Gold Nanoparticles for Targeting Varlitinib to Human Pancreatic Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
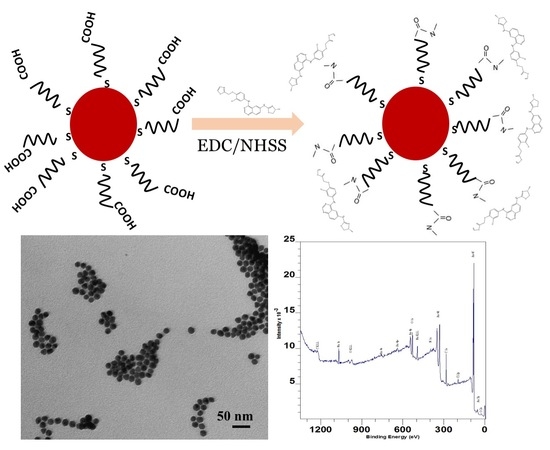
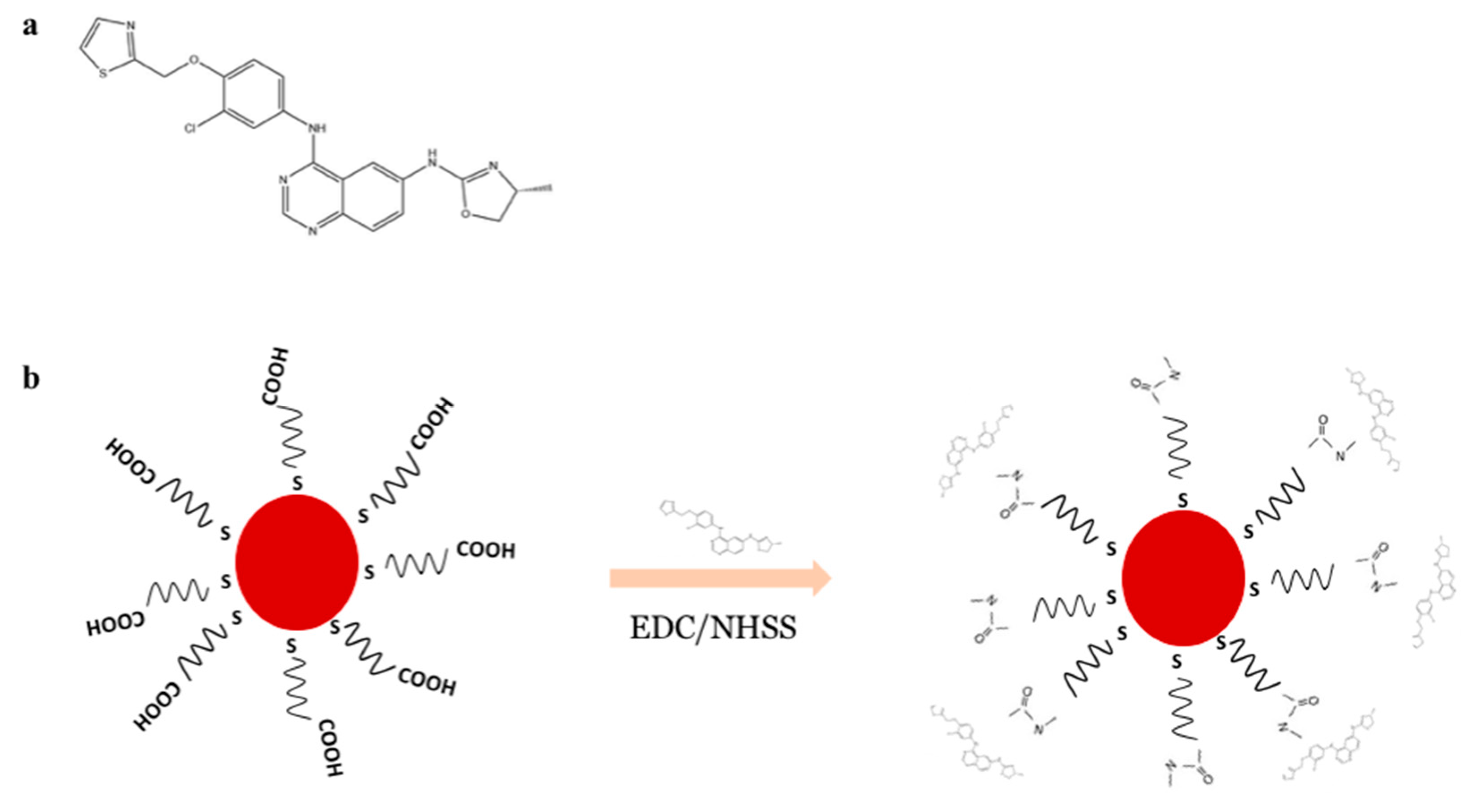
2.3. Synthesis of Pegylated Gold Nanoparticles
2.4. Conjugation of Varlitinib to PEGAuNP, PEGAUNPsVarl
2.5. Dynamic Light Scattering and Electrophoretic Mobility Measurements
2.6. Ultraviolet Visible Spectroscopy
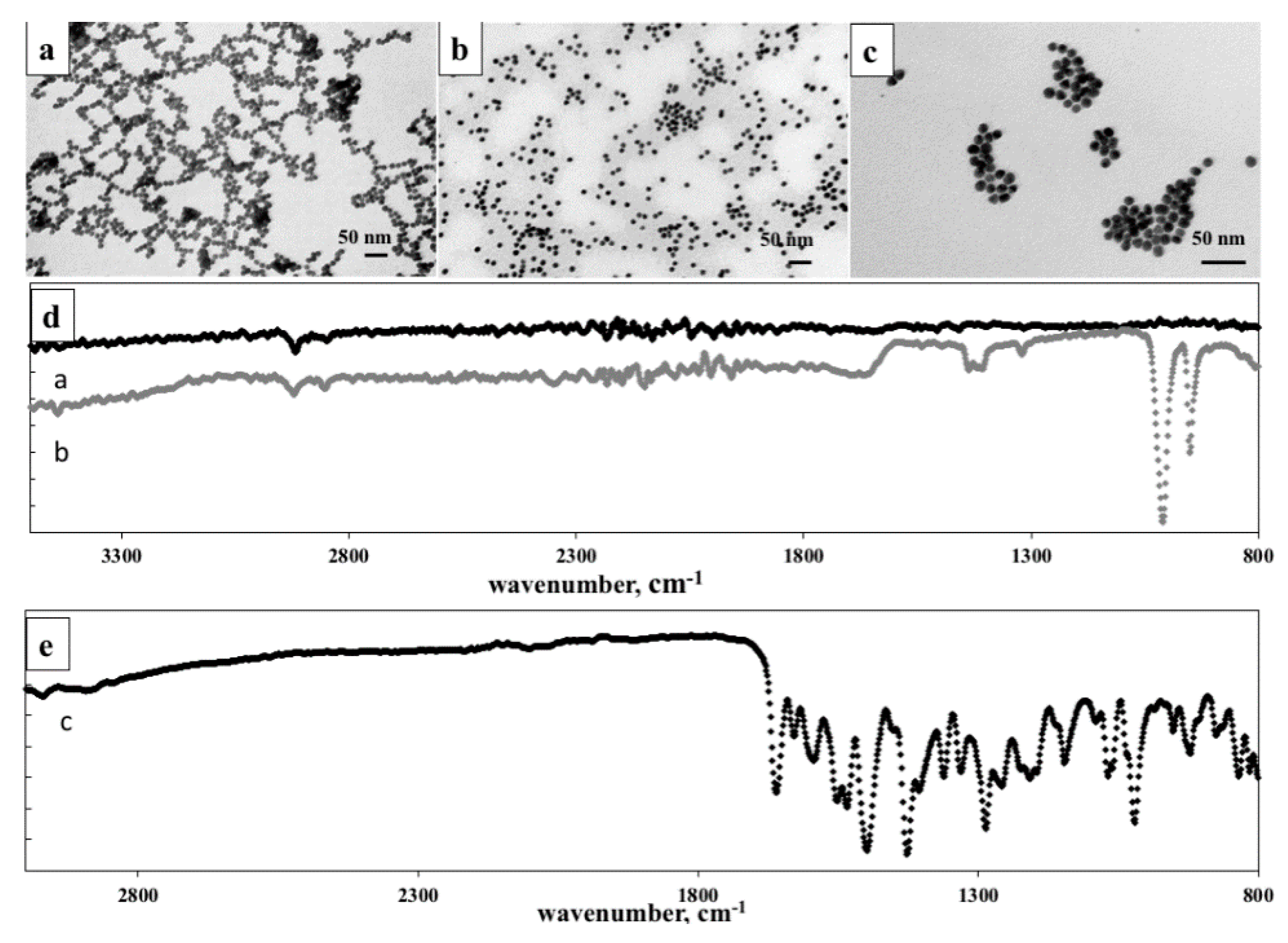
2.7. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.8. Transmission Electron Microscopy (TEM) Analysis
2.9. X-Ray Photoelectron Spectroscopy (XPS) Analysis
2.10. Varlitinib/PEGAuNPs Conjugation Efficiency
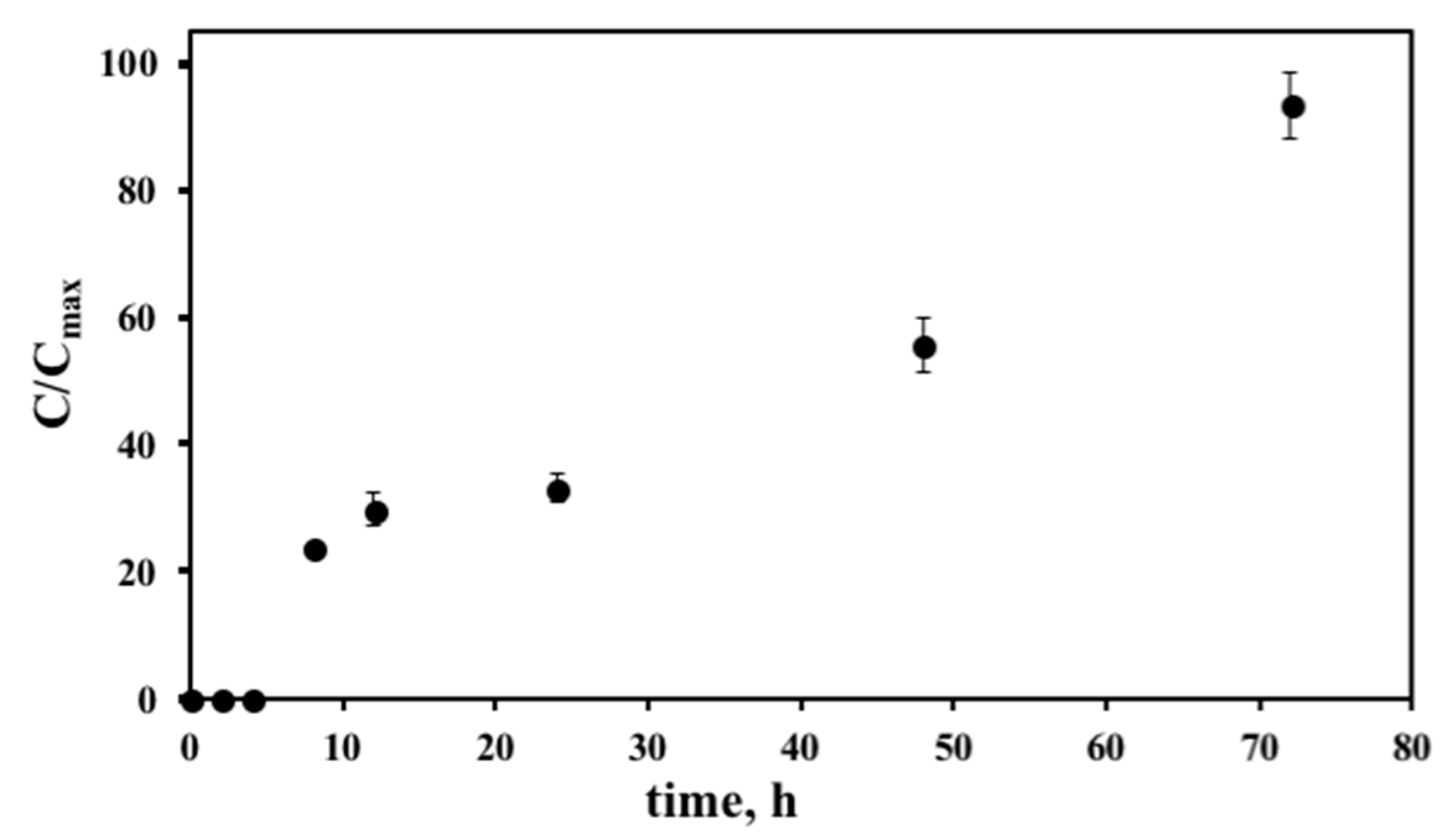
2.11. In Vitro Drug Release Studies
2.12. In Vitro Stability Studies
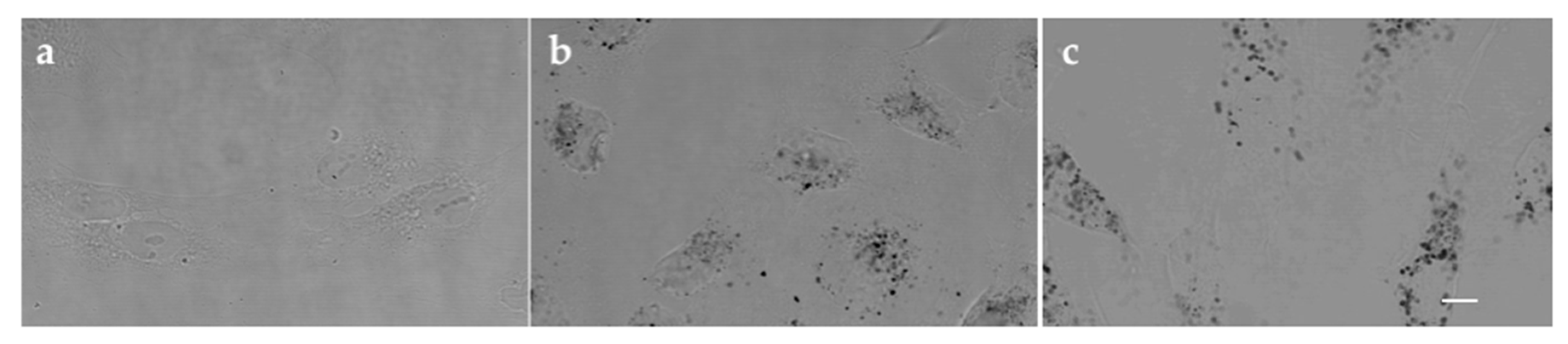
2.13. In Vitro Cytotoxicity Study
2.14. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ellard, S.; Rothenberg, M.; Cohen, R.; Taylor, M.; Hartt, N.; Berlin, J.; Murphy, P.; Kollmannsberger, C.; Maloney, L.; Ptaszynski, M.; et al. Abstract #3603: ARRY-334543 in ErbB2 positive metastatic breast cancer and other ErbB expressing-cancers: Experience from expansion cohorts on a phase I study. Cancer Res. 2009, 69, 3603. [Google Scholar]
- Wang, D.S.; Patel, A.; Sim, H.M.; Zhang, Y.K.; Wang, Y.J.; Kathawala, R.J.; Zhang, H.; Talele, T.T.; Ambudkar, S.V.; Xu, R.H.; et al. ARRY-334543 reverses multidrug resistance by antagonizing the activity of ATP-binding cassette subfamily G member 2. J. Cell. Biochem. 