Three-Step Thermal Drawing for Rapid Prototyping of Highly Customizable Microneedles for Vascular Tissue Insertion
Abstract
:1. Introduction
2. Materials, Principles and Methods
2.1. Materials
2.2. Thermal Drawing System
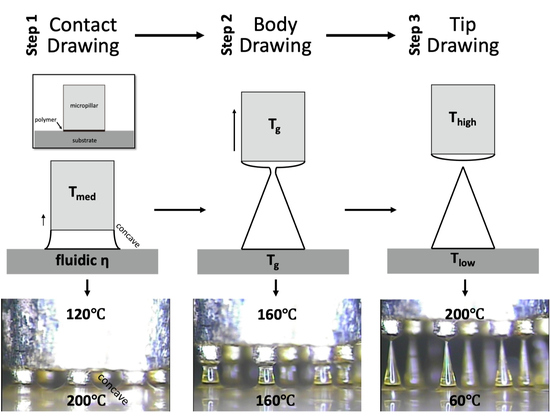
2.3. Principle of Three-Step Thermal Drawing for MN
2.4. Principle of Transfer Thermal Drawing
2.5. Ex Vivo Tissue Insertion Test
2.6. Optical Coherence Tomography Analysis
2.7. Statistical Analysis
3. Results
3.1. MN Fabrication Using Three-Step Thermal Drawing
3.2. Transfer Thermal Drawing onto Medical Balloon Surface
3.3. Mechanical Behavior of MN During Vascular Tissue Insertion
3.4. Tissue Wound with Respect to A/R Through OCT Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated microneedles: A novel approach to transdermal drug delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Hord, A.H.; Denson, D.D.; McAllister, D.V.; Smitra, S.; Allen, M.G.; Prausnitz, M.R. Lack of pain associated with microfabricated microneedles. Anesth. Analg. 2001, 92, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Gill, H.S.; Andrews, S.N.; Prausnitz, M.R. Kinetics of skin resealing after insertion of microneedles in human subjects. J. Control. Release 2011, 154, 148–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bal, S.M.; Caussin, J.; Pavel, S.; Bouwstra, J.A. In vivo assessment of safety of microneedle arrays in human skin. Eur. J. Pharm. Sci. 2008, 35, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-C.; Wang, K.-W.; Chen, D.-H.; Ling, M.-H.; Liu, C.-Y. Remotely triggered release of small molecules from LaB6@SiO2-loaded polycaprolactone microneedles. Acta Biomater. 2015, 13, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-M.; Todo, H.; Sugibayashi, K. Enhancement of skin permeation of high molecular compounds by a combination of microneedle pretreatment and iontophoresis. J. Control. Release 2007, 118, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, Y.H.; Yamada, M.; Lin, L.L.; Grice, J.E.; Roberts, M.S.; Raphael, A.P.; Benson, H.A.E.; Prow, T.W. Microneedle enhanced delivery of cosmeceutically relevant peptides in human skin. PLoS ONE 2014, 9, e101956. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-C.; Quan, F.-S.; Yoo, D.-G.; Compans, R.W.; Kang, S.-M.; Prausnitz, M.R. Improved influenza vaccination in the skin using vaccine coated microneedles. Vaccine 2009, 27, 6932–6938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Groot, A.M.; Du, G.; Mönkäre, J.; Platteel, A.C.M.; Broere, F.; Bouwstra, J.A.; Sijts, A.J.A.M. Hollow microneedle-mediated intradermal delivery of model vaccine antigen-loaded PLGA nanoparticles elicits protective T cell-mediated immunity to an intracellular bacterium. J. Control. Release 2017, 266, 27–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Q.; Zarnitsyn, V.G.; Ye, L.; Wen, Z.; Gao, Y.; Pan, L.; Skountzou, I.; Gill, H.S.; Prausnitz, M.R.; Yang, C.; et al. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc. Natl. Acad. Sci. USA 2009, 106, 7968–7973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.A.; McCrudden, C.M.; McCaffrey, J.; McBride, J.W.; Cole, G.; Dunne, N.J.; Robson, T.; Kissenpfennig, A.; Donnelly, R.F.; McCarthy, H.O. DNA vaccination for cervical cancer; a novel technology platform of RALA mediated gene delivery via polymeric microneedles. Nanomedicine 2017, 13, 921–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Kim, J.D.; Lee, C.Y.; Her, S.; Jung, H. A high-capacity, hybrid electro-microneedle for in-situ cutaneous gene transfer. Biomaterials 2011, 32, 7705–7710. