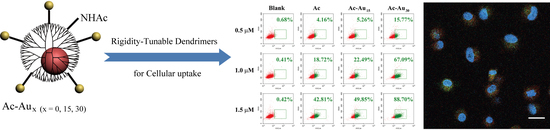
Cellular Uptake Behaviors of Rigidity-Tunable Dendrimers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of G5·NH2-FITC
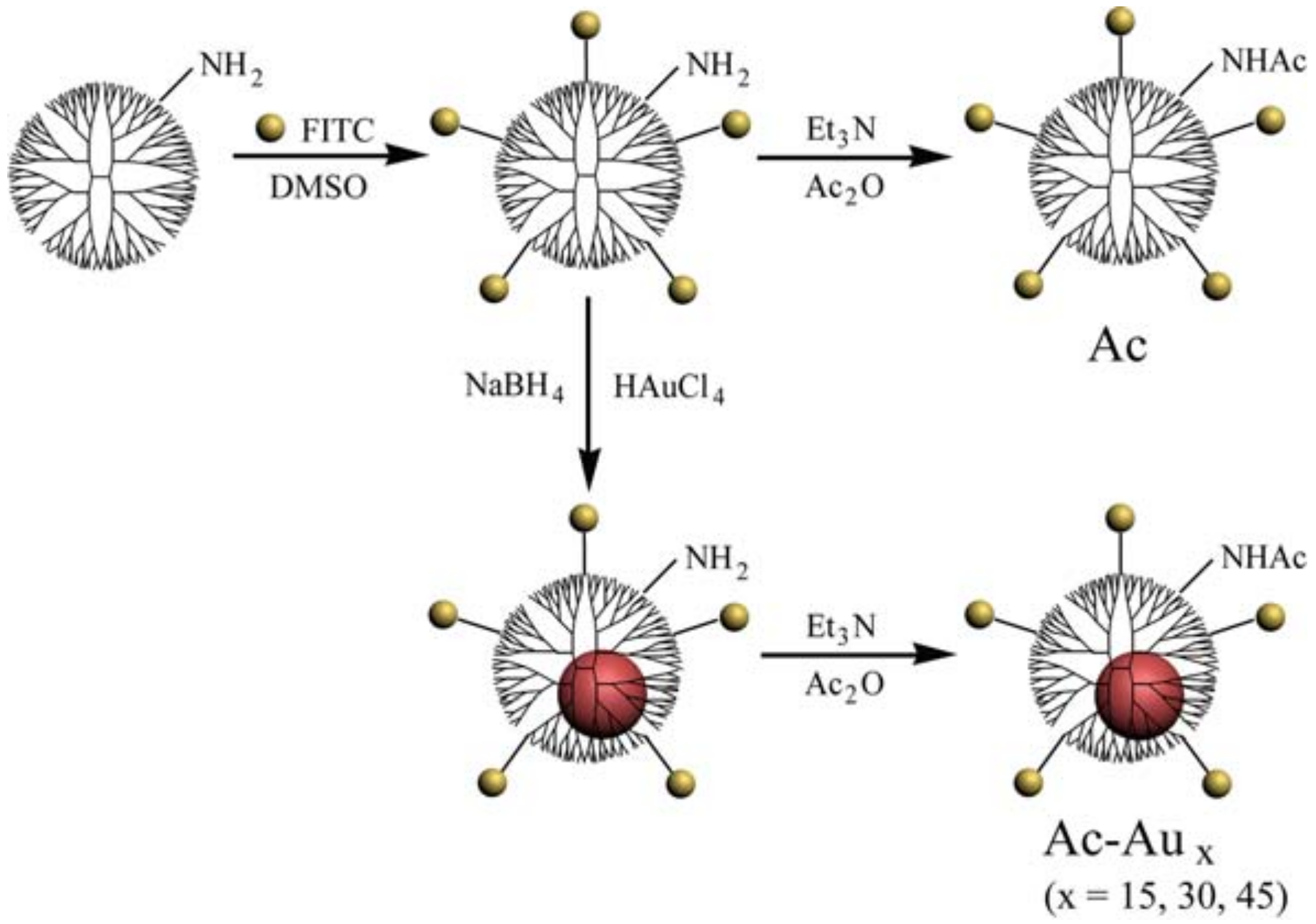
2.3. Preparation of Acetylated Fluorescent Dendrimers with Distinct Rigidity
2.4. Characterization Techniques
2.5. Cell Culture
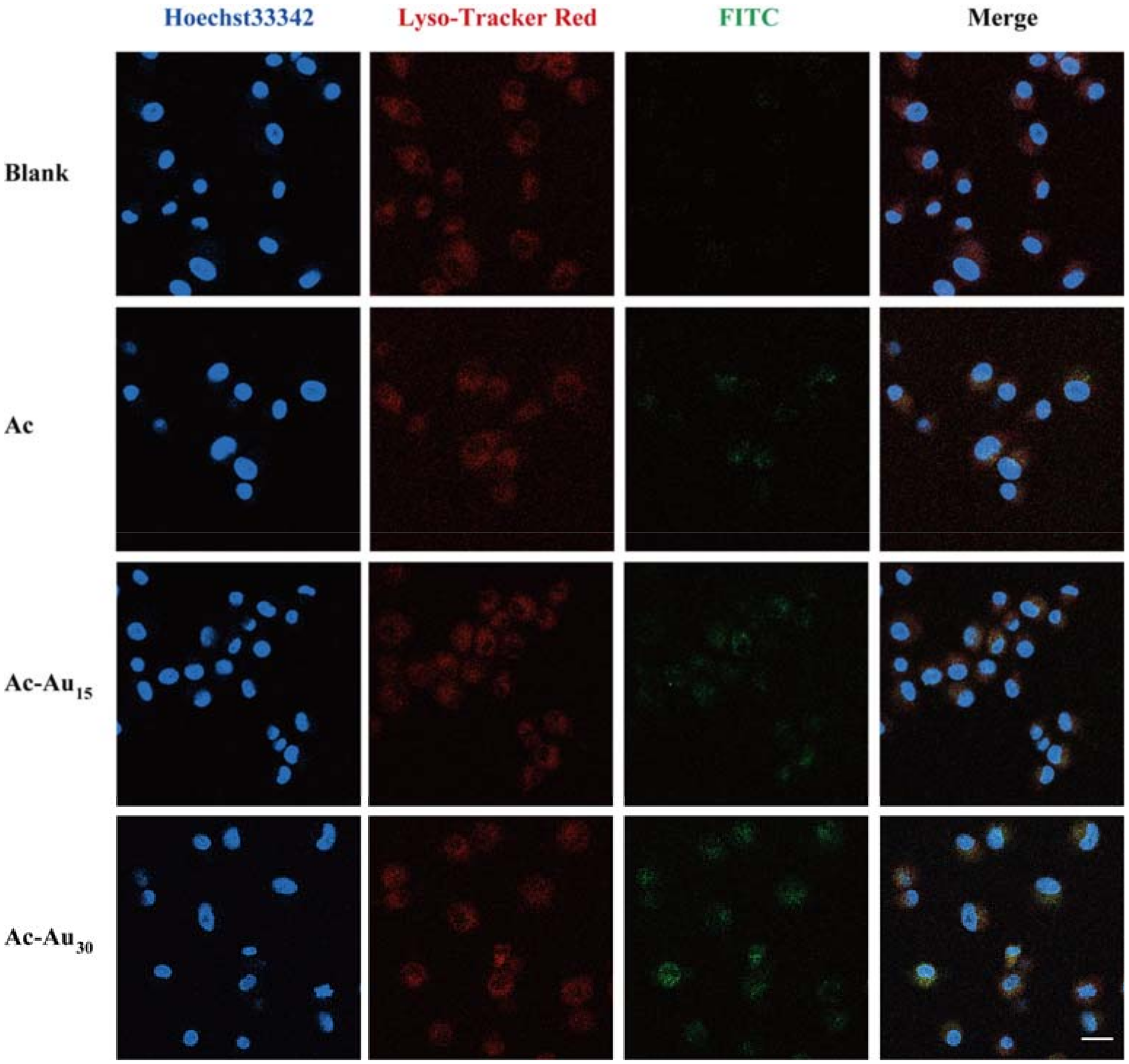
2.6. Analysis of Cellular Uptake Behaviors and Intracellular Localization
3. Results and Discussion
3.1. Preparation and Characterization of Ac-Aux Dendrimers
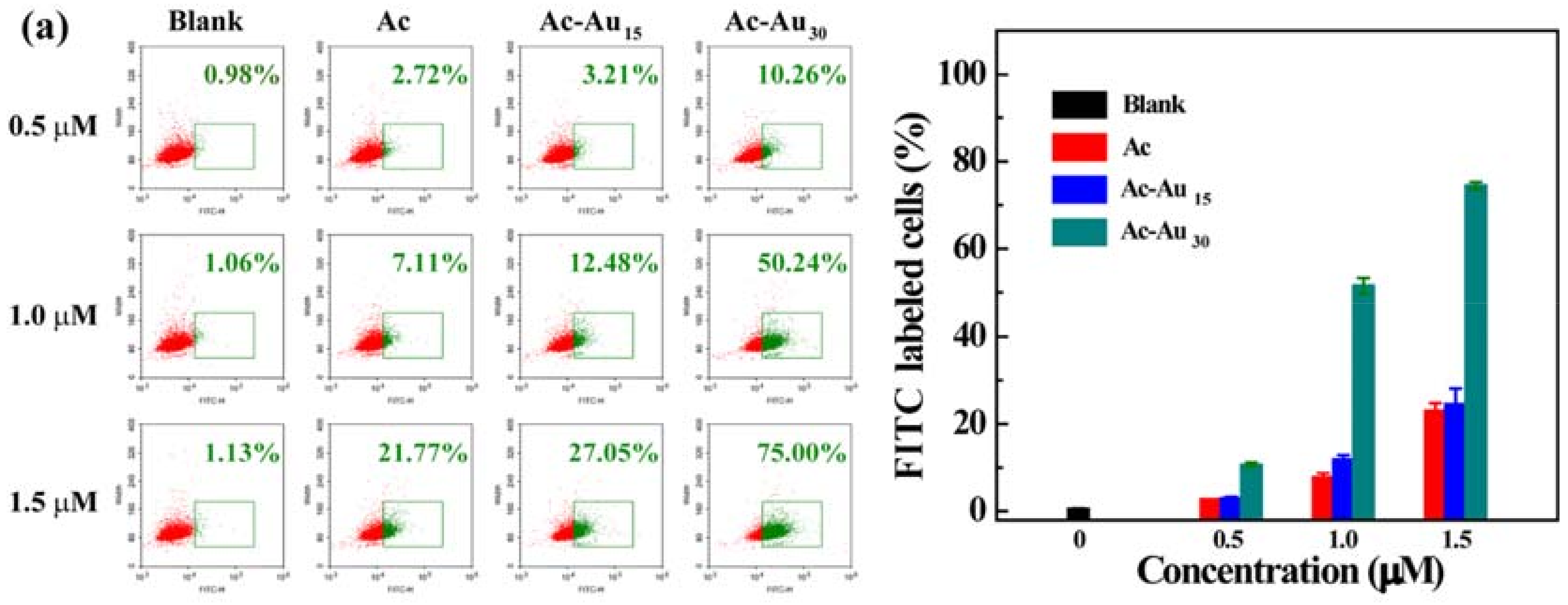
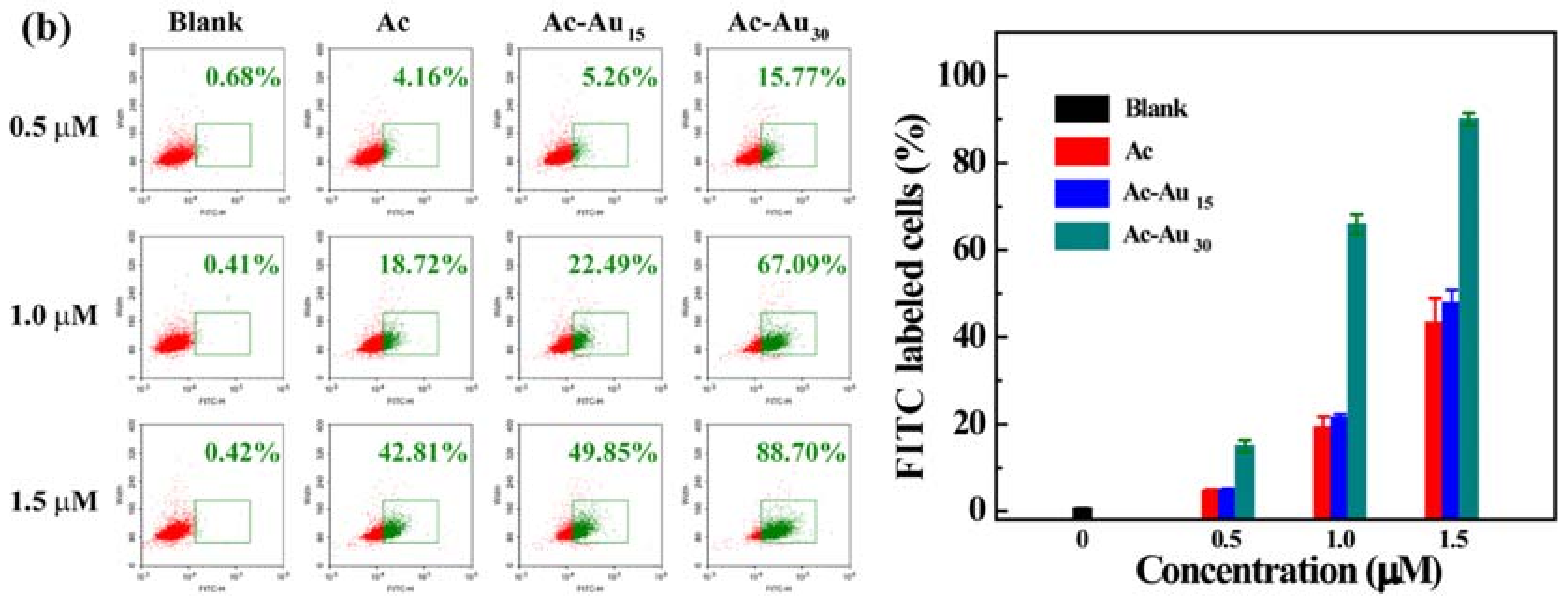
3.2. Analysis of Cellular Uptake Behaviors and Intracellular Localization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, Q.; Zhou, Z.; Qiu, N.; Shen, Y. Rational design of cancer nanomedicine: Nanoproperty integration and synchronization. Adv. Mater. 2017, 29, 1606628. [Google Scholar] [CrossRef] [PubMed]
- Doane, T.L.; Burda, C. The unique role of nanoparticles in nanomedicine: Imaging, drug delivery and therapy. Chem. Soc. Rev. 2012, 41, 2885–2911. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.R.; Gambhir, S.S. Nanomaterials for in vivo imaging. Chem. Rev. 2017, 117, 901–986. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Qian, J.; Zhou, L.; Su, Y.; Zhang, R.; Dong, C.M. Biopolymer-drug conjugate nanotheranostics for multimodal imaging-guided synergistic cancer photothermal-chemotherapy. ACS Appl. Mater. Interfaces 2017, 9, 31576–31588. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Han, Y.; Tang, J.; Piao, Y.; Liu, X.; Zhou, Z.; Gao, J.; Rao, J.; Shen, Y. A tumor-specific cascade amplification drug release nanoparticle for overcoming multidrug resistance in cancers. Adv. Mater. 2017, 29, 1702342. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Hu, X.; Hu, Y.; Wu, B.; Xing, D. Visible light-induced crosslinking and physiological stabilization of diselenide-rich nanoparticles for redox-responsive drug release and combination chemotherapy. Biomaterials 2017, 121, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hu, Y.; Sun, W.; Zhou, B.; Zhu, J.; Peng, C.; Shen, M.; Shi, X. Polyaniline-loaded gamma-polyglutamic acid nanogels as a platform for photoacoustic imaging-guided tumor photothermal therapy. Nanoscale 2017, 9, 12746–12754. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, J.; Wen, S.; Shen, M.; Zheng, L.; Zhang, G.; Shi, X. Targeted CT/MR dual mode imaging of human hepatocellular carcinoma using lactobionic acid-modified polyethyleneimine-entrapped gold nanoparticles. J. Mater. Chem. B 2017, 5, 2395–2401. [Google Scholar] [CrossRef]
- Kim, J.; Cho, H.R.; Jeon, H.; Kim, D.; Song, C.; Lee, N.; Choi, S.H.; Hyeon, T. Continuous O2-evolving MnFe2O4 nanoparticle-anchored mesoporous silica nanoparticles for efficient photodynamic therapy in hypoxic cancer. J. Am. Chem. Soc. 2017, 139, 10992–10995. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Han, X.; Li, Y.; Wang, H.; Ji, T.; Zhao, Y.; Nie, G. Photothermal effect enhanced cascade-targeting strategy for improved pancreatic cancer therapy by gold nanoshell@mesoporous silica nanorod. ACS Nano 2017, 11, 8103–8113. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Gao, H. Kinetics of receptor-mediated endocytosis of elastic nanoparticles. Nanoscale 2017, 9, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Stenzel, M.H. Entry of nanoparticles into cells: The importance of nanoparticle properties. Polym. Chem. 2018, 9, 259–272. [Google Scholar] [CrossRef]