2014, 115, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Miknis, G.; Wallace, E.; Lyssikatos, J.; Lee, P.; Zhao, Q.; Hans, J.; Topalov, G.; Buckmelter, A.; Tarlton, G.; Ren, L.; et al. ARRY-334543, A potent, orally active small molecule inhibitor of EGFR and ErbB-2. Cancer Res. 2005, 65, 801. [Google Scholar]
- Lee, P.; Anderson, D.; Avrutskaya, A.; White, A.; Pheneger, T.; Winkler, J. In vivo activity of ARRY-543, a potent, small molecule inhibitor of EGFR/ErbB-2 in combination with trastuzumab or docetaxel. Cancer Res. 2009, 69, 2150. [Google Scholar] [CrossRef]
- Fedele, C.; Tothill, R.W.; McArthur, G.A. Navigating the challenge of tumor heterogeneity in cancer therapy. Cancer Discov. 2014, 4, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Coelho, J.F.; Ferreira, P.C.; Alves, P.; Cordeiro, R.; Fonseca, A.C.; Góis, J.R.; Gil, M.H. Drug delivery systems: Advanced technologies potentially applicable in personalized treatments. EPMA J. 2010, 1, 164–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, S.C.; Pereira, M.C.; Juzeniene, A.; Juzenas, P.; Coelho, M.A. Supramolecular nanoscale assemblies for cancer diagnosis and therapy. J. Controll. Release 2015, 213, 152–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chidambaram, M.; Manavalan, R.; Kathiresan, K. Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J. Pharm. Pharm. Sci. 2011, 14, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. Gold Nanoparticles: Recent Advances in the Biomedical Applications. Cell Biochem. Biophys. 