Nanocrystals of Poorly Soluble Drugs: Drug Bioavailability and Physicochemical Stability
Abstract
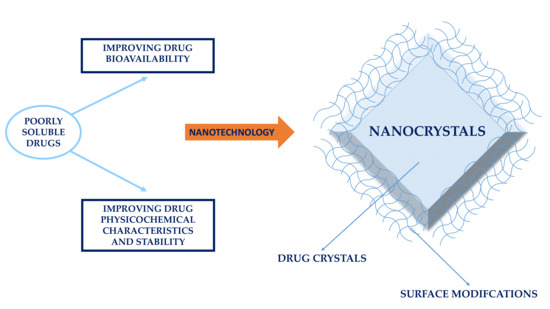
:1. Introduction
- ✓
- ✓
- ✓
- ✓
- ✓
- ✓
- ✓
- Reduced tissue irritation in case of subcutaneous/intramuscular administration [41];
- ✓
- ✓
- Fewer are the disadvantages, such as:
- ✓
- ✓
- ✓
- to overview the more recent information about the improvement in the dissolution rate and bioavailability of poorly soluble drugs, formulated as nanocrystals and administered through different administration routes;
- to review the physicochemical stability-related problems of nanocrystals and the methodological approaches to improve the physicochemical stability of formulations;
- to review the use of nanocrystal surface modifiers for different applications, from physicochemical stabilization to drug delivery and targeting.
2. Production Technologies of Nanocrystals
3. Nanocrystals (Nanosuspensions) and Bioavailability
- An increase in the particle curvature (particularly pronounced for colloidal particles) leads to an increase in dissolution pressure, according to the Kelvin’s equation [96];
- According to several authors, the transcellular uptake of nanocrystals through epithelial cells is another reason for the enhancement of bioavailability [9,102,103,104]. Nevertheless, Gao et al. [105] concluded that results are conflicting and confusing, and no further clarification could be highlighted;
- Nanocrystals can be administered by intravenous injection (nanosuspensions) and are able to efficiently reach the target tissue or organ with 100% bioavailability [10];
3.1. Oral Drug Delivery
3.2. Intravenous Drug Delivery
3.3. Pulmonary Drug Delivery
3.4. Ocular Drug Delivery
3.5. Dermal Drug Delivery
4. Physicochemical Stability
4.1. Particle Agglomeration and Stabilization
4.2. Amorphization and Crystallization
4.3. Particle Surface Modification
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Basic science and product development. J. Pharmacy Pharm. 2010, 62, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Keck, C.M.; Müller, R.H. Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. Eur. J. Pharm. Biopharm. 2006, 62, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, G.P.S.; Marcon, M.; Mocelin, R.; Herrmann, A.P.; Chaves, L.M.; Piato, A.L.; Lanza, M.; Oliveira, J.V. Micronization of n-acetylcysteine by supercritical fluid: Evaluation of in vitro and in vivo biological activity. J. Supercrit. Fluids 2017, 130, 282–291. [Google Scholar] [CrossRef]
- Jermain, S.V.; Brough, C.; Williams, R.O., III. Amorphous solid dispersions and nanocrystal technologies for poorly water-soluble drug delivery—An update. Int. J. Pharm. 2018, 535, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Junghanns, J.-U.A.; Müller, R.H. Nanocrystal technology, drug delivery and clinical applications. Int. J. Nanomed. 2008, 3, 295. [Google Scholar]
- Peltonen, L.; Hirvonen, J. Drug nanocrystals-versatile option for formulation of poorly soluble materials. Int. J. Pharm. 2018, 537, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, Y.; Zhang, J.; Hao, L.; Guo, H.; Lou, H.; Zhang, D. Bexarotene nanocrystal—Oral and parenteral formulation development, characterization and pharmacokinetic evaluation. Eur. J. Pharm. Biopharm. 2014, 87, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Staufenbiel, S.; Rühl, E.; Bodmeier, R. In situ determination of the saturation solubility of nanocrystals of poorly soluble drugs for dermal application. Int. J. Pharm. 2017, 521, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Sun, J.; Ai, X.; Zhang, P.; Li, M.; Wang, Y.; Liu, X.; Sun, Y.; Sui, X.; Sun, L. Nimodipine nanocrystals for oral bioavailability improvement: Role of mesenteric lymph transport in the oral absorption. Int. J. Pharm. 2013, 448, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Ganta, S.; Paxton, J.W.; Baguley, B.C.; Garg, S. Formulation and pharmacokinetic evaluation of an asulacrine nanocrystalline suspension for intravenous delivery. Int. J. Pharm. 2009, 367, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Chen, Y.; Thompson, D.H.; Park, K.; Li, T. Impact of surfactant treatment of paclitaxel nanocrystals on biodistribution and tumor accumulation in tumor-bearing mice. J. Control. Release 2016, 237, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Ige, P.P.; Baria, R.K.; Gattani, S.G. Fabrication of fenofibrate nanocrystals by probe sonication method for enhancement of dissolution rate and oral bioavailability. Colloids Surf. B Biointerfaces 2013, 108, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Vishakante, G.D.; Bathool, A. Development and characterization of pilocarpine loaded eudragit nanosuspensions for ocular drug delivery. J. Biomed. Nanotechnol. 2013, 9, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Han, M.; Tian, F.; Cun, D.; Rantanen, J.; Yang, M. Budesonide nanocrystal-loaded hyaluronic acid microparticles for inhalation: In vitro and in vivo evaluation. Carbohydr. Polym. 2018, 181, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, L.; Liu, F. Paclitaxel nanocrystals for overcoming multidrug resistance in cancer. Mol. Pharm. 2010, 7, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Mauludin, R.; Müller, R.H.; Keck, C.M. Development of an oral rutin nanocrystal formulation. Int. J. Pharm. 2009, 370, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Mitri, K.; Shegokar, R.; Gohla, S.; Anselmi, C.; Müller, R.H. Lutein nanocrystals as antioxidant formulation for oral and dermal delivery. Int. J. Pharm. 2011, 420, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.H.; Keck, C.M. Challenges and solutions for the delivery of biotech drugs—A review of drug nanocrystal technology and lipid nanoparticles. J. Biotechnol. 2004, 113, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Patravale, V.; Date, A.A.; Kulkarni, R. Nanosuspensions: A promising drug delivery strategy. J. Pharm. Pharmacol. 2004, 56, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Shegokar, R.; Singh, K.K. Surface modified nevirapine nanosuspensions for viral reservoir targeting: In vitro and in vivo evaluation. Int. J. Pharm. 2011, 421, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Vidlářová, L.; Romero, G.B.; Hanuš, J.; Štěpánek, F.; Müller, R.H. Nanocrystals for dermal penetration enhancement—Effect of concentration and underlying mechanisms using curcumin as model. Eur. J. Pharm. Biopharm. 