Synthesis, Structure–Activity Relationships and In Vitro Toxicity Profile of Lactose-Based Fatty Acid Monoesters as Possible Drug Permeability Enhancers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture Conditions
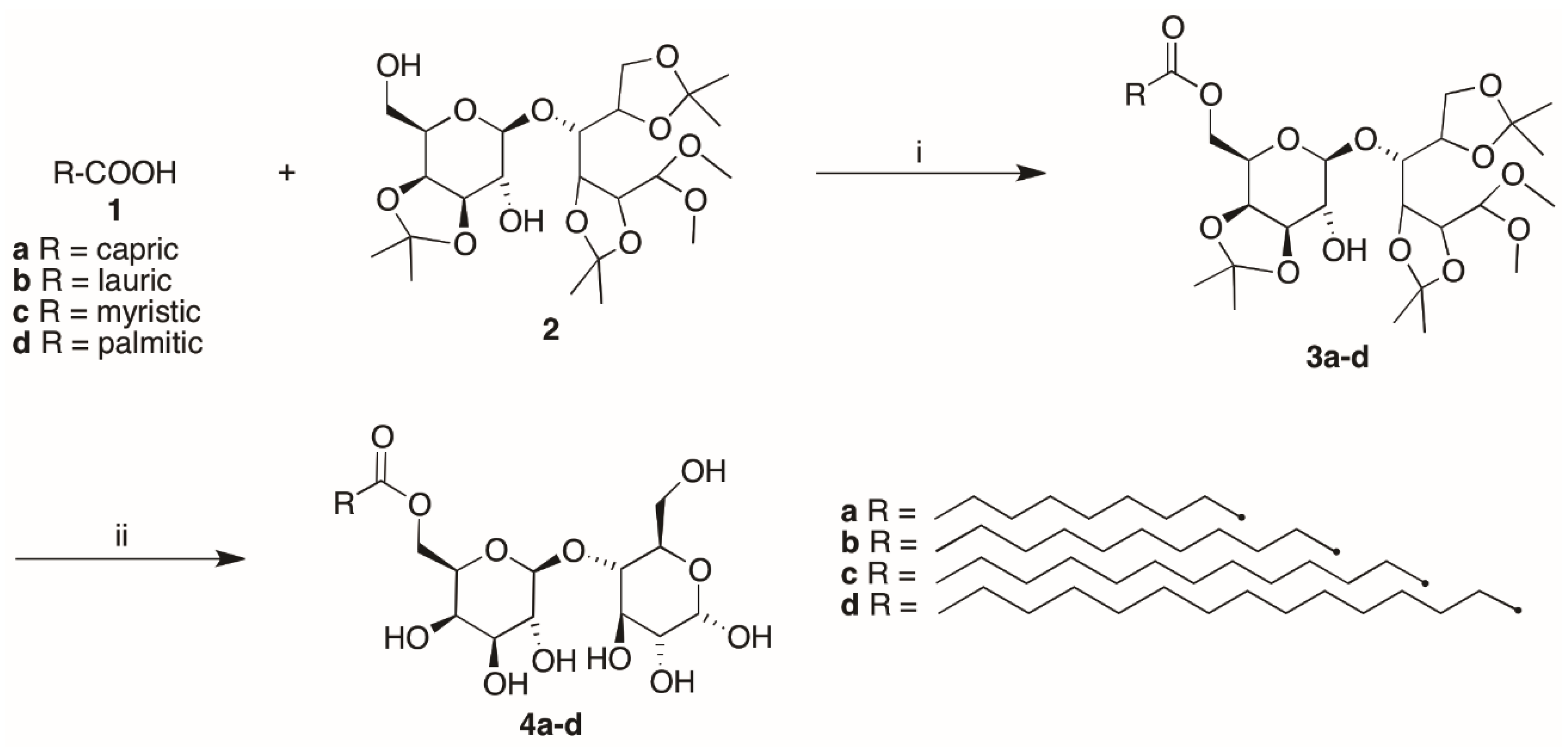
2.3. Synthesis of Lactose-Based Surfactants
2.3.1. General Procedure for the Synthesis of Lactose Tetra Acetal Monoesters
2.3.2. General Procedure for the Synthesis of Lactose Fatty Acid Monoesters
2.4. Surface Tension Measurements
2.5. MTT Cell Viability Assays
2.6. Lactate Dehydrogenase (LDH) Release Assay
2.7. Mitochondrial Membrane Potential (JC-1 Assay)
2.8. Caspase 3/7 Activation (CellEvent™ Assay)
2.9. Nuclear Membrane Permeability (CellTox™ Green Cytotoxicity Assay)
2.10. Measurement of Trans-Epithelial Electrical Resistance (TEER)
3. Results and Discussion
3.1. Surface Tension and CMC Determination
3.2. Biocompatibility Studies
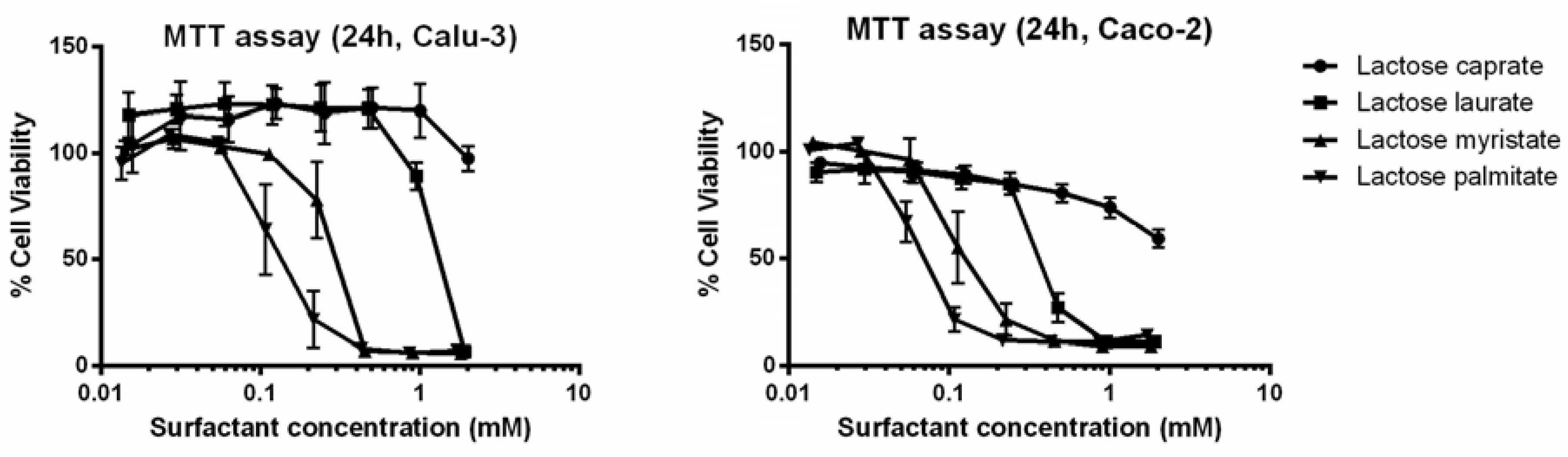
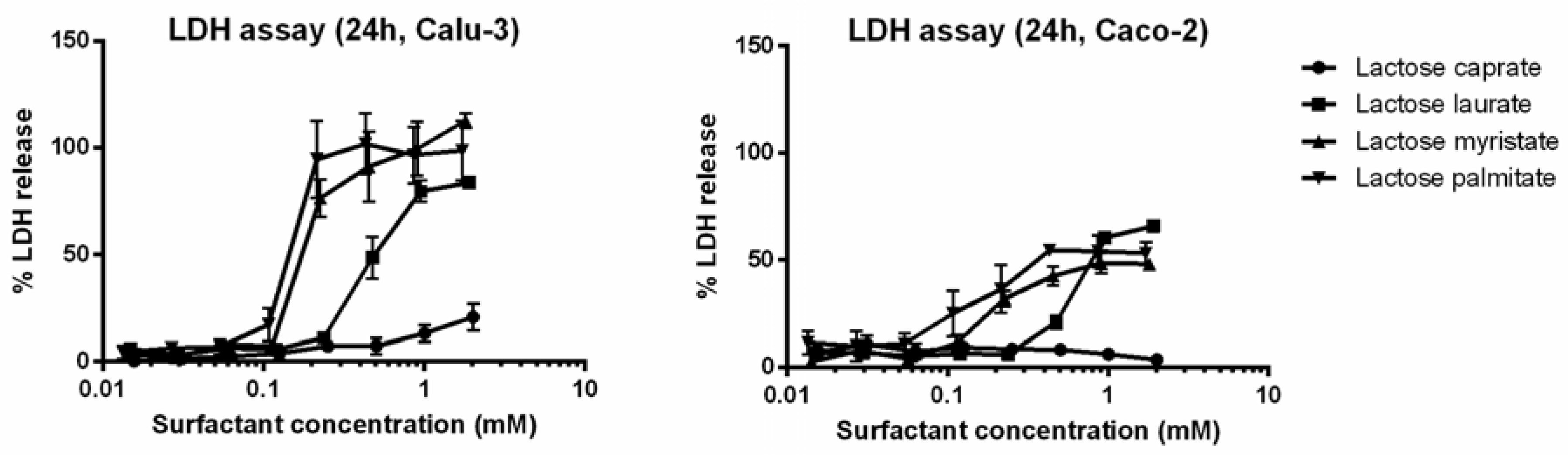
3.2.1. MTT and LDH Assays on Calu-3 and Caco-2 Cells
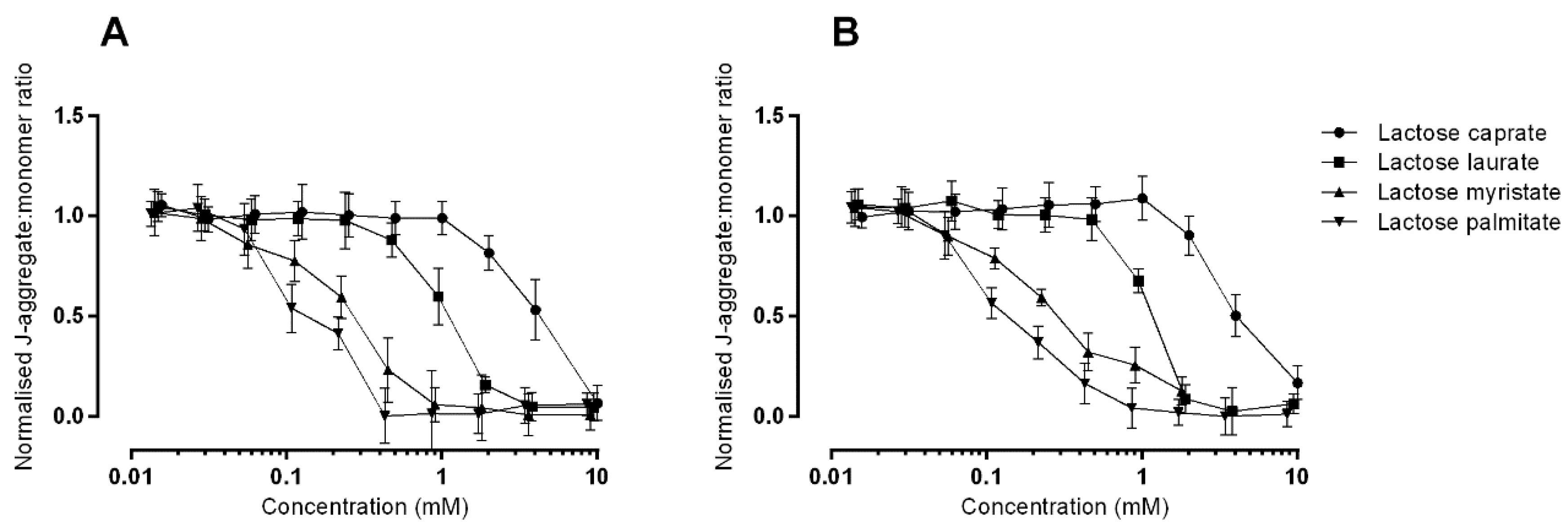
3.2.2. JC-1 Assay to Monitor Mitochondrial Health on Caco-2 and Calu-3
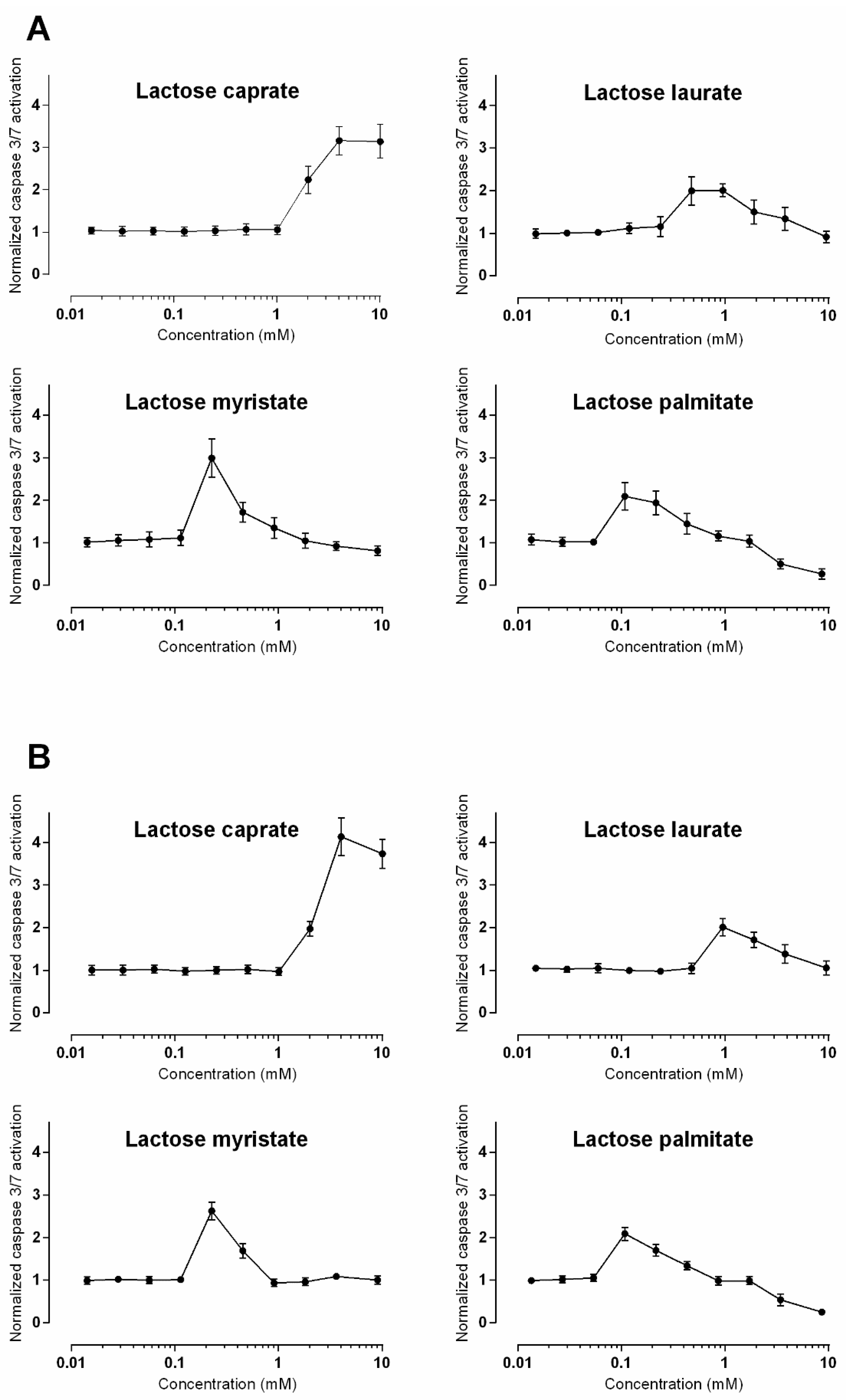
3.2.3. Caspase 3/7 Detection
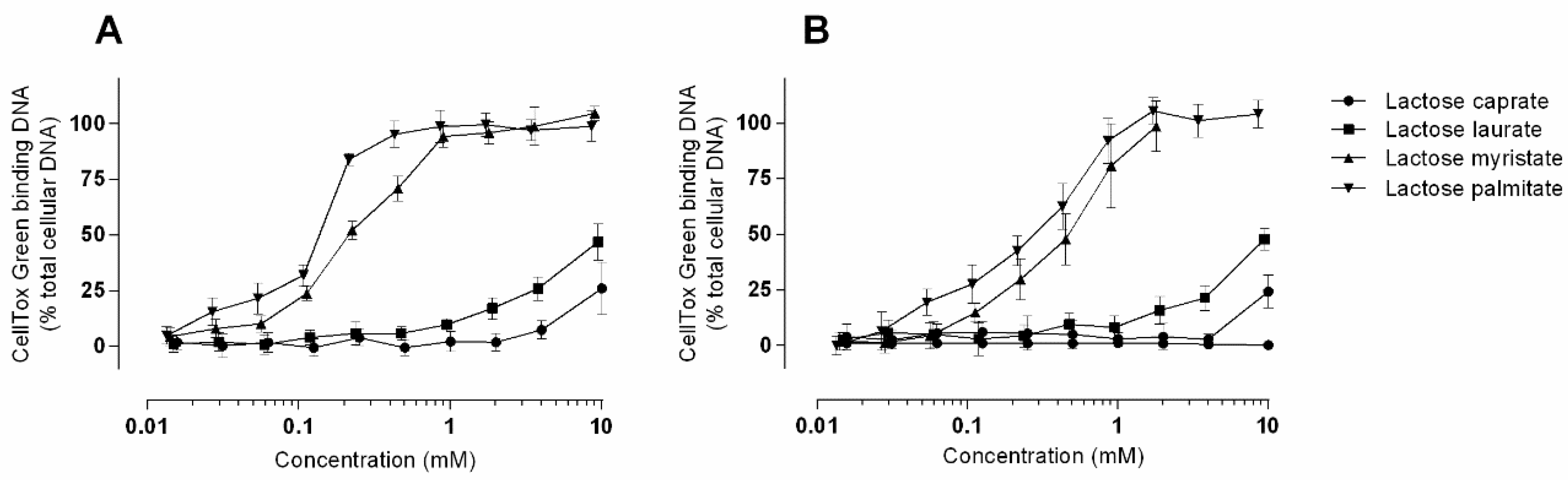
3.2.4. CellToxTM Green Cytotoxicity Assay
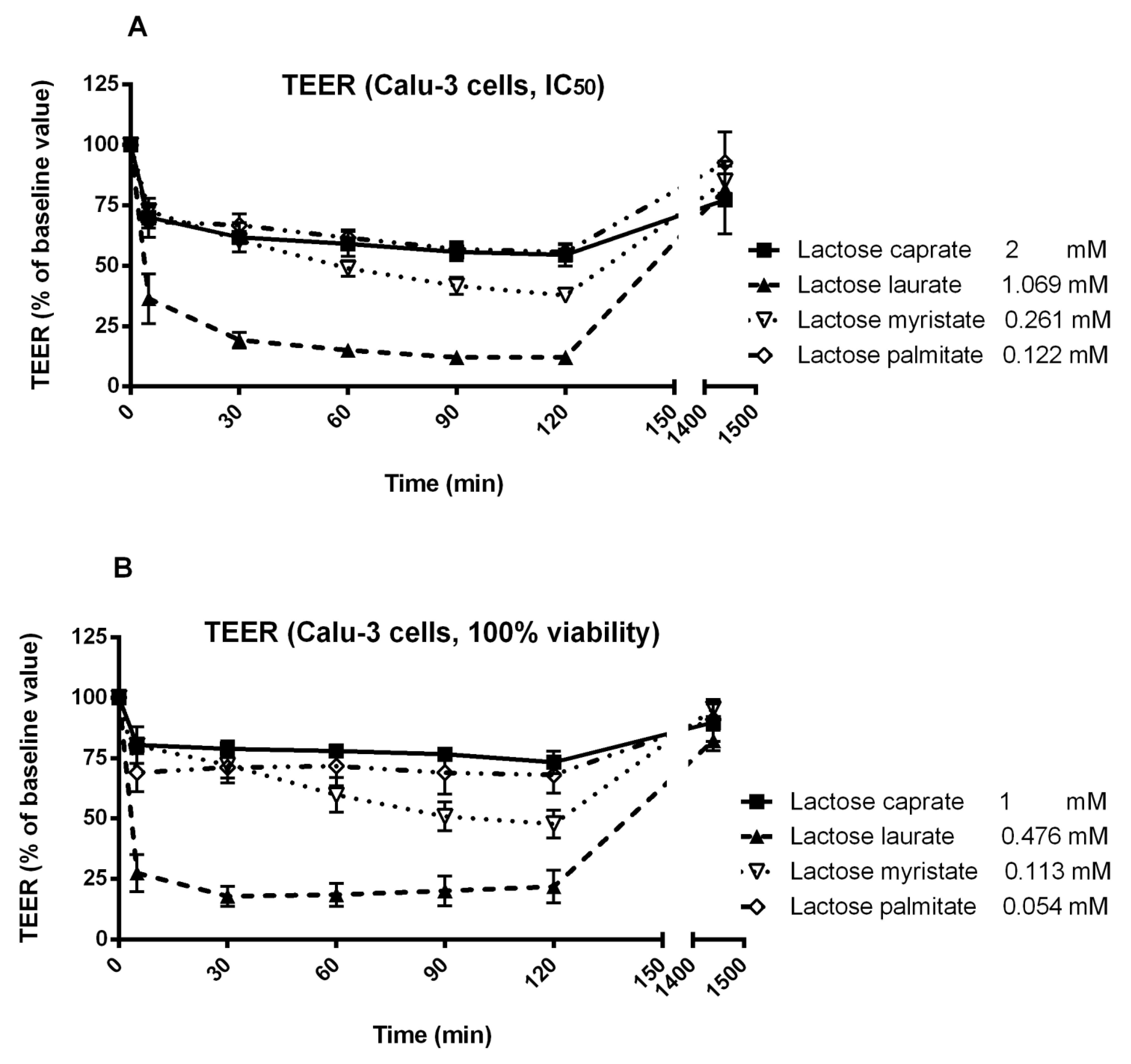
3.3. Trans-Epithelial Electrical Resistance (TEER) Studies in Calu-3 and Caco-2 Cells
