 1205–1213. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Ma, Y.J.; Zhou, M.; Li, L.; Chen, H. Determination of Ceftriaxone Sodium in Pharmaceutical Formulations by Flow Injection Analysis with Acid Potassium Permanganate Chemiluminescence Detection. Anal. Sci. 2006, 22, 183–186. [Google Scholar] [CrossRef]
- Luypaert, J.; Massart, D.L.; Heyden, Y.V. Near-infrared spectroscopy applications in pharmaceutical analysis. Talanta 2006, 72, 865–883. [Google Scholar]
- Omar, M.A.; Abdelmageed, O.H.; Attia, T.Z. Kinetic spectrofluorimetric determination of certain cephalosporins in human plasma. Int. J. Anal. Chem. 2009, 1–12. [Google Scholar]
- Chen, Y.R.; Lin, S.J.; Chou, Y.W.; Wu, H.L.; Chen, S.H. Simultaneous determination of cefepime and L-arginine by micellar electrokinetic chromatography and applications to commercial injections. J. Sep. Sci. 2005, 28, 2173–2179. [Google Scholar] [CrossRef]
- Gaspar, A.; Andrási, M.; Kardos, S. Application of capillary zone electrophoresis to the analysis and to a stability study of cephalosporins. J. Chromatogr. B 2002, 775, 239–246. [Google Scholar] [CrossRef]
- Sciacchitano, C.J.; Mopper, B.; Specchio, J.J. Identification and separation of five cephalosporins by micellar electrokinetic capillary chromatography. J. Chromatogr. B Biomed. Appl. 1994, 657, 395–399. [Google Scholar] [CrossRef]
- Brazilian Pharmacopeial Convention, Brazilian Pharmacopoeia, 5th ed; Anvisa: Brasília, Brazil, 2010.
- The United States Pharmacopeia, The National Formulary (NF24). By Authority of the United States Pharmacopeial Convention; United States Pharmacopeial Convention: Rockville, MD, USA, 2010; pp. 1697–1698.
- Hewitt, W. Microbiological Assay for Pharmaceutical Analysis: A Rational Approach; Interpharm/CRC Press: Boca Raton, FL, USA, 2003; pp. 97–115. [Google Scholar]
- International Conference on Harmonization (ICH), Q2(R1): Validation of Analytical Procedures: Text and Methodology; ICH: Geneva, Switzerland, 2005.
- Gomes, G.C.; Salgado, H.R.N. Microbiological assay for determination of lomefloxacin in coated tablets. J. AOAC Int. 2006, 89, 1077–1079. [Google Scholar]
- Salgado, H.R.N.; Lopes, C.C.G.O.; Lucchesi, M.B.B. Microbiological assay for gatifloxacin in pharmaceutical formulations. J. Pharm. Biomed. Anal. 2006, 40, 443–446. [Google Scholar] [CrossRef]
- Moreno, A.R.; Salgado, H.R.N. Microbiological assay for ceftazidime injection. J. AOAC Int. 2007, 90, 1379–1382. [Google Scholar]
- Tozo, G.C.G.; Salgado, H.R.N. Microbiological assay for cefoxitin sodium in dosage form. J. AOAC Int. 2007, 90, 452–455. [Google Scholar]
- Marona, H.R.N.; Schapoval, E.E.S. Desarrollo de análisis microbiológico para la determinación de esparfloxacino en polvo y en comprimidos de 200 mg. Inf. Tecnol. 1998, 9, 251–254. [Google Scholar]
- Lopes, C.C.G.O.; Salgado, H.R.N. Development and validation of a stability-indicative Agar diffusion assay to determine the potency of linezolid in tablets in the presence of photodegradation products. Talanta 2010, 82, 918–922. [Google Scholar] [CrossRef]
- Cazedey, E.C.L.; Salgado, H.R.N. Development and validation of a microbiological agar assay for determination of orbifloxacin in pharmaceutical preparations. Pharmaceutics 2011, 3, 572–580. [Google Scholar] [CrossRef]
- Resolução no 899 de 29 de maio de 2003. Guia para validação de métodos analíticos e bioanalíticos (in Portuguese), Diário Oficial da República Federativa do Brasil, DF, 2 Jun. 2003.
- Baird, R.M.; Hodges, N.A.; Denyer, S.P. Handbook of Microbiological Quality Control: Pharmaceuticals and Medical Devices; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Aléssio, P.V.; Salgado, H.R.N. Development and Validation of a Successful Microbiological Agar Assay for Determination of Ceftriaxone Sodium in Powder for Injectable Solution. Pharmaceutics 2012, 4, 334-342. https://doi.org/10.3390/pharmaceutics4030334
Aléssio PV, Salgado HRN. Development and Validation of a Successful Microbiological Agar Assay for Determination of Ceftriaxone Sodium in Powder for Injectable Solution. Pharmaceutics. 2012; 4(3):334-342. https://doi.org/10.3390/pharmaceutics4030334
Chicago/Turabian StyleAléssio, Patrícia V., and Hérida R. N. Salgado. 2012. "Development and Validation of a Successful Microbiological Agar Assay for Determination of Ceftriaxone Sodium in Powder for Injectable Solution" Pharmaceutics 4, no. 3: 334-342. https://doi.org/10.3390/pharmaceutics4030334