2016, 104, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Johnston, K.P.; Williams, R.O., III. Comparison of bioavailability of amorphous versus crystalline itraconazole nanoparticles via pulmonary administration in rats. Eur. J. Pharm. Biopharm. 2010, 75, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Lademann, J.; Keck, C.M.; Müller, R.H. Dermal nanocrystals from medium soluble actives–physical stability and stability affecting parameters. Eur. J. Pharm. Biopharm. 2014, 88, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Hollis, C.P.; Zhang, H.; Sun, L.; Gemeinhart, R.A.; Li, T. Hybrid nanocrystals: Achieving concurrent therapeutic and bioimaging functionalities toward solid tumors. Mol. Pharm. 2011, 8, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Baba, K.; Pudavar, H.E.; Roy, I.; Ohulchanskyy, T.Y.; Chen, Y.; Pandey, R.K.; Prasad, P.N. New method for delivering a hydrophobic drug for photodynamic therapy using pure nanocrystal form of the drug. Mol. Pharm. 2007, 4, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Liversidge, G.G.; Cundy, K.C. Particle size reduction for improvement of oral bioavailability of hydrophobic drugs: I. Absolute oral bioavailability of nanocrystalline danazol in beagle dogs. Int. J. Pharm. 1995, 125, 91–97. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; Sarpotdar, P.; Bruno, J.; Hajj, S.; Wei, L.; Peltier, N.; Rake, J.; Shaw, J.; Pugh, S.; Polin, L. Formulation and antitumor activity evaluation of nanocrystalline suspensions of poorly soluble anticancer drugs. Pharm. Res. 1996, 13, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Moschwitzer, J.; Muller, R.H. Spray coated pellets as carrier system for mucoadhesive drug nanocrystals. Eur. J. Pharm. Biopharm. 2006, 62, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Gao, S.; Dagnæs-Hansen, F.; Jakobsen, M.; Kjems, J. Impact of peg chain length on the physical properties and bioactivity of pegylated chitosan/sirna nanoparticles in vitro and in vivo. ACS Appl. Mater. Interfaces 2017, 9, 12203–12216. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, D.; Chen, M.; Zheng, T.; Wang, S. Preparation and characterization of an oridonin nanosuspension for solubility and dissolution velocity enhancement. Drug Dev. Ind. Pharmacy 2007, 33, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Romero, G.B.; Keck, C.M.; Müller, R.H.; Bou-Chacra, N.A. Development of cationic nanocrystals for ocular delivery. Eur. J. Pharm. Biopharm. 2016, 107, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Shegokar, R.; Müller, R.H. Nanocrystals: Industrially feasible multifunctional formulation technology for poorly soluble actives. Int. J. Pharm. 2010, 399, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Martena, V.; Censi, R.; Hoti, E.; Malaj, L.; Martino, P.D. Physicochemical characterization of nicergoline and cabergoline in its amorphous state. J. Therm. Anal. Calorim. 2012, 108, 323–332. [Google Scholar] [CrossRef]
- Sun, J.; Wang, F.; Sui, Y.; She, Z.; Zhai, W.; Wang, C.; Deng, Y. Effect of particle size on solubility, dissolution rate, and oral bioavailability: Evaluation using coenzyme q10 as naked nanocrystals. Int. J. Nanomed. 2012, 7, 5733. [Google Scholar]
- Yu, L. Amorphous pharmaceutical solids: Preparation, characterization and stabilization. Adv. Drug Deliv. Rev. 2001, 48, 27–42. [Google Scholar] [CrossRef]
- Wang, T.; Qi, J.; Ding, N.; Dong, X.; Zhao, W.; Lu, Y.; Wang, C.; Wu, W. Tracking translocation of self-discriminating curcumin hybrid nanocrystals following intravenous delivery. In. J. Pharm. 2018, 546, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Singh, J.; Verma, A.; Teja, V.; Shukla, R.P.; Singh, S.; Sharma, V.; Konwar, R.; Mishra, P. Hyaluronic acid anchored paclitaxel nanocrystals improves chemotherapeutic efficacy and inhibits lung metastasis in tumor-bearing rat model. RSC Adv. 2016, 6, 73083–73095. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Y.; Wu, W. Injected nanocrystals for targeted drug delivery. Acta Pharm. Sin. B 2016, 6, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Peng, X.; Wang, Y.; Wang, Y.; Shin, D.M.; El-Sayed, M.A.; Nie, S. A reexamination of active and passive tumor targeting by using rod-shaped gold nanocrystals and covalently conjugated peptide ligands. ACS Nano 2010, 4, 5887–5896. [Google Scholar] [CrossRef] [PubMed]
- Pawar, V.K.; Singh, Y.; Meher, J.G.; Gupta, S.; Chourasia, M.K. Engineered nanocrystal technology: In-vivo fate, targeting and applications in drug delivery. J. Control. Release 2014, 183, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Chaubal, M.V. Application of formulation technologies in lead candidate selection and optimization. Drug Discov. Today 2004, 9, 603–609. [Google Scholar] [CrossRef]
- Shi, W.; Zeng, H.; Sahoo, Y.; Ohulchanskyy, T.Y.; Ding, Y.; Wang, Z.L.; Swihart, M.; Prasad, P.N. A general approach to binary and ternary hybrid nanocrystals. Nano Lett. 2006, 6, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Hollis, C.P.; Weiss, H.L.; Leggas, M.; Evers, B.M.; Gemeinhart, R.A.; Li, T. Biodistribution and bioimaging studies of hybrid paclitaxel nanocrystals: Lessons learned of the epr effect and image-guided drug delivery. J. Control. Release 2013, 172, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Sailor, M.J.; Park, J.H. Hybrid nanoparticles for detection and treatment of cancer. Adv. Mater. 2012, 24, 3779–3802. [Google Scholar] [CrossRef] [PubMed]
- Hancock, B.C.; Parks, M. What is the true solubility advantage for amorphous pharmaceuticals? Pharm. Res. 2000, 17, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Merisko-Liversidge, E.; Liversidge, G.G. Nanosizing for oral and parenteral drug delivery: A perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv. Drug Deliv. Rev. 2011, 63, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Trasi, N.S.; Byrn, S.R. Mechanically induced amorphization of drugs: A study of the thermal behavior of cryomilled compounds. AAPS PharmSciTech 2012, 13, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.B.; Kagan, C.R.; Bawendi, M.G. Synthesis and characterization of monodisperse nanocrystals and close-packed nanocrystal asssemblies. Annu. Rev. Mater. Sci. 2000, 30, 545–610. [Google Scholar] [CrossRef]
- Vishal, P.; Abhale, V.N. Nanocrystal technology: A particle engineering formulation strategy for the poorly water soluble drugs. Int. J. Pharm. 2016, 8, 384–392. [Google Scholar]