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Plou, F.J.; Cruces, M.A.; Ferrer, M.; Fuentes, G.; Pastor, E.; Bernabé, M.; Christensen, M.; Comelles, F.; Parra, J.L.; Ballesteros, A. Enzymatic acylation of di- and trisaccharides with fatty acids: Choosing the appropriate enzyme, support and solvent. J. Biotechnol. 2002, 96, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Hill, K.; Rhode, O. Sugar-based surfactants for consumer products and technical applications. Lipid 1999, 101, 25–33. [Google Scholar] [CrossRef]
- Neta, N.S.; Teixeira, J.A.; Rodrigues, L.R. Sugar Ester Surfactants: Enzymatic Synthesis and Applications in Food Industry. Crit. Rev. Food Sci. Nutr. 2015, 55, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Plat, T.; Linhardt, R.J. Syntheses and applications of sucrose-based esters. J. Surfactants Deterg. 2001, 4, 415–421. [Google Scholar] [CrossRef]
- Szuts, A.; Szabó-Révész, P. Sucrose esters as natural surfactants in drug delivery systems—A mini-review. Int. J. Pharm. 2012, 433, 1–9. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Malik, S.; Ghosh, A.; Saha, R.; Saha, B. A review on natural surfactants. RSC Adv. 2015, 5, 65757–65767. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, W.; Cao, X.; Feng, F. Characterization of enzymatically prepared sugar medium-chain fatty acid monoesters. J. Sci. Food Agric. 2015, 95, 1631–1637. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.-H.; Cai, Y.-Z.; Lin, X.-S.; Xiong, J.; Halling, P.; Yang, Z. Enzymatic Synthesis of Glucose-Based Fatty Acid Esters in Bisolvent Systems Containing Ionic Liquids or Deep Eutectic Solvents. Molecules 2016, 21, 1294. [Google Scholar] [CrossRef] [PubMed]
- Cruces, M.A.; Plou, F.J.; Ferrer, M.; Bernabé, M.; Ballesteros, A. Improved synthesis of sucrose fatty acid monoesters. J. Am. Oil Chem. Soc. 2001, 78, 541–546. [Google Scholar] [CrossRef]