- Li, J.; Mao, H.; Kawazoe, N.; Chen, G. Insight into the interactions between nanoparticles and cells. Biomater. Sci. 2017, 5, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zheng, J. Clearance pathways and tumor targeting of imaging nanoparticles. ACS Nano 2015, 9, 6655–6674. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Mintri, S.; Menon, A.V.; Lee, H.Y.; Choi, H.S.; Kim, J. Pharmacokinetics, pharmacodynamics and toxicology of theranostic nanoparticles. Nanoscale 2015, 7, 18848–18862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, L.; He, Q.; Liu, J.; Chen, Y.; Ma, M.; Zhang, L.; Shi, J. Nuclear-targeted drug delivery of TAT peptide-conjugated monodisperse mesoporous silica nanoparticles. J. Am. Chem. Soc. 2012, 134, 5722–5725. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, F.; Tian, R.; Zhang, L.; Fu, G.; Yang, L.; Zhu, L. Nanotubes-embedded indocyanine green-hyaluronic acid nanoparticles for photoacoustic-imaging-guided phototherapy. ACS Appl. Mater. Interfaces 2016, 8, 5608–5617. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Y.; Li, G.; Zhang, P.; Jin, C.; Zeng, L.; Ji, L.; Chao, H. Ruthenium(II) polypyridyl complexes as mitochondria-targeted two-photon photodynamic anticancer agents. Biomaterials 2015, 56, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Shi, X.; Gao, H. Cellular uptake of elastic nanoparticles. Phys. Rev. Lett. 2011, 107, 098101. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Gao, H. Cell membrane wrapping of a spherical thin elastic shell. Soft Matter 2015, 11, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Liu, D.; Subramanyam, K.; Wang, B.; Yang, J.; Huang, J.; Auguste, D.T.; Moses, M.A. Nanoparticle elasticity directs tumor uptake. Nat. Commun. 2018, 9, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stern, T.; Kaner, I.; Laser Zer, N.; Shoval, H.; Dror, D.; Manevitch, Z.; Chai, L.; Brill-Karniely, Y.; Benny, O. Rigidity of polymer micelles affects interactions with tumor cells. J. Control. Release 2017, 257, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, L.; Wang, J.; Feng, Q.; Liu, D.; Yin, Q.; Xu, D.; Wei, Y.; Ding, B.; Shi, X.; et al. Tunable rigidity of (polymeric core)-(lipid shell) nanoparticles for regulated cellular uptake. Adv. Mater. 