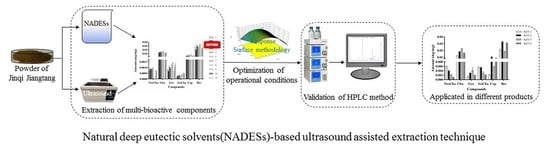
Natural Deep Eutectic Solvents for Simultaneous Extraction of Multi-Bioactive Components from Jinqi Jiangtang Preparations
Abstract
:1. Introduction
2. Methods and Materials
2.1. Materials
2.2. Apparatus and Conditions
2.3. Sample Extraction Procedure
2.4. Preparation of NADESs
2.5. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Selection of Compounds for Evaluating the Extraction Efficiency with Different Solvents
3.2. Comparison of Extractability of Multi-Compounds from JQJT with Different Solvents
3.3. Optimization of the NADESs Extraction Condition
3.4. Validation of the HPLC-UV Method
3.5. Analysis of Four Commercial JQJT Preparations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huie, C.W. A review of modern sample-preparation techniques for the extraction and analysis of medicinal plants. Anal. Bioanal. Chem. 2002, 373, 23–30. [Google Scholar] [CrossRef]
- Grodowska, K.; Parczewski, A. Organic solvents in the pharmaceutical industry. Acta Pol. Pharm. 2010, 67, 3–12. [Google Scholar]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.-J.; Verpoorte, R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef]
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. New natural and renewable low transition temperature mixtures (LTTMs): Screening as solvents for lignocellulosic biomass processing. Green Chem. 2012, 14, 2153–2157. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Lores, H.; Romero, V.; Costas, I.; Bendicho, C.; Lavilla, I. Natural deep eutectic solvents in combination with ultrasonic energy as a green approach for solubilisation of proteins: Application to gluten determination by immunoassay. Talanta 2017, 162, 453–459. [Google Scholar] [CrossRef]
- Cicco, L.; Rodríguez-Álvarez, M.J.; Perna, F.M.; García-Álvarez, J.; Capriati, V. One-pot sustainable synthesis of tertiary alcohols by combining ruthenium-catalysed isomerisation of allylic alcohols and chemoselective addition of polar organometallic reagents in deep eutectic solvents. Green Chem. 2017, 19, 3069–3077. [Google Scholar] [CrossRef]
- Marset, X.; Khoshnood, A.; Sotorríos, L.; Gómez-Bengoa, E.; Alonso, D.A.; Ramón, D.J. Deep Eutectic Solvent Compatible Metallic Catalysts: Cationic Pyridiniophosphine Ligands in Palladium Catalyzed Cross-Coupling Reactions. ChemCatChem 2017, 9, 1269–1275. [Google Scholar] [CrossRef] [Green Version]
- Massolo, E.; Palmieri, S.; Benaglia, M.; Capriati, V.; Perna, F.M. Stereoselective organocatalysed reactions in deep eutectic solvents: Highly tunable and biorenewable reaction media for sustainable organic synthesis. Green Chem. 2016, 18, 792–797. [Google Scholar] [CrossRef]
- Martínez, R.; Berbegal, L.; Guillena, G.; Ramón, D.J. Bio-renewable enantioselective aldol reaction in natural deep eutectic solvents. Green Chem. 2016, 18, 1724–1730. [Google Scholar] [CrossRef] [Green Version]
- Dilauro, G.; García, S.M.; Tagarelli, D.; Vitale, P.; Perna, F.M.; Capriati, V. Ligand-Free Bioinspired Suzuki–Miyaura Coupling Reactions using Aryltrifluoroborates as Effective Partners in Deep Eutectic Solvents. ChemSusChem 2018, 11, 3495–3501. [Google Scholar] [CrossRef]
- Messa, F.; Perrone, S.; Capua, M.; Tolomeo, F.; Troisi, L.; Capriati, V.; Salomone, A. Towards a sustainable synthesis of amides: Chemoselective palladium-catalysed aminocarbonylation of aryl iodides in deep eutectic solvents. Chem. Commun. 2018, 54, 8100–8103. [Google Scholar] [CrossRef]
- Boldrini, C.L.; Manfredi, N.; Perna, F.M.; Trifiletti, V.; Capriati, V.; Abbotto, A. Dye-Sensitized Solar Cells that use an Aqueous Choline Chloride-Based Deep Eutectic Solvent as Effective Electrolyte Solution. Energy Technol. 2017, 5, 345–353. [Google Scholar] [CrossRef]