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Park, S.H.; Lee, J.Y.; Joo, H.C.; Jang, E.H.; Youn, Y.-N.; Ryu, W. Perivascular biodegradable microneedle cuff for reduction of neointima formation after vascular injury. J. Control. Release 2014, 192, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Jang, E.; Lee, K.; Lee, J.; Park, S.; Seo, I.; Lee, K.; Lee, S.; Ryu, W.; Youn, Y.-N. A biodegradable microneedle cuff for comparison of drug effects through perivascular delivery to balloon-injured arteries. Polymers 2017, 9, 56. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.-H.; Lee, K.J.; Seo, I.H.; Park, S.H.; Jang, E.H.; Park, Y.; Youn, Y.-N.; Ryu, W. Transfer-molded wrappable microneedle meshes for perivascular drug delivery. J. Control. Release 2017, 268, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Gill, H.S.; Ghate, D.; McCarey, B.E.; Patel, S.R.; Edelhauser, H.F.; Prausnitz, M.R. Coated microneedles for drug delivery to the eye. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4038–4043. [Google Scholar] [CrossRef] [PubMed]
- Song, H.B.; Lee, K.J.; Seo, I.H.; Lee, J.Y.; Lee, S.-M.; Kim, J.H.; Kim, J.H.; Ryu, W. Impact insertion of transfer-molded microneedle for localized and minimally invasive ocular drug delivery. J. Control. Release 2015, 209, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Song, H.B.; Cho, W.; Kim, J.H.; Kim, J.H.; Ryu, W. Intracorneal injection of a detachable hybrid microneedle for sustained drug delivery. Acta Biomater. 2018, 80, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Lin, A.S.P.; Edelhauser, H.F.; Prausnitz, M.R. Suprachoroidal drug delivery to the back of the eye using hollow microneedles. Pharm. Res. 2010, 28, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Lee, K.; You, Y.S.; Lee, S.H.; Jung, H. Tower microneedle via reverse drawing lithography for innocuous intravitreal drug delivery. Adv. Healthc. Mater. 2013, 2, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; O’Cearbhaill, E.D.; Sisk, G.C.; Park, K.M.; Cho, W.K.; Villiger, M.; Bouma, B.E.; Pomahac, B.; Karp, J.M. A bio-inspired swellable microneedle adhesive for mechanical interlocking with tissue. Nat. Commun. 2013, 4, 1702. [Google Scholar] [CrossRef] [PubMed]
- Mukerjee, E.V.; Collins, S.D.; Isseroff, R.R.; Smith, R.L. Microneedle array for transdermal biological fluid extraction and in situ analysis. Sens. Actuators A Phys. 2004, 114, 267–275. [Google Scholar] [CrossRef]
- Chang, H.; Zheng, M.; Yu, X.; Than, A.; Seeni, R.Z.; Kang, R.; Tian, J.; Khanh, D.P.; Liu, L.; Chen, P.; et al. A swellable microneedle patch to rapidly extract skin interstitial fluid for timely metabolic analysis. Adv. Mater. 2017, 29, 1702243. [Google Scholar] [CrossRef] [PubMed]
- Jina, A.; Tierney, M.J.; Tamada, J.A.; McGill, S.; Desai, S.; Chua, B.; Chang, A.; Christiansen, M. Design, development, and evaluation of a novel microneedle array-based continuous glucose monitor. J. Diabetes Sci. Technol. 2014, 8, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Yen, S.-C.; Xue, N.; Sun, T.; Tsang, W.M.; Zhang, S.; Liao, L.-D.; Thakor, N.V.; Lee, C. Ultra-thin flexible polyimide neural probe embedded in a dissolvable maltose-coated microneedle. J. Micromech. Microeng. 2014, 24, 065015. [Google Scholar] [CrossRef]