2015, 27, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Shi, X. Dendrimer-based molecular imaging contrast agents. Prog. Polym. Sci. 2015, 44, 1–27. [Google Scholar] [CrossRef]
- Wen, S.; Li, K.; Cai, H.; Chen, Q.; Shen, M.; Huang, Y.; Peng, C.; Hou, W.; Zhu, M.; Zhang, G.; et al. Multifunctional dendrimer-entrapped gold nanoparticles for dual mode CT/MR imaging applications. Biomaterials 2013, 34, 1570–1580. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Huang-Fu, M.Y.; Guo, W.W.; Guo, N.N.; Chen, J.; Liu, H.N.; Xie, Z.Q.; Lin, M.T.; Wei, Q.C.; Gao, J.Q. MMP-2-sensitive HA end-conjugated poly(amidoamine) dendrimers via click reaction to enhance drug penetration into solid tumor. ACS Appl. Mater. Interfaces 2017, 9, 42459–42470. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, Y.; Yan, Y.; Zhang, Q.; Cheng, Y. Dendrimer-templated ultrasmall and multifunctional photothermal agents for efficient tumor ablation. ACS Nano 2016, 10, 4863–4872. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Shi, X. Dendrimer-based organic/inorganic hybrid nanoparticles in biomedical applications. Nanoscale 2010, 2, 1596–1610. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, H.; Xu, Y.H.; Shen, M.W.; Zhao, J.L.; Zhang, G.X.; Shi, X. Synthesis of PEGylated low generation dendrimer-entrapped gold nanoparticles for CT imaging applications. Nanoscale 2014, 6, 4521–4526. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, L.; Peng, C.; Guo, R.; Shen, M.; Shi, X.; Zhang, G. Computed tomography imaging of cancer cells using acetylated dendrimer-entrapped gold nanoparticles. Biomaterials 2011, 32, 2979–2988. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Gothwal, A.; Iyer, A.K.; Jain, K.; Chourasia, M.K.; Gupta, U. Dendrimer nanohybrid carrier systems: An expanding horizon for targeted drug and gene delivery. Drug Discov. Today 2017, 23, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.; Rodrigues, J.; Tomas, H.; Zablocka, M.; Shi, X.; Caminade, A.-M.; Majoral, J.-P. Dendrimers in combination with natural products and analogues as anti-cancer agents. Chem. Soc. Rev. 2018, 47, 514–532. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Sun, W.; Shi, X. Design and biomedical applications of poly(amidoamine)-dendrimer-based hybrid nanoarchitectures. Small Methods 2017, 1, 1700224. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, L.; Peng, C.; Shen, M.; Shi, X.; Zhang, G. Folic acid-modified dendrimer-entrapped gold nanoparticles as nanoprobes for targeted CT imaging of human lung adencarcinoma. Biomaterials 2013, 34, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, J. Loading IR820 using multifunctional dendrimers with enhanced stability and specificity. Pharmaceutics 2018, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Yang, X.; Liu, R.; Ruan, S.; Zhou, Y.; Xiao, W.; Yu, W.; Yang, C.; Gao, H. Coadministration of iRGD with multistage responsive nanoparticles enhanced tumor targeting and penetration abilities for breast cancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 22571–22579. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Fu, F.; Zhu, J.; Huang, R.; Zhu, Y.; Liu, Z.; Wang, J.; Conti, P.S.; Shi, X.; Chen, K. Cu-64-Labeled multifunctional dendrimers for targeted tumor PET imaging. Nanoscale 2018, 10, 6113–6124. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, H.; Xu, Y.H.; Guo, R.; Wen, S.H.; Huang, Y.P.; Liu, W.N.; Shen, M.W.; Zhao, J.L.; Zhang, G.X.; et al. Lactobionic acid-modified dendrimer-entrapped gold nanoparticles for targeted computed tomography imaging of human hepatocellular carcinoma. ACS Appl. Mater. Interfaces 2014, 6, 6944–6953. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, S.; Meshinchi, S.; Van Antwerp, M.E.; Bi, X.; Lee, I.; Baker, J.R., Jr. Dendrimer-entrapped gold nanoparticles as a platform for cancer-cell targeting and imaging. Small 2007, 3, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Wang, Y.; Zhu, J.; Li, X.; He, Y.; Qu, J.; Shen, M.; Xia, J.; Shi, X. Dendrimers meet zwitterions: Development of a unique antifouling nanoplatform for enhanced blood pool, lymph node and tumor CT imaging. Nanoscale 2017, 9, 12295–12301. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shen, M.W.; Zhao, J.L.; Zhu, J.Y.; Xiao, T.T.; Cao, X.Y.; Zhang, G.X.; Shi, X.Y. Facile formation of folic acid-modified dendrimer-stabilized gold–silver alloy nanoparticles for potential cellular computed tomography imaging applications. Analyst 2013, 138, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zheng, L.; Wen, S.; Tang, Y.; Shen, M.; Zhang, G.; Shi, X. Targeted cancer theranostics using alpha-tocopheryl succinate-conjugated multifunctional dendrimer-entrapped gold nanoparticles. Biomaterials 2014, 35, 7635–7646. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Wang, J.; Li, W.; Hu, J.; Wang, M.; Kang, Y. Cellular Uptake Behaviors of Rigidity-Tunable Dendrimers. Pharmaceutics 2018, 10, 99. https://doi.org/10.3390/pharmaceutics10030099
Liu H, Wang J, Li W, Hu J, Wang M, Kang Y. Cellular Uptake Behaviors of Rigidity-Tunable Dendrimers. Pharmaceutics. 2018; 10(3):99. https://doi.org/10.3390/pharmaceutics10030099
Chicago/Turabian StyleLiu, Hui, Jingjing Wang, Wenchao Li, Jie Hu, Min Wang, and Yuejun Kang. 2018. "Cellular Uptake Behaviors of Rigidity-Tunable Dendrimers" Pharmaceutics 10, no. 3: 99. https://doi.org/10.3390/pharmaceutics10030099