- Pardeike, J.; Strohmeier, D.M.; Schrodl, N.; Voura, C.; Gruber, M.; Khinast, J.G.; Zimmer, A. Nanosuspensions as advanced printing ink for accurate dosing of poorly soluble drugs in personalized medicines. Int. J. Pharm. 2011, 420, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Martena, V.; Censi, R.; Hoti, E.; Malaj, L.; Di Martino, P. A new nanospray drying method for the preparation of nicergoline pure nanoparticles. J. Nanopart. Res. 2012, 14, 934. [Google Scholar] [CrossRef]
- Bhakay, A.; Rahman, M.; Dave, R.N.; Bilgili, E. Bioavailability enhancement of poorly water-soluble drugs via nanocomposites: Formulation processing aspects and challenges. Pharmaceutics 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Chin, W.W.L.; Parmentier, J.; Widzinski, M.; Tan, E.H.; Gokhale, R. A brief literature and patent review of nanosuspensions to a final drug product. J. Pharm. Sci. 2014, 103, 2980–2999. [Google Scholar] [CrossRef] [PubMed]
- Malamatari, M.; Taylor, K.M.; Malamataris, S.; Douroumis, D.; Kachrimanis, K. Pharmaceutical nanocrystals: Production by wet milling and applications. Drug Discov. Today 2018, 23, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, N.; Deecaraman, M.; Rani, C. Nanosuspension technology and its applications in drug delivery. Asian J. Pharm. 2014, 3. [Google Scholar] [CrossRef]
- Cooper, E.R. Nanoparticles: A personal experience for formulating poorly water soluble drugs. J. Control. Release 2010, 141, 300–302. [Google Scholar] [CrossRef] [PubMed]
- Merisko-Liversidge, E.M.; Liversidge, G.G. Drug nanoparticles: Formulating poorly water-soluble compounds. Toxicol. Pathol. 2008, 36, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Möschwitzer, J. Particle size reduction technologies in the pharmaceutical development process. Am. Pharm. Rev. 2010, 2010, 54–59. [Google Scholar]
- Möschwitzer, J.P. Drug nanocrystals in the commercial pharmaceutical development process. Int. J. Pharm. 2013, 453, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Texter, J. Precipitation and condensation of organic particles. J. Dispers. Sci. Technol. 2001, 22, 499–527. [Google Scholar] [CrossRef]
- Fontana, F.; Figueiredo, P.; Zhang, P.; Hirvonen, J.T.; Liu, D.; Santos, H.A. Production of pure drug nanocrystals and nano co-crystals by confinement methods. Adv. Drug Deliv. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Arzi, R.S.; Sosnik, A. Electrohydrodynamic atomization and spray-drying for the production of pure drug nanocrystals and co-crystals. Adv. Drug Deliv. Rev. 2018. [Google Scholar] [CrossRef]
- Salazar, J.; Müller, R.H.; Möschwitzer, J.P. Combinative particle size reduction technologies for the production of drug nanocrystals. J. Pharm. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Martena, V.; Censi, R.; Hoti, E.; Malaj, L.; Di Martino, P. Indomethacin nanocrystals prepared by different laboratory scale methods: Effect on crystalline form and dissolution behavior. J. Nanopart. Res. 2012, 14, 1275. [Google Scholar] [CrossRef]
- Van Eerdenbrugh, B.; Froyen, L.; Van Humbeeck, J.; Martens, J.A.; Augustijns, P.; Van den Mooter, G. Drying of crystalline drug nanosuspensions—The importance of surface hydrophobicity on dissolution behavior upon redispersion. Eur. J. Pharm. Sci. 2008, 35, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-Y.; Park, C.H.; Lee, J. Effect of polymer molecular weight on nanocomminution of poorly soluble drug. Drug Deliv. 2008, 15, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, J. Effective polymeric dispersants for vacuum, convection and freeze drying of drug nanosuspensions. Int. J. Pharm. 2010, 397, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.-K.; Kwok, P.C.L. Production methods for nanodrug particles using the bottom-up approach. Adv. Drug Deliv. Rev. 2011, 63, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.; Bingham, D.; Juan, L.D.; Tang, K. Production of protein nanoparticles by electrospray drying. J. Aerosol Sci. 1998, 29, 561–574. [Google Scholar] [CrossRef]
- Basa, S.; Muniyappan, T.; Karatgi, P.; Prabhu, R.; Pillai, R. Production and in vitro characterization of solid dosage form incorporating drug nanoparticles. Drug Dev. Ind. Pharm. 2008, 34, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Bhakay, A.; Davé, R.; Bilgili, E. Recovery of bcs class II drugs during aqueous redispersion of core–shell type nanocomposite particles produced via fluidized bed coating. Powder Technol. 2013, 236, 221–234. [Google Scholar] [CrossRef]
- Krull, S.M.; Ammirata, J.; Bawa, S.; Li, M.; Bilgili, E.; Davé, R.N. Critical material attributes of strip films loaded with poorly water-soluble drug nanoparticles: II. Impact of polymer molecular weight. J. Pharm. Sci. 2017, 106, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Krull, S.M.; Moreno, J.; Li, M.; Bilgili, E.; Davé, R.N. Critical material attributes (cmas) of strip films loaded with poorly water-soluble drug nanoparticles: III. Impact of drug nanoparticle loading. Int. J. Pharm. 2017, 523, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Krull, S.M.; Patel, H.V.; Li, M.; Bilgili, E.; Davé, R.N. Critical material attributes (cmas) of strip films loaded with poorly water-soluble drug nanoparticles: I. Impact of plasticizer on film properties and dissolution. Eur. J. Pharm. Sci. 2016, 92, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Sievens-Figueroa, L.; Bhakay, A.; Jerez-Rozo, J.I.; Pandya, N.; Romañach, R.J.; Michniak-Kohn, B.; Iqbal, Z.; Bilgili, E.; Davé, R.N. Preparation and characterization of hydroxypropyl methyl cellulose films containing stable bcs class ii drug nanoparticles for pharmaceutical applications. Int. J. Pharm. 2012, 423, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Susarla, R.; Afolabi, A.; Patel, D.; Bilgili, E.; Davé, R.N. Novel use of superdisintegrants as viscosity enhancing agents in biocompatible polymer films containing griseofulvin nanoparticles. Powder Technol. 2015, 285, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, R.; Eitzlmayr, A.; Matsko, N.; Tetyczka, C.; Khinast, J.; Roblegg, E. Nano-extrusion: A promising tool for continuous manufacturing of solid nano-formulations. Int. J. Pharm. 2014, 477, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Khinast, J.; Baumgartner, R.; Roblegg, E. Nano-extrusion: A one-step process for manufacturing of solid nanoparticle formulations directly from the liquid phase. AAPS PharmSciTech 2013, 14, 601–604. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ioannidis, N.; Gogos, C.; Bilgili, E. A comparative assessment of nanocomposites vs. Amorphous solid dispersions prepared via nanoextrusion for drug dissolution enhancement. Eur. J. Pharm. Biopharm. 2017, 119, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Patil, H.; Feng, X.; Tiwari, R.V.; Lu, J.; Gryczke, A.; Kolter, K.; Langley, N.; Majumdar, S.; Neupane, D. Conjugation of hot-melt extrusion with high-pressure homogenization: A novel method of continuously preparing nanocrystal solid dispersions. AAPS PharmSciTech 2016, 17, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hu, H.; Zhang, H.; Dai, W.; Wang, X.; Wang, X.; Zhang, Q. Effects of pegylated paclitaxel nanocrystals on breast cancer and its lung metastasis. Nanoscale 2015, 7, 10790–10800. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-F.; Zhang, J.-Y.; Shen, Z.-G.; Zhong, J.; Yun, J. Preparation and characterization of amorphous cefuroxime axetil drug nanoparticles with novel technology: High-gravity antisolvent precipitation. Ind. Eng. Chem. Res. 2006, 45, 8723–8727. [Google Scholar] [CrossRef]