- Nam, M.W.; Zhao, J.; Lee, M.S.; Jeong, J.H.; Lee, J. Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: Application to flavonoid extraction from Flos sophorae. Green Chem. 2015, 17, 1718–1727. [Google Scholar] [CrossRef]
- Dai, Y.T.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as a New Extraction Media for Phenolic Metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar] [CrossRef]
- Duan, L.; Dou, L.-L.; Guo, L.; Li, P.; Liu, E.H. Comprehensive Evaluation of Deep Eutectic Solvents in Extraction of Bioactive Natural Products. ACS Sustain. Chem. Eng. 2016, 4, 2405–2411. [Google Scholar] [CrossRef]
- Cao, H.B.; Ren, M.; Guo, L.P.; Shang, H.C.; Zhang, J.H.; Song, Y.Z.; Wang, H.; Wang, B.H.; Li, X.T.; Hu, J.; et al. JinQi-Jiangtang tablet, a Chinese patent medicine, for pre-diabetes: A randomized controlled trial. Trials 2010, 11, 27. [Google Scholar] [CrossRef]
- Gao, L.H.; Liu, Q.; Liu, S.N.; Chen, Z.Y.; Li, C.N.; Lei, L.; Sun, S.J.; Li, L.Y.; Liu, J.L.; Shen, Z.F. A refined-JinQi-JiangTang tablet ameliorates prediabetes by reducing insulin resistance and improving beta cell function in mice. J. Ethnopharmacol. 2014, 151, 675–685. [Google Scholar] [CrossRef]
- Liu, P.; Yang, H.; Long, F.; Hao, H.-P.; Xu, X.; Liu, Y.; Shi, X.-W.; Zhang, D.-D.; Zheng, H.-C.; Wen, Q.-Y. Bioactive equivalence of combinatorial components identified in screening of an herbal medicine. Pharm. Res. 2014, 31, 1788–1800. [Google Scholar] [CrossRef]
- Chang, Y.-X.; Ge, A.-H.; Donnapee, S.; Li, J.; Bai, Y.; Liu, J.; He, J.; Yang, X.; Song, L.-J.; Zhang, B.-L. The multi-targets integrated fingerprinting for screening anti-diabetic compounds from a Chinese medicine Jinqi Jiangtang Tablet. J. Ethnopharmacol. 2015, 164, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Bajkacz, S.; Adamek, J. Evaluation of new natural deep eutectic solvents for the extraction of isoflavones from soy products. Talanta 2017, 168, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Sirviö, J.A.; Visanko, M.; Liimatainen, H. Deep eutectic solvent system based on choline chloride-urea as a pre-treatment for nanofibrillation of wood cellulose. Green Chem. 2015, 17, 3401–3406. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Ionic liquids and deep eutectic solvents in natural products research: Mixtures of solids as extraction solvents. J. Nat. Prod. 2013, 76, 2162–2173. [Google Scholar] [CrossRef] [PubMed]
- Maran, J.P.; Manikandan, S.; Thirugnanasambandham, K.; Nivetha, C.V.; Dinesh, R. Box–Behnken design based statistical modeling for ultrasound-assisted extraction of corn silk polysaccharide. Carbohydr. Polym. 2013, 92, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Zhang, M.; Tan, T.; Chen, J.; Li, Z.; Zhang, Q.; Qiu, H. Deep eutectic solvents as novel extraction media for phenolic compounds from model oil. Chem. Commun. 2014, 50, 11749–11752. [Google Scholar] [CrossRef]
- Habibi, E.; Ghanemi, K.; Fallah-Mehrjardi, M.; Dadolahi-Sohrab, A. A novel digestion method based on a choline chloride–oxalic acid deep eutectic solvent for determining Cu, Fe, and Zn in fish samples. Anal. Chim. Acta 2013, 762, 61–67. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Bi, W.; Tian, M.; Row, K.H. Evaluation of alcohol-based deep eutectic solvent in extraction and determination of flavonoids with response surface methodology optimization. J. Chromatogr. A 2013, 1285, 22–30. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Zhang, Y.; Xia, Q.; Bi, W.; Yang, X.; Chen, D.D.Y. Fast environment-friendly ball mill-assisted deep eutectic solvent-based extraction of natural products. J. Chromatogr. A 2016, 1443, 262–266. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Hammond, O.S.; Bowron, D.T.; Edler, K.J. The effect of water upon deep eutectic solvent nanostructure: An unusual transition from ionic mixture to aqueous solution. Angew. Chem. Int. Ed. 2017, 56, 9782–9785. [Google Scholar] [CrossRef] [PubMed]