- Wilke, N.; Hibert, C.; O’Brien, J.; Morrissey, A. Silicon microneedle electrode array with temperature monitoring for electroporation. Sens. Actuators A Phys. 2005, 123–124, 319–325. [Google Scholar] [CrossRef]
- Roxhed, N.; Griss, P.; Stemme, G. A method for tapered deep reactive ion etching using a modified Bosch process. J. Micromech. Microeng. 2007, 17, 1087–1092. [Google Scholar] [CrossRef]
- Griss, P.; Stemme, G. Side-opened out-of-plane microneedles for microfluidic transdermal liquid transfer. J. Microelectromech. Syst. 2003, 12, 296–301. [Google Scholar] [CrossRef]
- Ji, J.; Tay, F.E.H.; Miao, J. Microfabricated hollow microneedle array using ICP etcher. J. Phys. Conf. Ser. 2006, 34, 1132–1136. [Google Scholar] [CrossRef] [Green Version]
- Roxhed, N. A Fully Integrated Microneedle-based Transdermal Drug Delivery System; KTH-Royal Institute of Technology: Stockhelm, Sweden, 2007; ISBN 9789171787514. [Google Scholar]
- Bassous, E. Fabrication of novel three-dimensional microstructures by the anisotropic etching of (100) and (110) silicon. IEEE Trans. Electron Devices 1978, 25, 1178–1185. [Google Scholar] [CrossRef]
- Trimmer, W.; Ling, P.; Chin, C.-K.; Orton, P.; Gaugler, R.; Hashmi, S.; Hashmi, G.; Brunett, B.; Reed, M. Injection of DNA into plant and animal tissues with micromechanical piercing structures. In Proceedings of the IEEE Micro Electro Mechanical Systems, Amsterdam, The Netherlands, 29 January–2 February 1995. [Google Scholar]
- Wilke, N.; Reed, M.L.; Morrissey, A. The evolution from convex corner undercut towards microneedle formation: theory and experimental verification. J. Micromech. Microeng. 2006, 16, 808–814. [Google Scholar] [CrossRef]
- Wilke, N.; Morrissey, A. Silicon microneedle formation using modified mask designs based on convex corner undercut. J. Micromech. Microeng. 2006, 17, 238–244. [Google Scholar] [CrossRef]
- Shikida, M.; Odagaki, M.; Todoroki, N.; Ando, M.; Ishihara, Y.; Ando, T.; Sato, K. Non-photolithographic pattern transfer for fabricating arrayed three-dimensional microstructures by chemical anisotropic etching. Sens. Actuators A Phys. 2004, 116, 264–271. [Google Scholar] [CrossRef]
- Lee, K.; Lee, H.C.; Lee, D.-S.; Jung, H. Drawing lithography: Three-dimensional fabrication of an ultrahigh-aspect-ratio microneedle. Adv. Mater. 2010, 22, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.K.; Lee, K.J.; Youn, Y.N.; Jang, E.H.; Kim, W.; Min, B.-K.; Ryu, W. Spatially discrete thermal drawing of biodegradable microneedles for vascular drug delivery. Eur. J. Pharm. Biopharm. 2013, 83, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Kim, M.; Yang, H.; Lee, K.; Jung, H. Droplet-born air blowing: Novel dissolving microneedle fabrication. J. Control. Release 2013, 170, 430–436. [Google Scholar] [CrossRef]