- Zhong, J.; Shen, Z.; Yang, Y.; Chen, J. Preparation and characterization of uniform nanosized cephradine by combination of reactive precipitation and liquid anti-solvent precipitation under high gravity environment. Int. J. Pharm. 2005, 30, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, J.-X.; Wang, Q.-A.; Chen, J.-F.; Yun, J. Controlled liquid antisolvent precipitation of hydrophobic pharmaceutical nanoparticles in a microchannel reactor. Ind. Eng. Chem. Res. 2007, 46, 8229–8235. [Google Scholar] [CrossRef]
- Chiou, H.; Li, L.; Hu, T.; Chan, H.K.; Chen, J.F.; Yun, J. Production of salbutamol sulfate for inhalation by high-gravity controlled antisolvent precipitation. Int. J. Pharm. 2007, 331, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Chiou, H.; Chan, H.K.; Heng, D.; Prud’homme, R.K.; Raper, J.A. A novel production method for inhalable cyclosporine a powders by confined liquid impinging jet precipitation. J. Aerosol Sci. 2008, 39, 500–509. [Google Scholar] [CrossRef]
- Kakran, M.; Shegokar, R.; Sahoo, N.G.; Gohla, S.; Li, L.; Muller, R.H. Long-term stability of quercetin nanocrystals prepared by different methods. J. Pharm. Pharmacol. 2012, 64, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Kobierski, S.; Ofori-Kwakye, K.; Müller, R.; Keck, C. Resveratrol nanosuspensions for dermal application–production, characterization, and physical stability. Die Pharm. Int. J. Pharm. Sci. 2009, 64, 741–747. [Google Scholar]
- Lemke, A.; Kiderlen, A.F.; Petri, B.; Kayser, O. Delivery of amphotericin b nanosuspensions to the brain and determination of activity against balamuthia mandrillaris amebas. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.R.; Al Shaal, L.; Müller, R.H.; Keck, C.M. Production and characterization of hesperetin nanosuspensions for dermal delivery. Int. J. Pharm. 2009, 371, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Denny, W.A.; Garg, S. Effect of wet milling process on the solid state of indomethacin and simvastatin. Int. J. Pharm. 2009, 380, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Al Shaal, L.; Shegokar, R.; Müller, R.H. Production and characterization of antioxidant apigenin nanocrystals as a novel uv skin protective formulation. Int. J. Pharm. 2011, 420, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Martena, V.; Censi, R.; Hoti, E.; Malaj, L.; Di Martino, P. Preparation of glibenclamide nanocrystals by a simple laboratory scale ultra cryo-milling. J. Nanopart. Res. 2013, 15, 1712. [Google Scholar] [CrossRef]
- Dressman, J.; Butler, J.; Hempenstall, J.; Reppas, C. The bcs: Where do we go from here? Pharm. Technol. 2001, 25, 68–77. [Google Scholar]
- Noyes, A.A.; Whitney, W.R. The rate of solution of solid substances in their own solutions. J. Am. Chem. Soc. 1897, 19, 930–934. [Google Scholar] [CrossRef]
- Muller, R. Drug nanocrystals of poorly soluble drugs. Encycl. Nanosci. Nanotechnol. 2004, 627–638. [Google Scholar]
- Gao, L.; Zhang, D.; Chen, M. Drug nanocrystals for the formulation of poorly soluble drugs and its application as a potential drug delivery system. J. Nanopart. Res. 2008, 10, 845–862. [Google Scholar] [CrossRef]
- Junyaprasert, V.B.; Morakul, B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian J. Pharm. Sci. 2015, 10, 13–23. [Google Scholar] [CrossRef]
- Moschwitzer, J.; Muller, R. Drug nanocrystals-the universal formulation approach for poorly soluble drugs. Drugs Pharm. Sci. 2007, 166, 71. [Google Scholar]
- Ponchel, G.; Montisci, M.-J.; Dembri, A.; Durrer, C.; Duchêne, D. Mucoadhesion of colloidal particulate systems in the gastro-intestinal tract. Eur. J. Pharm. Biopharm. 1997, 44, 25–31. [Google Scholar] [CrossRef]
- Jacobs, C.; Kayser, O.; Muller, R.H. Production and characterisation of mucoadhesive nanosuspensions for the formulation of bupravaquone. Int. J. Pharm. 2001, 214, 3–7. [Google Scholar] [CrossRef]
- Delie, F. Evaluation of nano-and microparticle uptake by the gastrointestinal tract. Adv. Drug Deliv. Rev. 1998, 34, 221–233. [Google Scholar] [CrossRef]
- Des Rieux, A.; Fievez, V.; Garinot, M.; Schneider, Y.-J.; Préat, V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J. Control. Release 2006, 116, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Grama, C.; Ankola, D.; Kumar, M.R. Poly(lactide-co-glycolide) nanoparticles for peroral delivery of bioactives. Curr. Opin. Colloid Interface Sci. 2011, 16, 238–245. [Google Scholar] [CrossRef]
- Gao, L.; Liu, G.; Ma, J.; Wang, X.; Zhou, L.; Li, X. Drug nanocrystals: In vivo performances. J. Control. Release 2012, 160, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Park, J.Y.; Zhang, Y.; Conwell, C.; Liu, Y.; Bathula, S.R.; Huang, L. Targeted cancer therapy with novel high drug-loading nanocrystals. J. Pharm. Sci. 2010, 99, 3542–3551. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, W.; Huang, Y.; Li, Z.; Jiang, Y.; Xie, Q. Facile encapsulation of hydroxycamptothecin nanocrystals into zein-based nanocomplexes for active targeting in drug delivery and cell imaging. Acta Biomater. 