2015, 72, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Patra, C.R.; Earl, A.; Wang, S.; Katarya, A.; Lu, L.; Kizhakkedathu, J.N.; Yaszemski, M.J.; Greipp, P.R.; Mukhopadhyay, D.; et al. Attaching folic acid on gold nanoparticles using noncovalent interaction via different polyethylene glycol backbones and targeting of cancer cells. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 224–238. [Google Scholar] [CrossRef]
- Aryal, S.; Grailer, J.J.; Pilla, S.; Steeber, D.A.; Gong, S. Doxorubicin conjugated gold nanoparticles as water-soluble and pH-responsive anticancer drug nanocarriers. J. Mater. Chem. 2009, 19, 7879–7884. [Google Scholar] [CrossRef]
- Murugan, M.; Anthony, K.J.P.; Jeyaraj, M.; Rathinam, N.K.; Gurunathan, S. Biofabrication of gold nanoparticles and its biocompatibility in human breast adenocarcinoma cells (MCF-7). J. Ind. Eng. Chem. 2014, 20, 1713–1719. [Google Scholar] [CrossRef]
- Coelho, S.C.; Rocha, S.; Pereira, M.C.; Juzenas, P.; Coelho, M.A. Enhancing proteasome-lnhibitor effect by functionalized gold nanoparticles. J. Biomed. Nanotechnol. 2014, 10, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Duncan, B.; Kim, C.; Rotello, V.M. Gold nanoparticle platforms as drug and biomacromolecule delivery systems. J. Controll. Release 2010, 148, 122–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Goulet, P.J.G.; Lennox, R.B. New Insights into Brust−Schiffrin Metal Nanoparticle Synthesis. J. Am. Chem. Soc. 2010, 132, 9582–9584. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Hirst, D.G.; O’Sullivan, J.M. Gold nanoparticles as novel agents for cancer therapy. Br. J. Radiol. 2012, 85, 101–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chhour, P.; Kim, J.; Benardo, B.; Tovar, A.; Mian, S.; Litt, H.I.; Ferrari, V.A.; Cormode, D.P. Effect of Gold Nanoparticle Size and Coating on Labeling Monocytes for CT Tracking. Bioconjugate Chem. 2017, 28, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Patra, C.R.; Bhattacharya, R.; Wang, E.; Katarya, A.; Lau, J.S.; Dutta, S.; Muders, M.; Wang, S.; Buhrow, S.A.; Safgren, S.L.; et al. Targeted Delivery of Gemcitabine to Pancreatic Adenocarcinoma Using Cetuximab as a Targeting Agent. Cancer Res. 2008, 68, 1970–1978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, S.C.; Almeida, G.M.; Santos-Silva, F.; Pereira, M.C.; Coelho, M.A. Enhancing the efficiency of bortezomib conjugated to pegylated gold nanoparticles: An in vitro study on human pancreatic cancer cells and adenocarcinoma human lung alveolar basal epithelial cells. Expert Opin. Drug Deliv. 2016, 13, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Daduang, J.; Palasap, A.; Daduang, S.; Boonsiri, P.; Suwannalert, P.; Limpaiboon, T. Gallic acid enhancement of gold nanoparticle anticancer activity in cervical cancer cells. Asian Pac. J. Cancer Prev. 2015, 16, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Zhang, Q.; Xu, H.; Yan, Z. Effects of nanoparticle size on antitumor activity of 10-hydroxycamptothecin-conjugated gold nanoparticles: In vitro and in vivo studies. Int. J. Nanomed. 2016, 11, 929–940. [Google Scholar]