- Zhang, X.; Song, F.; Taxipalati, M.; Wei, W.; Feng, F. Comparative Study of Surface-Active Properties and Antimicrobial Activities of Disaccharide Monoesters. PLoS ONE 2014, 9, e114845. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Comelles, F.; Plou, F.J.; Cruces, M.A.; Fuentes, G.; Parra, J.L.; Ballesteros, A. Comparative Surface Activities of Di- and Trisaccharide Fatty Acid Esters. Langmuir 2002, 18, 667–673. [Google Scholar] [CrossRef]
- Lucarini, S.; Fagioli, L.; Campana, R.; Cole, H.; Duranti, A.; Baffone, W.; Vllasaliu, D.; Casettari, L. Unsaturated fatty acids lactose esters: Cytotoxicity, permeability enhancement and antimicrobial activity. Eur. J. Pharm. Biopharm. 2016, 107, 88–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perinelli, D.R.; Lucarini, S.; Fagioli, L.; Campana, R.; Vllasaliu, D.; Duranti, A.; Casettari, L. Lactose oleate as new biocompatible surfactant for pharmaceutical applications. Eur. J. Pharm. Biopharm. 2018, 124, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Kiss, L.; Hellinger, É.; Pilbat, A.-M.; Kittel, Á.; Török, Z.; Füredi, A.; Szakács, G.; Veszelka, S.; Sipos, P.; Ózsvári, B.; et al. Sucrose esters increase drug penetration, but do not inhibit p-glycoprotein in caco-2 intestinal epithelial cells. J. Pharm. Sci. 2014, 103, 3107–3119. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; Heade, J.; McCartney, F.; Waters, S.; Bleiel, S.B.; Brayden, D.J. Effects of surfactant-based permeation enhancers on mannitol permeability, histology, and electrogenic ion transport responses in excised rat colonic mucosae. Int. J. Pharm. 