2017, 61, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-L.; John, M.; Lee, S.L.; Tyner, K.M. Development considerations for nanocrystal drug products. AAPS J. 2017, 19, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Sun, J.; Zhang, D.; Li, M.; Wang, Y.; Ling, G.; Liu, X.; Sun, Y.; Sui, X.; Luo, C. Nimodipine nanocrystals for oral bioavailability improvement: Preparation, characterization and pharmacokinetic studies. Colloids Surf. B Biointerfaces 2013, 109, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Sarnes, A.; Kovalainen, M.; Häkkinen, M.R.; Laaksonen, T.; Laru, J.; Kiesvaara, J.; Ilkka, J.; Oksala, O.; Rönkkö, S.; Järvinen, K. Nanocrystal-based per-oral itraconazole delivery: Superior in vitro dissolution enhancement versus sporanox® is not realized in in vivo drug absorption. J. Control. Release 2014, 180, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, I.; Schenck, D.; Bose, S.; Ruegger, C. Optimization of formulation and process parameters for the production of nanosuspension by wet media milling technique: Effect of vitamin e tpgs and nanocrystal particle size on oral absorption. Eur. J. Pharm. Sci. 2012, 47, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Miura, S.; Danjo, K. Universal wet-milling technique to prepare oral nanosuspension focused on discovery and preclinical animal studies—Development of particle design method. Int. J. Pharm. 2011, 405, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Tuomela, A.; Laaksonen, T.; Laru, J.; Antikainen, O.; Kiesvaara, J.; Ilkka, J.; Oksala, O.; Rönkkö, S.; Järvinen, K.; Hirvonen, J. Solid formulations by a nanocrystal approach: Critical process parameters regarding scale-ability of nanocrystals for tableting applications. Int. J. Pharm. 2015, 485, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Hollis, C.P.; Weiss, H.L.; Evers, B.M.; Gemeinhart, R.A.; Li, T. In vivo investigation of hybrid paclitaxel nanocrystals with dual fluorescent probes for cancer theranostics. Pharm. Res. 2014, 31, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Gohla, S.; Keck, C.M. State of the art of nanocrystals—Special features, production, nanotoxicology aspects and intracellular delivery. Eur. J. Pharm. Biopharm. 2011, 78, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Progress in nanomedicine: Approved and investigational nanodrugs. Pharm. Ther. 2017, 42, 742. [Google Scholar]
- Havel, H.A. Where are the nanodrugs? An industry perspective on development of drug products containing nanomaterials. AAPS J. 2016, 18, 1351–1353. [Google Scholar] [CrossRef] [PubMed]
- Gaul, R.; Ramsey, J.M.; Heise, A.; Cryan, S.-A.; Greene, C.M. Nanotechnology approaches to pulmonary drug delivery: Targeted delivery of small molecule and gene-based therapeutics to the lung. In Design of Nanostructures for Versatile Therapeutic Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 221–253. [Google Scholar]
- Araújo, J.; Gonzalez, E.; Egea, M.A.; Garcia, M.L.; Souto, E.B. Nanomedicines for ocular nsaids: Safety on drug delivery. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, S.; Zhang, Y. Advance of the application of nano-controlled release system in ophthalmic drug delivery. Drug Deliv. 2016, 23, 2897–2901. [Google Scholar] [CrossRef] [PubMed]
- Kassem, M.; Rahman, A.A.; Ghorab, M.; Ahmed, M.; Khalil, R. Nanosuspension as an ophthalmic delivery system for certain glucocorticoid drugs. Int. J. Pharm. 2007, 340, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Tuomela, A.; Liu, P.; Puranen, J.; Rönkkö, S.; Laaksonen, T.; Kalesnykas, G.; Oksala, O.; Ilkka, J.; Laru, J.; Järvinen, K. Brinzolamide nanocrystal formulations for ophthalmic delivery: Reduction of elevated intraocular pressure in vivo. Int. J. Pharm. 2014, 467, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R. Nanocrystals for Use in Topical Formulations and Method of Production Thereof. Germany Patent US9114077B2, 17 November 2006. [Google Scholar]
- Bahl, D.; Bogner, R.H. Amorphization of indomethacin by co-grinding with neusilin us2: Amorphization kinetics, physical stability and mechanism. Pharm. Res. 2006, 23, 2317–2325. [Google Scholar] [CrossRef] [PubMed]
- Blagden, N.; de Matas, M.; Gavan, P.T.; York, P. Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates. Adv. Drug Deliv. Rev. 2007, 59, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Martino, P.D.; Magnoni, F.; Peregrina, D.V.; Gigliobianco, M.R.; Censi, R.; Malaj, L. Formation, physicochemical characterization, and thermodynamic stability of the amorphous state of drugs and excipients. Curr. Pharm. Des. 2016, 22, 4959–4974. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Nakanishi, Y.; Danjo, K. One-step preparation of pharmaceutical nanocrystals using ultra cryo-milling technique in liquid nitrogen. Eur. J. Pharm. Sci. 2010, 41, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Willart, J.F.; Descamps, M. Solid state amorphization of pharmaceuticals. Mol. Pharm. 2008, 5, 905–920. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.G.Z.; Law, D.; Schmitt, E.A.; Qiu, Y. Phase transformation considerations during process development and manufacture of solid oral dosage forms. Adv. Drug Deliv. Rev. 2004, 56, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Derjaguin, B.; Landau, L. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Prog. Surf. Sci. 1993, 43, 30–59. [Google Scholar] [CrossRef]
- Verwey, E.J.W.; Overbeek, J.T.G. Long distance forces acting between colloidal particles. Trans. Faraday Soc. 1946, 42, B117–B123. [Google Scholar] [CrossRef]
- Van Eerdenbrugh, B.; Van den Mooter, G.; Augustijns, P. Top-down production of drug nanocrystals: Nanosuspension stabilization, miniaturization and transformation into solid products. Int. J. Pharm. 2008, 364, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, L.; Hirvonen, J. Pharmaceutical nanocrystals by nanomilling: Critical process parameters, particle fracturing and stabilization methods. J. Pharm. Pharmacol. 2010, 62, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Mu, S.; Li, M.; Guo, M.; Yang, W.; Wang, Y.; Li, J.; Fu, Q.; He, Z. Spironolactone nanocrystals for oral administration: Different pharmacokinetic performances induced by stabilizers. Colloids Surf. B Biointerfaces 2016, 147, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-Y.; Yoo, J.Y.; Kwak, H.-S.; Uk Nam, B.; Lee, J. Role of polymeric stabilizers for drug nanocrystal dispersions. Curr. Appl. Phys. 2005, 5, 472–474. [Google Scholar] [CrossRef]
- Liu, P.; Rong, X.; Laru, J.; van Veen, B.; Kiesvaara, J.; Hirvonen, J.; Laaksonen, T.; Peltonen, L. Nanosuspensions of poorly soluble drugs: Preparation and development by wet milling. Int. J. Pharm. 2011, 411, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Hancock, B.C.; Zografi, G. Characteristics and significance of the amorphous state in pharmaceutical systems. J. Pharm. Sci. 1997, 86, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Brough, C.; Williams, R.O., III. Amorphous solid dispersions and nano-crystal technologies for poorly water-soluble drug delivery. Int. J. Pharm. 2013, 453, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Liversidge, G.G.; Cundy, K.C.; Bishop, J.F.; Czekai, D.A.; STWB Inc. Czekai Surface Modified Drug Nanoparticles. U.S. Patent 5,145,684, 25 January 1991. [Google Scholar]