- Coelho, S.C.; Almeida, G.M.; Pereira, M.C.; Santos-Silva, F.; Coelho, M.A. Functionalized gold nanoparticles improve afatinib delivery into cancer cells. Expert Opin. Drug Deliv. 2016, 13, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Park, T.G. Folate-receptor-targeted delivery of doxorubicin nano-aggregates stabilized by doxorubicin-PEG-folate conjugate. J. Controll. Release 2004, 100, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Solaro, R.; Chiellini, F.; Battisti, A. Targeted Delivery of Protein Drugs by Nanocarriers. Materials 2010, 3, 1928–1980. [Google Scholar] [CrossRef] [Green Version]
- Hermanson, G.T. Chapter 3—Zero-Length Crosslinkers. In Bioconjugate Techniques, 2nd ed.; Academic Press: New York, NY, USA, 2008; pp. 213–233. [Google Scholar]
- Buchler, P.; Reber, H.A.; Buchler, M.C.; Roth, M.A.; Buchler, M.W.; Friess, H.; Isacoff, W.H.; Hines, O.J. Therapy for pancreatic cancer with a recombinant humanized anti-HER2 antibody (herceptin). J. Gastrointest. Surg. 2001, 5, 139–146. [Google Scholar] [CrossRef]
- Komoto, M.; Nakata, B.; Nishii, T.; Kawajiri, H.; Shinto, O.; Amano, R.; Yamada, N.; Yashiro, M.; Hirakawa, K. In vitro and in vivo evidence that a combination of lapatinib plus S-1 is a promising treatment for pancreatic cancer. Cancer Sci. 2010, 101, 468–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, H.; Grippo, P. Drug