- Vecchione, R.; Coppola, S.; Esposito, E.; Casale, C.; Vespini, V.; Grilli, S.; Ferraro, P.; Netti, P.A. Electro-drawn drug-loaded biodegradable polymer microneedles as a viable route to hypodermic injection. Adv. Funct. Mater. 2014, 24, 3515–3523. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, L.; Li, J.; Yao, L.; Chen, Y.; Liu, B.; Jiang, L. Rapid fabrication of microneedles using magnetorheological drawing lithography. Acta Biomater. 2018, 65, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kim, S.; Kang, G.; Lahiji, S.F.; Jang, M.; Kim, Y.M.; Kim, J.-M.; Cho, S.-N.; Jung, H. Centrifugal lithography: Self-shaping of polymer microstructures encapsulating biopharmaceutics by centrifuging polymer drops. Adv. Healthc. Mater. 2017, 6, 1700326. [Google Scholar] [CrossRef]
- Nerem, R.M. Tissue engineering a blood vessel substitute: the role of biomechanics. Yonsei Med. J. 2000, 41, 735–739. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; O’Rourke, M.; Nichols, W.W. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Chabri, F.; Bouris, K.; Jones, T.; Barrow, D.; Hann, A.; Allender, C.; Brain, K.; Birchall, J. Microfabricated silicon microneedles for nonviral cutaneous gene delivery. Br. J. Dermatol. 2004, 150, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Campbell, S.A. Preparation of hollow hafnium oxide microneedles for transdermal drug delivery. In Proceedings of the 2016 9th International Congress on Image and Signal Processing, BioMedical Engineering and Informatics (CISP-BMEI), Datong, China, 15–17 October 2016. [Google Scholar]
- Dee, G.T.; Sauer, B.B. The surface tension of polymer liquids. Macromol. Symp. 1999, 139, 115–123. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Cheung, L.K.; Park, C.B.; Neumann, A.W. Study on the surface tensions of polymer melts using axisymmetric drop shape analysis. Polym. Eng. Sci. 1998, 38, 757–764. [Google Scholar] [CrossRef]
- Oliveira, M.S.N.; Yeh, R.; McKinley, G.H. Iterated stretching, extensional rheology and formation of beads-on-a-string structures in polymer solutions. J. Non-Newton. Fluid Mech. 2006, 137, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Rothstein, J.P.; Miller, E.; Moldenaers, P.; Clasen, C.; Co, A.; Leal, G.L.; Colby, R.H.; Jeffrey Giacomin, A. The effect of step-stretch parameters on capillary breakup extensional rheology (CaBER) measurements. In Proceedings of the AIP Conference Proceedings, Monterey, CA, USA, 7 July 2008. [Google Scholar]
- Campo-Deaño, L.; Clasen, C. The slow retraction method (SRM) for the determination of ultra-short relaxation times in capillary breakup extensional rheometry experiments. J. Non-Newton. Fluid Mech. 2010, 165, 1688–1699. [Google Scholar] [CrossRef] [Green Version]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.; Park, S.H.; Lee, J.; Ryu, S.; Joo, C.; Ryu, W. Three-Step Thermal Drawing for Rapid Prototyping of Highly Customizable Microneedles for Vascular Tissue Insertion. Pharmaceutics 2019, 11, 100. https://doi.org/10.3390/pharmaceutics11030100
Lee K, Park SH, Lee J, Ryu S, Joo C, Ryu W. Three-Step Thermal Drawing for Rapid Prototyping of Highly Customizable Microneedles for Vascular Tissue Insertion. Pharmaceutics. 2019; 11(3):100. https://doi.org/10.3390/pharmaceutics11030100
Chicago/Turabian StyleLee, KangJu, Seung Hyun Park, JiYong Lee, Suho Ryu, Chulmin Joo, and WonHyoung Ryu. 2019. "Three-Step Thermal Drawing for Rapid Prototyping of Highly Customizable Microneedles for Vascular Tissue Insertion" Pharmaceutics 11, no. 3: 100. https://doi.org/10.3390/pharmaceutics11030100