- Kayaert, P.; Van den Mooter, G. Is the amorphous fraction of a dried nanosuspension caused by milling or by drying? A case study with naproxen and cinnarizine. Eur. J. Pharm. Biopharm. 2012, 81, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Shakhtshneider, T.P.; Danède, F.; Capet, F.; Willart, J.F.; Descamps, M.; Paccou, L.; Surov, E.V.; Boldyreva, E.V.; Boldyrev, V.V. Grinding of drugs with pharmaceutical excipients at cryogenic temperatures. J. Therm. Anal. Calorim. 2007, 89, 709–715. [Google Scholar] [CrossRef]
- Xiong, R.; Lu, W.; Li, J.; Wang, P.; Xu, R.; Chen, T. Preparation and characterization of intravenously injectable nimodipine nanosuspension. Int. J. Pharm. 2008, 350, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, S.-J.; Choi, J.-Y.; Yoo, J.Y.; Ahn, C.-H. Amphiphilic amino acid copolymers as stabilizers for the preparation of nanocrystal dispersion. Eur. J. Pharm. Sci. 2005, 24, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, Z.-H.; Li, T.; McNally, H.; Park, K.; Sturek, M. Development and evaluation of transferrin-stabilized paclitaxel nanocrystal formulation. J. Control. Release 2014, 176, 76–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanki, K.; Gangwal, R.P.; Sangamwar, A.T.; Jain, S. Oral delivery of anticancer drugs: Challenges and opportunities. J. Control. Release 2013, 170, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Jacobs, C.; Kayser, O. Nanosuspensions as particulate drug formulations in therapy: Rationale for development and what we can expect for the future. Adv. Drug Deliv. Rev. 2001, 47, 3–19. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, D.; Chen, M.; Duan, C.; Dai, W.; Jia, L.; Zhao, W. Studies on pharmacokinetics and tissue distribution of oridonin nanosuspensions. Int. J. Pharm. 2008, 355, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, K.; Schulz, J.D.; Gauthier, M.A.; Leroux, J.-C. Peg nanocages as non-sheddable stabilizers for drug nanocrystals. ACS Nano 2012, 6, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Shubar, H.M.; Lachenmaier, S.; Heimesaat, M.M.; Lohman, U.; Mauludin, R.; Mueller, R.H.; Fitzner, R.; Borner, K.; Liesenfeld, O. Sds-coated atovaquone nanosuspensions show improved therapeutic efficacy against experimental acquired and reactivated toxoplasmosis by improving passage of gastrointestinal and blood-brain barriers. J. Drug Target. 2011, 19, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.; Mittapelly, N.; Banala, V.T.; Mishra, P.R. Multifunctional glycoconjugate assisted nanocrystalline drug delivery for tumor targeting and permeabilization of lysosomal-mitochondrial membrane. ACS Appl. Mater. Interfaces 2018, 10, 16964–16976. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.M.; Kumar, M.P.; Apte, S. Formulation of nanosuspensions of albendazole for oral administration. Curr. Nanosci. 2008, 4, 53–58. [Google Scholar] [CrossRef]
- Langguth, P.; Hanafy, A.; Frenzel, D.; Grenier, P.; Nhamias, A.; Ohlig, T.; Vergnault, G.; Spahn-Langguth, H. Nanosuspension formulations for low-soluble drugs: Pharmacokinetic evaluation using spironolactone as model compound. Drug Dev. Ind. Pharm. 2005, 31, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Rachmawati, H.; Al Shaal, L.; Müller, R.H.; Keck, C.M. Development of curcumin nanocrystal: Physical aspects. J. Pharm. Sci. 2013, 102, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R. Development of an oral curcumin nanocrystal formulation. J. Nanotechnol. Eng. Med. 2012, 3, 041007. [Google Scholar] [CrossRef]
- Moorthi, C.; Kathiresan, K. Fabrication of highly stable sonication assisted curcumin nanocrystals by nanoprecipitation method. Drug Invent. Today 2013, 5, 66–69. [Google Scholar] [CrossRef]
- Quan, P.; Shi, K.; Piao, H.; Piao, H.; Liang, N.; Xia, D.; Cui, F. A novel surface modified nitrendipine nanocrystals with enhancement of bioavailability and stability. Int. J. Pharm. 2012, 430, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Koradia, K.D.; Parikh, R.H.; Koradia, H.D. Albendazole nanocrystals: Optimization, spectroscopic, thermal and anthelmintic studies. J. Drug Deliv. Sci. Technol. 2018, 43, 369–378. [Google Scholar] [CrossRef]
- Xia, D.; Quan, P.; Piao, H.; Piao, H.; Sun, S.; Yin, Y.; Cui, F. Preparation of stable nitrendipine nanosuspensions using the precipitation-ultrasonication method for enhancement of dissolution and oral bioavailability. Eur. J. Pharm. Sci. 2010, 40, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Quan, P.; Xia, D.; Piao, H.; Piao, H.; Shi, K.; Jia, Y.; Cui, F. Nitrendipine nanocrystals: Its preparation, characterization, and in vitro—In vivo evaluation. AAPS PharmSciTech 2011, 12, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Kakran, M.; Shegokar, R.; Sahoo, N.G.; Al Shaal, L.; Li, L.; Müller, R.H. Fabrication of quercetin nanocrystals: Comparison of different methods. Eur. J. Pharm. Biopharm. 2012, 80, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Kobierski, S.; Ofori-Kwakye, K.; Müller, R.; Keck, C. Resveratrol nanosuspensions: Interaction of preservatives with nanocrystal production. Die Pharm. Int. J. Pharm. Sci. 2011, 66, 942–947. [Google Scholar]
- Bi, Y.; Liu, J.; Wang, J.; Hao, J.; Li, F.; Wang, T.; Sun, H.W.; Guo, F. Particle size control and the interactions between drug and stabilizers in an amorphous nanosuspension system. J. Drug Deliv. Sci. Technol. 2015, 29, 167–172. [Google Scholar] [CrossRef]