2018, 539, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, T.A.S.; Rosa, M.; Guterres, S.S.; Pohlmann, A.R.; Coulter, I.; Brayden, D.J. Investigation of coco-glucoside as a novel intestinal permeation enhancer in rat models. Eur. J. Pharm. Biopharm. 2014, 88, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.; Mrsny, R.J.; Brayden, D.J. Intestinal permeation enhancers for oral peptide delivery. Adv. Drug Deliv. Rev. 2016, 106, 277–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCartney, F.; Gleeson, J.P.; Brayden, D.J. Safety concerns over the use of intestinal permeation enhancers: A mini-review. Tissue Barriers 2016, 4, e1176822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touitou, E. Enhancement of intestinal peptide absorption. J. Control. Release 1992, 21, 139–144. [Google Scholar] [CrossRef]
- Ghadiri, M.; Canney, F.; Pacciana, C.; Colombo, G.; Young, P.M.; Traini, D. The use of fatty acids as absorption enhancer for pulmonary drug delivery. Int. J. Pharm. 2018, 541, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Scholnick, F.; Sucharski, M.K.; Linfield, W.M. Lactose-derived surfactants (I) fatty esters of lactose. J. Am. Oil Chem. Soc. 1974, 51, 8–11. [Google Scholar] [CrossRef]
- Staroń, J.; Dąbrowski, J.M.; Cichoń, E.; Guzik, M. Lactose esters: Synthesis and biotechnological applications. Crit. Rev. Biotechnol. 2018, 38, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Drummond, C.J.; Wells, D. Nonionic lactose and lactitol based surfactants: Comparison of some physico-chemical properties. Colloids Surf. A Physicochem. Eng. Asp. 1998, 141, 131–142. [Google Scholar] [CrossRef]