Discovery in Pancreatic Cancer: Models and Techniques; Springer: New York, NY, USA, 2010. [Google Scholar]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich Method for Gold Nanoparticle Synthesis Revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S.C.; Rocha, S.; Juzenas, P.; Sampaio, P.; Almeida, G.M.; Silva, F.S.; Pereira, M.C.; Coelho, M.A.N. Gold nanoparticle delivery-enhanced proteasome inhibitor effect in adenocarcinoma cells. Expert Opin. Drug Deliv. 2013, 10, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Baptista, P.; Doria, G.; Henriques, D.; Pereira, E.; Franco, R. Colorimetric detection of eukaryotic gene expression with DNA-derivatized gold nanoparticles. J. Biotechnol. 2005, 119, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Borzenkov, M.; Chirico, G.; D’Alfonso, L.; Sironi, L.; Collini, M.; Cabrini, E.; Dacarro, G.; Milanese, C.; Pallavicini, P.; Taglietti, A.; et al. Thermal and Chemical Stability of Thiol Bonding on Gold Nanostars. Langmuir 2015, 31, 8081–8091. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Gao, Z.; Bae, Y.H. Recent progress in tumor pH targeting nanotechnology. J. Controll. Release 2008, 132, 164–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, J.; Lane, L.A.; Nie, S. Stimuli-responsive nanoparticles for targeting the tumor microenvironment. J. Controll. Release 2015, 219, 205–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, H.-M.; Ma, Y.-Q. Controlling Cellular Uptake of Nanoparticles with pH-Sensitive Polymers. Sci. Rep. 2013, 3, 2804. [Google Scholar] [CrossRef] [PubMed]