- Onoue, S.; Takahashi, H.; Kawabata, Y.; Seto, Y.; Hatanaka, J.; Timmermann, B.; Yamada, S. Formulation design and photochemical studies on nanocrystal solid dispersion of curcumin with improved oral bioavailability. J. Pharm. Sci. 2010, 99, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Döge, N.; Hönzke, S.; Schumacher, F.; Balzus, B.; Colombo, M.; Hadam, S.; Rancan, F.; Blume-Peytavi, U.; Schäfer-Korting, M.; Schindler, A. Ethyl cellulose nanocarriers and nanocrystals differentially deliver dexamethasone into intact, tape-stripped or sodium lauryl sulfate-exposed ex vivo human skin-assessment by intradermal microdialysis and extraction from the different skin layers. J. Control. Release 2016, 242, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Pireddu, R.; Caddeo, C.; Valenti, D.; Marongiu, F.; Scano, A.; Ennas, G.; Lai, F.; Fadda, A.M.; Sinico, C. Diclofenac acid nanocrystals as an effective strategy to reduce in vivo skin inflammation by improving dermal drug bioavailability. Colloids Surf. B Biointerfaces 2016, 143, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Pireddu, R.; Sinico, C.; Ennas, G.; Marongiu, F.; Muzzalupo, R.; Lai, F.; Fadda, A.M. Novel nanosized formulations of two diclofenac acid polymorphs to improve topical bioavailability. Eur. J. Pharm. Sci. 2015, 77, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Dhapte, V.; Pokharkar, V. Polyelectrolyte stabilized antimalarial nanosuspension using factorial design approach. J. Biomed. Nanotechnol. 2011, 7, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Hecq, J.; Deleers, M.; Fanara, D.; Vranckx, H.; Amighi, K. Preparation and characterization of nanocrystals for solubility and dissolution rate enhancement of nifedipine. Int. J. Pharm. 2005, 299, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Hecq, J.; Nollevaux, G.; Deleers, M.; Fanara, D.; Vranckx, H.; Peulen, O.; Dandrifosse, G.; Amighi, K. Nifedipine nanocrystals: Pharmacokinetic evaluation in the rat and permeability studies in caco-2/ht29-5 m21 (co)-cultures. J. Drug Deliv. Sci. Technol. 2006, 16, 437–442. [Google Scholar] [CrossRef]
- Yang, H.; Teng, F.; Wang, P.; Tian, B.; Lin, X.; Hu, X.; Zhang, L.; Zhang, K.; Zhang, Y.; Tang, X. Investigation of a nanosuspension stabilized by soluplus(r) to improve bioavailability. Int. J. Pharm. 2014, 477, 88–95. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Ghosh, I. Identifying the correlation between drug/stabilizer properties and critical quality attributes (cqas) of nanosuspension formulation prepared by wet media milling technology. Eur. J. Pharm. Sci. 2013, 48, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Ahmadipour, S.; Tabbakhian, M.; Ahmadipour, S. Nanocrystalization of pioglitazone by precipitation method. Drug Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Pini, E.; Angioni, G.; Manca, M.L.; Perricci, J.; Sinico, C.; Fadda, A. Nanocrystals as tool to improve piroxicam dissolution rate in novel orally disintegrating tablets. Eur. J. Pharm. Biopharm. 2011, 79, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Iurian, S.; Bogdan, C.; Tomuță, I.; Szabó-Révész, P.; Chvatal, A.; Leucuța, S.E.; Moldovan, M.; Ambrus, R. Development of oral lyophilisates containing meloxicam nanocrystals using qbd approach. Eur. J. Pharm. Sci. 2017, 104, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.H.; Parmentier, J.; Low, A.; Möschwitzer, J.P. Downstream drug product processing of itraconazole nanosuspension: Factors influencing tablet material properties and dissolution of compacted nanosuspension-layered sugar beads. Int. J. Pharm. 2017, 532, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Shariare, M.H.; Sharmin, S.; Jahan, I.; Reza, H.; Mohsin, K. The impact of process parameters on carrier free paracetamol nanosuspension prepared using different stabilizers by antisolvent precipitation method. J. Drug Deliv. Sci. Technol. 2018, 43, 122–128. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Cao, F.; Miao, X.; Chen, T.; Chang, Q.; Zheng, Y. Formulation of 20 (s)-protopanaxadiol nanocrystals to improve oral bioavailability and brain delivery. Int. J. Pharm. 2016, 497, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Tang, D.; Wang, F.; Zhang, J.; Zhang, J.; Wang, J.; Xu, X.; Zhang, J. Enhancing both oral bioavailability and brain penetration of puerarin using borneol in combination with preparation technologies. Drug Deliv. 2017, 24, 422–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Ma, Y.; Xu, J.; Chen, Y.; Xie, J.; Yue, P.; Zheng, Q.; Yang, M. Apolipoproteins adsorption and brain-targeting evaluation of baicalin nanocrystals modified by combination of tween80 and tpgs. Colloids Surf. B Biointerfaces 2017, 160, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Karmali, P.P.; Kotamraju, V.R.; Kastantin, M.; Black, M.; Missirlis, D.; Tirrell, M.; Ruoslahti, E. Targeting of albumin-embedded paclitaxel nanoparticles to tumors. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 73–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias, J.L.; Reddy, L.H.; Couvreur, P. Magnetoresponsive squalenoyl gemcitabine composite nanoparticles for cancer active targeting. Langmuir 2008, 24, 7512–7519. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, J. Folate-targeted drug-delivery systems prepared by nano-comminution. Drug Dev. Ind. Pharm. 2011, 37, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Liang, J.F. Extreme activity of drug nanocrystals coated with a layer of non-covalent polymers from self-assembled boric acid. Sci. Rep. 2016, 6, 38668. [Google Scholar] [CrossRef] [PubMed]
| Technique | Drug | References | ||
|---|---|---|---|---|
| Bottom-Up | Evaporation methods | Spray-drying | Budesonide, nicergolide, indomethacin, cinnarizine, griseofulvin, mebendazole | [14,33,51,64,65] |
| Freeze-drying | Fenofibrate | [12] | ||
| Vacuum-drying | Itraconazole, naproxen, sofalcone, colostazol | [66,67] | ||
| Aerosol flow reactor | Beclomethasone dipropionate | [68] | ||
| Electrospraying | Insulin | [69] | ||
| Fluid bed coating/granulation/drying | Hydrocortisone acetate, ketoconazole, griseofulvin, phenylbutazone | [28,70,71] | ||
| Wet-casting drying | Griseofulvin, naproxen, fenofibrate, | [72,73,74,75,76] | ||
| Nanoextrusion | Phenytoin, efavirenz, griseofulvin | [77,78,79,80] | ||
| Precipitation methods | Solvent–antisolvent precipitation | Paclitaxel | [81] | |
| High gravity precipitation | Cefuroxime axetil, cephradine, azithromycin, danazol, Salbutamol Sulphate | [82,83,84,85] | ||
| Flash Precipitation | Cyclosporine A | [86] | ||
| Sonoprecipitation | Fenofibrate, paclitaxel | [12,81] | ||
| Supercritical Fluid | Apigenin | [9] | ||
| Top-Down | High pressure homogenization | Microfluidification | Bexarotene | [7] |
| Piston gap homogenization | Nimodipine, lutein, asulacrine, baicalein, apigenin, quercetin, hesperetin, resveratrol, indomethacin, hydrocortisone acetate, nevirapine, amphotericin B | [9,10,17,20,24,28,87,88,89,90,91] | ||
| Bead milling | Apigenin, dexamethasone, ibuprofen, tacrolimus, quercetin | [8,87,92] | ||
| Cryomilling | Indomethacin, glibenclamide, ketoconazole, ursodiol, indomethacin, griseofulvin, carbamazepine, piroxicam | [47,64,93] | ||
| Main Instability | Techniques Provoking the Instability | References |
|---|---|---|
| particle aggregation | Wet comminution | [8,9,16,17,20,21,24,28,64,87,92] |