- Becerra, N.; Toro, C.; Zanocco, A.L.; Lemp, E.; Günther, G. Characterization of micelles formed by sucrose 6-O-monoesters. Colloids Surf. A Physicochem. Eng. Asp. 2008, 327, 134–139. [Google Scholar] [CrossRef]
- Neta, N.D.A.S.; dos Santos, J.C.S.; de Oliveira Sancho, S.; Rodrigues, S.; Gonçalves, L.R.B.; Rodrigues, L.R.; Teixeira, J.A. Enzymatic synthesis of sugar esters and their potential as surface-active stabilizers of coconut milk emulsions. Food Hydrocoll. 2012, 27, 324–331. [Google Scholar] [CrossRef] [Green Version]
- Thelwall, L.A.W.; Hough, L.; Richardson, A.C. Sugar Acetals, Their Preparation and Use. U.S. Patent 4,284,763, 2 April 1980. [Google Scholar]
- Sarney, D.B.; Kapeller, H.; Fregapane, G.; Vulfson, E.N. Chemo-enzymatic synthesis of disaccharide fatty acid esters. J. Am. Oil Chem. Soc. 1994, 71, 711–714. [Google Scholar] [CrossRef]
- Mukherjee, I.; Moulik, S.P.; Rakshit, A.K. Tensiometric determination of Gibbs surface excess and micelle point: A critical revisit. J. Colloid Interface Sci. 2013, 394, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Garofalakis, G.; Murray, B.S.; Sarney, D.B. Surface Activity and Critical Aggregation Concentration of Pure Sugar Esters with Different Sugar Headgroups. J. Colloid Interface Sci. 2000, 229, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Vayssade, M.; Miao, Y.; Chagnault, V.; Grand, E.; Wadouachi, A.; Postel, D.; Drelich, A.; Egles, C.; Pezron, I. Physico-chemical properties and cytotoxic effects of sugar-based surfactants: Impact of structural variations. Colloids Surf. B. Biointerfaces 2016, 145, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Turánek, J.; Knötigová, P.; Kudláčková, H.; Mašek, J.; Parkin, S.; Rankin, S.E.; Knutson, B.L.; Lehmler, H.