| Physical Characteristics | AuNPs | PEGAuNPs | PEGAuNPsVarl |
|---|---|---|---|
| size, nm | 20.0 ± 0.2 | 27 ± 2 | 28 ± 2 |
| polydispersity index | 0.2 | 0.3 | 0.7 |
| zeta potential, mV | −37 ± 3 | −34 ± 1 | −33 ± 1 |
| Element | AuNPs | PEGAuNPs | PEGAuNPsVarl |
|---|---|---|---|
| C 1s | 60.44 | 65.05 | 65.06 |
| N 1s | 0.06 | - | 1.60 |
| Au 4f | 16.77 | 4.82 | 13.80 |
| O 1s | 22.73 | 30.14 | 19.55 |
| Parametric Analysis | MIA PaCa-2 | hTERT-HPNE | ||
|---|---|---|---|---|
| PEGAuNPsVarl | varlitinib | PEGAuNPsVarl | varlitinib | |
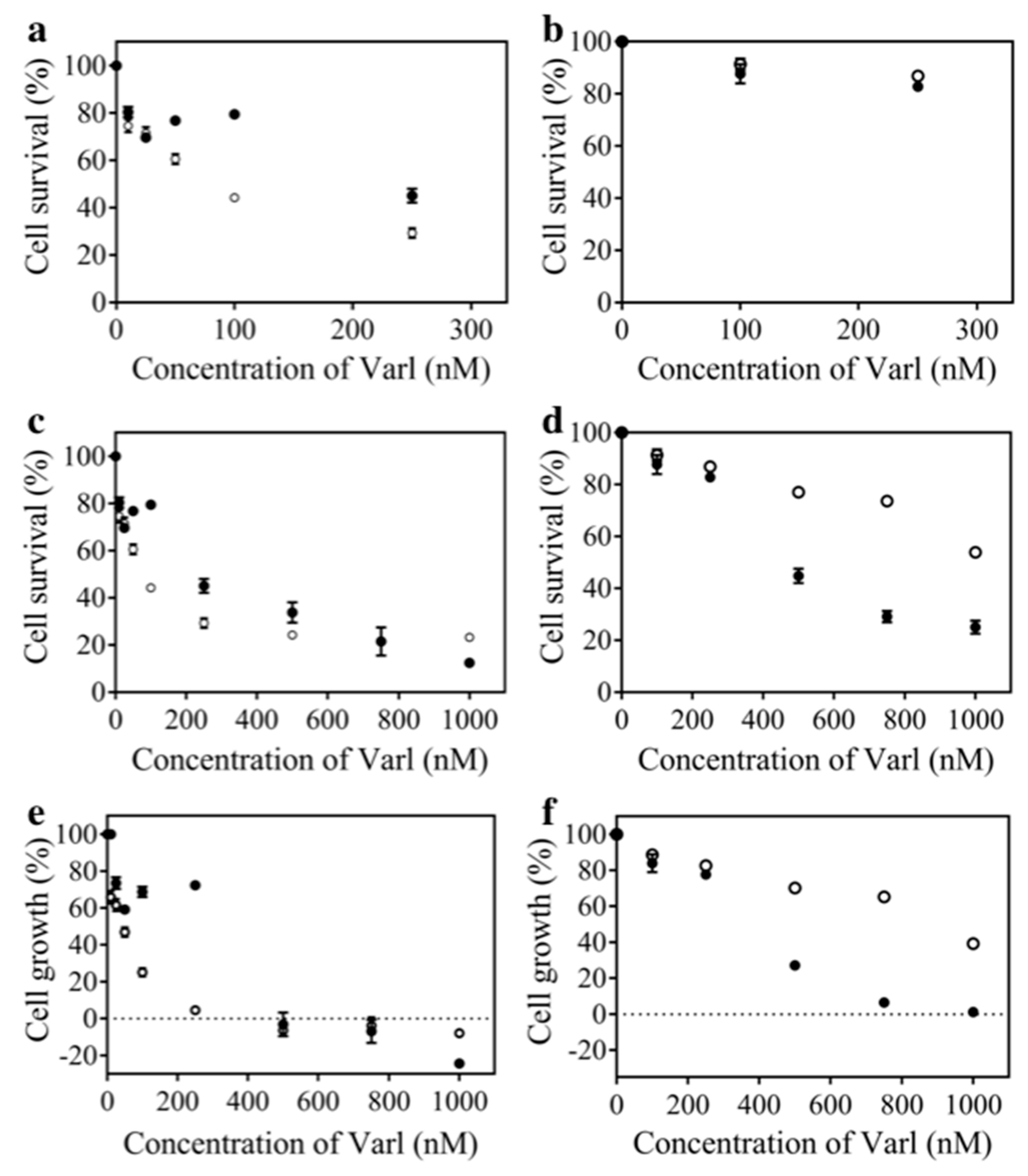
| IC50 (nM) | 80 ± 4 | 259.1 ± 0.4 | 1186 ± 4 | 478 ± 5 |
| GI50 (nM) | 40 ± 1 | 268 ± 7 | 916 ± 3 | 354 ± 5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, S.C.; Reis, D.P.; Pereira, M.C.; Coelho, M.A.N. Gold Nanoparticles for Targeting Varlitinib to Human Pancreatic Cancer Cells. Pharmaceutics 2018, 10, 91. https://doi.org/10.3390/pharmaceutics10030091
Coelho SC, Reis DP, Pereira MC, Coelho MAN. Gold Nanoparticles for Targeting Varlitinib to Human Pancreatic Cancer Cells. Pharmaceutics. 2018; 10(3):91. https://doi.org/10.3390/pharmaceutics10030091
Chicago/Turabian StyleCoelho, Sílvia Castro, Daniel Pires Reis, Maria Carmo Pereira, and Manuel A. N. Coelho. 2018. "Gold Nanoparticles for Targeting Varlitinib to Human Pancreatic Cancer Cells" Pharmaceutics 10, no. 3: 91. https://doi.org/10.3390/pharmaceutics10030091