| No. | Solvents | Mole Ratio |
|---|---|---|
| 1 | 70% MeOH | |
| 2 | Water | |
| 3 | ChCl-La | 1:2 |
| 4 | ChCl-Gly | 1:2 |
| 5 | ChCl-Glu | 1:1 |
| 6 | ChCl-Glu-Ma | 1:1:1 |
| 7 | ChCl-Pro | 1:2 |
| 8 | Pro-La | 1:2 |
| 9 | Pro-Gly | 1:1 |
| 10 | Pro-Glu | 1:1 |
| 11 | Pro-Ma | 1:1 |
| Variables | Unit | −1 | 0 | 1 |
|---|---|---|---|---|
| water | % | 25 | 50 | 75 |
| solid/solvent | mg/mL | 8 | 16 | 24 |
| time | min | 20 | 40 | 60 |
| Title | Sum of | Mean | F | p-Value | Significance | |
|---|---|---|---|---|---|---|
| Source | Squares | df | Square | Value | Prob > F | |
| Model | 2.96E-04 | 9 | 3.29E-05 | 14.18 | 0.001 | significant |
| A-A | 3.34E-05 | 1 | 3.34E-05 | 14.41 | 0.0068 | |
| B-B | 2.24E-05 | 1 | 2.24E-05 | 9.67 | 0.0171 | |
| C-C | 1.04E-05 | 1 | 1.04E-05 | 4.47 | 0.0722 | |
| AB | 2.93E-05 | 1 | 2.93E-05 | 12.65 | 0.0093 | |
| AC | 1.08E-05 | 1 | 1.08E-05 | 4.65 | 0.0679 | |
| BC | 3.25E-06 | 1 | 3.25E-06 | 1.4 | 0.2748 | |
| A^2 | 1.79E-04 | 1 | 1.79E-04 | 77.1 | <0.0001 | |
| B^2 | 1.70E-07 | 1 | 1.70E-07 | 7.40E-02 | 0.7941 | |
| C^2 | 1.18E-05 | 1 | 1.18E-05 | 5.1 | 0.0585 | |
| Residual | 1.62E-05 | 7 | 2.32E-06 | |||
| Lack of Fit | 9.10E-06 | 3 | 3.03E-06 | 1.71 | 0.3029 | not significant |
| Pure Error | 7.12E-06 | 4 | 1.78E-06 | |||
| Cor Total | 3.12E-04 | 16 | ||||
| R² | 0.95 | |||||
| CV | 2.41 |
| Compounds | Calibration Curve | Linear Range (μg/mL) | R² | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|
| Neochlorogenic acid | y = 14280x − 4.4111 | 0.16–42 | 0.9999 | 0.024 | 0.1 |
| Chlorogenic acid | y = 15806x − 4.5094 | 0.16–167 | 1 | 0.024 | 0.1 |
| Groenlandicine | y = 7672x − 0.7485 | 0.5–142 | 1 | 0.1 | 0.2 |
| Isochlorogenic acid B | y = 16377x − 23.675 | 0.16–167 | 0.9990 | 0.02 | 0.1 |
| Coptisine | y = 6957.4x − 6.4411 | 0.16–167 | 0.9995 | 0.04 | 0.1 |
| Berberine | y = 15899x − 15.411 | 0.16–167 | 0.9995 | 0.09 | 0.16 |
| Compounds | Concentration (mg/mL) | Precision | Repeatability (n = 3) | Stability (n = 3) | Recovery (n = 3) | |
|---|---|---|---|---|---|---|
| Intra-Day (RSD%) | Inter-Day (RSD%) | RSD% | RSD% | Mean ± SD, % | ||
| Neochlorogenic acid | 0.000425 | 0.82 | 1.19 | 4.82 | 2.09 | 100.44 ± 5.14 |
| 0.00095 | 2.50 | 1.78 | ||||
| 0.01 | 0.29 | 7.93 | ||||
| Chlorogenic acid | 0.000425 | 1.42 | 4.28 | 4.34 | 1.19 | 101.18 ± 3.14 |
| 0.00095 | 2.45 | 2.00 | ||||
| 0.01 | 0.55 | 7.78 | ||||
| Groenlandicine | 0.000425 | 6.30 | 4.28 | 4.38 | 1.59 | 100.25 ± 3.57 |
| 0.00095 | 1.56 | 2.66 | ||||
| 0.01 | 0.54 | 8.02 | ||||
| Isochlorogenic acid B | 0.0003 | 5.34 | 3.23 | 5.62 | 5.34 | 98.50 ± 3.03 |
| 0.01 | 0.68 | 8.01 | ||||
| 0.1 | 7.15 | 8.24 | ||||
| Coptisine | 0.000425 | 5.37 | 3.60 | 2.85 | 1.42 | 103.15 ± 0.29 |
| 0.01 | 0.48 | 8.15 | ||||
| 0.1 | 7.09 | 8.18 | ||||
| Berberine | 0.0008 | 0.51 | 5.32 | 4.01 | 1.68 | 102.06 ± 2.16 |
| 0.01 | 1.01 | 8.41 | ||||
| 0.1 | 5.81 | 7.79 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Li, L.; Hu, H.; Wan, J.; Li, P. Natural Deep Eutectic Solvents for Simultaneous Extraction of Multi-Bioactive Components from Jinqi Jiangtang Preparations. Pharmaceutics 2019, 11, 18. https://doi.org/10.3390/pharmaceutics11010018
Yang L, Li L, Hu H, Wan J, Li P. Natural Deep Eutectic Solvents for Simultaneous Extraction of Multi-Bioactive Components from Jinqi Jiangtang Preparations. Pharmaceutics. 2019; 11(1):18. https://doi.org/10.3390/pharmaceutics11010018
Chicago/Turabian StyleYang, Lele, Ling Li, Hao Hu, Jianbo Wan, and Peng Li. 2019. "Natural Deep Eutectic Solvents for Simultaneous Extraction of Multi-Bioactive Components from Jinqi Jiangtang Preparations" Pharmaceutics 11, no. 1: 18. https://doi.org/10.3390/pharmaceutics11010018