-J. Hydrophobic tail length, degree of fluorination and headgroup stereochemistry are determinants of the biocompatibility of (fluorinated) carbohydrate surfactants. Colloids Surf. B Biointerfaces 2009, 73, 65–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perinelli, D.R.; Casettari, L.; Cespi, M.; Fini, F.; Man, D.K.W.; Giorgioni, G.; Canala, S.; Lam, J.K.W.; Bonacucina, G.; Palmieri, G.F. Chemical–physical properties and cytotoxicity of N-decanoyl amino acid-based surfactants: Effect of polar heads. Colloids Surf. A Physicochem. Eng. Asp. 2016, 492, 38–46. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Cespi, M.; Casettari, L.; Vllasaliu, D.; Cangiotti, M.; Ottaviani, M.F.; Giorgioni, G.; Bonacucina, G.; Palmieri, G.F. Correlation among chemical structure, surface properties and cytotoxicity of N-acyl alanine and serine surfactants. Eur. J. Pharm. Biopharm. 2016, 109, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Sakamuru, S.; Li, X.; Attene-Ramos, M.S.; Huang, R.; Lu, J.; Shou, L.; Shen, M.; Tice, R.R.; Austin, C.P.; Xia, M. Application of a homogenous membrane potential assay to assess mitochondrial function. Physiol. Genom. 2012, 44, 495–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzo, I.; Brenner, C.; Zamzami, N.; Jürgensmeier, J.M.; Susin, S.A.; Vieira, H.L.; Prévost, M.C.; Xie, Z.; Matsuyama, S.; et al. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science 1998, 281, 2027–2031. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial Membrane Permeabilization in Cell Death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patterson, S.D.; Spahr, C.S.; Daugas, E.; Susin, S.A.; Irinopoulou, T.; Koehler, C.; Kroemer, G. Mass spectrometric identification of proteins released from mitochondria undergoing permeability transition. Cell Death Differ. 2000, 7, 137–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Loo, G.; Demol, H.; van Gurp, M.; Hoorelbeke, B.; Schotte, P.; Beyaert, R.; Zhivotovsky, B.; Gevaert, K.; Declercq, W.; Vandekerckhove, J.; et al. A matrix-assisted laser desorption ionization post-source decay (MALDI-PSD) analysis of proteins released from isolated liver mitochondria treated with recombinant truncated Bid. Cell Death Differ. 2002, 9, 301–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inácio, Â.S.; Costa, G.N.; Domingues, N.S.; Santos, M.S.; Moreno, A.J.M.; Vaz, W.L.C.; Vieira, O.V. Mitochondrial dysfunction is the focus of quaternary ammonium surfactant toxicity to mammalian epithelial cells. Antimicrob. Agents Chemother. 2013, 57, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- McDougall, M.; Dwight, S. Nucleic Acid Binding Dyes and Uses Therefor. U.S. Patent 8,598,198 B2, 3 December 2013. [Google Scholar]
- Heerklotz, H. Interactions of surfactants with lipid membranes. Q. Rev. Biophys. 2008, 41, 205–264. [Google Scholar] [CrossRef] [PubMed]
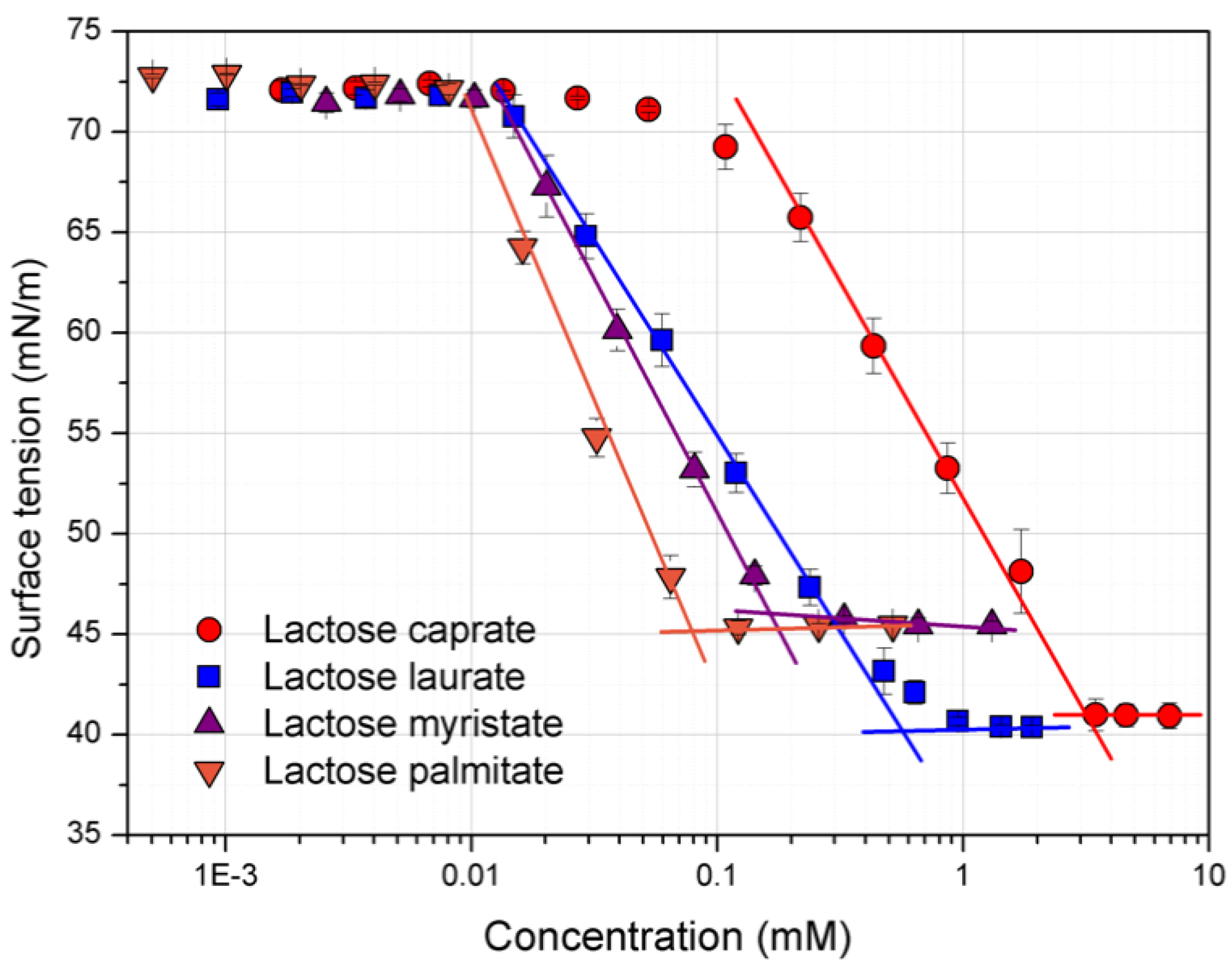

| Entry | CMC (mM) | γCMC (mN/m) | Γmax × 10−6 (mol/m2) | Amin (Å2) |
|---|---|---|---|---|
| Lactose caprate | 2.58 ± 0.32 | 40.6 ± 0.04 | 9.52 ± 0.11 | 17.46 ± 0.25 |
| Lactose laurate | 0.55 ± 0.02 | 40.4 ± 0.02 | 9.52 ± 0.05 | 17.45 ± 0.09 |
| Lactose myristate | 0.14 ± 0.05 | 45.6 ± 0.19 | 8.37 ± 0.46 | 19.86 ± 0.80 |
| Lactose palmitate | 0.08 ± 0.03 | 45.1 ± 0.33 | 6.46 ± 0.11 | 25.69 ± 0.43 |
| Entry | MTT Assay IC50 (mM) | LDH Assay IC50 (mM) | ||
|---|---|---|---|---|
| Calu-3 | Caco-2 | Calu-3 | Caco-2 | |
| Lactose caprate | >2 | >2 | >2 | >2 |
| Lactose laurate | 1.069 | 0.376 | 0.452 | 0.597 |
| Lactose myristate | 0.261 | 0.112 | 0.189 | 0.194 |
| Lactose palmitate | 0.122 | 0.060 | 0.142 | 0.163 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucarini, S.; Fagioli, L.; Cavanagh, R.; Liang, W.; Perinelli, D.R.; Campana, M.; Stolnik, S.; Lam, J.K.W.; Casettari, L.; Duranti, A. Synthesis, Structure–Activity Relationships and In Vitro Toxicity Profile of Lactose-Based Fatty Acid Monoesters as Possible Drug Permeability Enhancers. Pharmaceutics 2018, 10, 81. https://doi.org/10.3390/pharmaceutics10030081
Lucarini S, Fagioli L, Cavanagh R, Liang W, Perinelli DR, Campana M, Stolnik S, Lam JKW, Casettari L, Duranti A. Synthesis, Structure–Activity Relationships and In Vitro Toxicity Profile of Lactose-Based Fatty Acid Monoesters as Possible Drug Permeability Enhancers. Pharmaceutics. 2018; 10(3):81. https://doi.org/10.3390/pharmaceutics10030081
Chicago/Turabian StyleLucarini, Simone, Laura Fagioli, Robert Cavanagh, Wanling Liang, Diego Romano Perinelli, Mario Campana, Snjezana Stolnik, Jenny K. W. Lam, Luca Casettari, and Andrea Duranti. 2018. "Synthesis, Structure–Activity Relationships and In Vitro Toxicity Profile of Lactose-Based Fatty Acid Monoesters as Possible Drug Permeability Enhancers" Pharmaceutics 10, no. 3: 81. https://doi.org/10.3390/pharmaceutics10030081