| Lyophilization | ||
| High-pressure homogenization | ||
| Bead milling | ||
| Cavi-precipitation | ||
| Dehydration of the surfactant | ||
| amorphization | Spray-drying | [10,14,16,22,47,64,93,124,125,126,127,128,129] |
| Lyophilization | ||
| Dry milling | ||
| Cryomilling | ||
| Wet milling | ||
| crystallization | Antisolvent | [24,64] |
| High-pressure homogenization | ||
| Nanospray drying underwent |
| Type of Nanocrystal Surface Modifier | Mechanism | Drug—Active Compound | Applied Technology | References |
|---|---|---|---|---|
| Ionic surfactants/charged polymers: sodium cholate, sodium deoxycholate, sodium lauryl sulfate, sodium dodecyl sulfate, sodium poly(ethylene imine), chitosan | Electrostatic repulsion (prevent aggregation) | Albendazole | Nanoprecipitation | [151] |
| Sonication | ||||
| High-pressure homogenization | ||||
| High-speed homogenization | ||||
| Milling | ||||
| Spironolactone | Wet milling | [134] | ||
| High-pressure homogenization | [152] | |||
| Curcumin | High-speed homogenization | [153] | ||
| High-pressure homogenization | [154] | |||
| Nanoprecipitation method | [155] | |||
| Nitrendipine | Precipitation + high-pressure homogenization | [156] | ||
| Rutin | High-pressure homogenization | [16] | ||
| Non ionic surfactant/polymers: celluloses, polyvinyl alcohol, polyvinyl pyrrolidone, polysorbates, pluronic, poloxamers, triblock-copolymers of polyoxyethylene and polyoxypropylene, hydroxypropyl methylcellulose | Steric barrier against aggregation (prevent aggregation) | Albendazole | Nanoprecipitation | [151] |
| Sonication | ||||
| High-pressure homogenization | ||||
| High-speed homogenization | ||||
| Milling | ||||
| Antisolvent precipitation method | [157] | |||
| Nitrendipine | Precipitation + high-pressure homogenization | [158,159] | ||
| Ibuprofen | Wet comminution | [135] | ||
| Naproxen | ||||
| Prednisolone acetate | ||||
| Hydrocortisone acetate | ||||
| Anthracene | ||||
| Itraconazole | Wet comminution/Wet milling | [135,136] | ||
| Indomethacin | Wet milling | [136] | ||
| Quercetin | High-pressure homogenization | [87,160] | ||
| Bead milling | ||||
| Cavi-precipitation | ||||
| Apigenin | Bead milling + high-pressure homogenization | [92] | ||
| Hesperetin | High-pressure homogenization | [90] | ||
| Resveratrol | High-pressure homogenization | [88,161] | ||
| Precipitation | [162] | |||
| Caffeine | Pearl milling | [23] | ||
| Curcumin | High-speed homogenization | [153] | ||
| Wet milling | [163] | |||
| High-pressure homogenization | [154] | |||
| Nanoprecipitation | [155] | |||
| Dexamethasone | Wet milling | [164] | ||
| Diclofenac | Wet milling | [165,166] | ||
| Pyrimethamine | Nanoprecipitation + high-pressure homogenization | [167] | ||
| Nifedipine | High-pressure homogenization | [168,169] | ||
| Spironolactone | Wet milling | [134] | ||
| Alkyl polyglucoside (Plantacare® 2000), hydroxypropyl methyl cellulose (HPMC 2910), polyvinyl pyrrolidone, and poloxamer | Formation of an amorphous solid dispersion at the interface (prevent amorphization) | Apigenin | Bead milling + high-pressure homogenization | [92] |
| Cinnarizine and naproxen | Ball milling | [140] | ||
| Indomethacin | Dry milling | [124] | ||
| Wet milling | [91] | |||
| Fenofibrate | Milling | [170] | ||
| Arginine, amphiphilic amino acid copolymers (albumin, leucin), vitamin E, polyethylene glycol succinate (TPGS), lecithin, hydroxypropyl methyl cellulose, sodium cholic acid | Biological active providing additional functions to nanocrystals (promotion of a stable formulation) | Nimodipine | High-pressure homogenization | [142] |
| Naproxen | Wet comminution | [143,171] | ||
| Amoitone B | High-pressure homogenization | [40] | ||
| Prednisolone, carbamazepine, itraconazole, baicalin, cyclosporine | High-pressure homogenization | [146] | ||
| Paclitaxel | Antisolvent precipitation + sonication | [144] | ||
| Curcumin | High-pressure homogenization | [153] | ||
| Caffeine | Pearl milling | [23] | ||
| Chitosan (amino group), Carbopol | Mucoadhesion (promote absorption) | Hydrocortisone acetate | High-pressure homogenization | [28,146] |
| Buparvaquone | High-pressure homogenization | [101,146] | ||
| Cetylpyridinium chloride (CPC) and benzalkonium chloride (BAC) | Mucoadhesion by the positively charged nanocrystal formulation | Dexamethasone acetate | Wet bead milling | [31] |
| Hyaluronic acid | Mucoadhesion by gelation (promote absorption) | Budesonide | Wet ball milling | [14] |
| Improving long circulation and interaction with specific receptors | Paclitaxel | High-pressure homogenization | [37] | |
| Polyethylene glycol (PEG) | Improving biological stability | Nevirapine | High-pressure homogenization | [20] |
| Pioglitazone | Precipitation | [172] | ||
| Piroxicam | High-pressure homogenisation | [173] | ||
| Meloxicam | High-pressure homogenization | [174] | ||
| Itraconazole | Nanomilling | [175] | ||
| Paracetamol | Precipitation | [176] | ||
| Camptothecin | Three-phase nanoparticle engineering technology (3PNET) | [106] | ||
| Paclitaxel | Antisolvent precipitation + sonication | [81] | ||
| Wet milling | [148] | |||
| Three-phase nanoparticle engineering technology (3PNET) | [106] | |||
| Antisolvent precipitation | [11] | |||
| Surfactants: sodium dodecyl sulfate, polysorbate, sodium cholate, d-α-tocopheryl polyethylene glycol 1000 succinate | Modify the permeation at the blood–brain barrier (promote targeting) | Atovaquone | High-pressure homogenization | [149] |
| 20(S)-protopanaxadiol | Antisolvent precipitation | [177] | ||
| Puerarin | Ultrasonic | [178] | ||
| Amphotericin B | High-pressure homogenization | [89] | ||
| Single-chain variable fragment (ScFv) peptide, amino terminal fragment (ATF) peptide, cyclic RGD peptides, folate ligand (F127), serum albumin, dextran, Crystal lattice, Apo A-I and Apo A-IV, folate-conjugated polydopamine (PFA), chondroitin sulfate A, chimeric DNA molecules, 3-chloro-2-hydroxy-1-propanesulfonic, acid sodium salt hydrate (CPSA) and 4-sulfophenyl isothiocyanate sodium salt monohydrate (4-SPITC), acid folic, apolipoprotein E | Promote the specific interaction with the biological substrate (promote targeting) | Gold nanorods | Precipitation | [39] |
| Baicalin | High-pressure homogenization | [179] | ||
| Paclitaxel and camptothecin | 3-Phase nanoparticle engineering technology (3 PNET) | [106] | ||
| Nevirapine | Cold high-pressure homogenization | [20] | ||
| Paclitaxel | Antisolvent process: sonication | [24,180] | ||
| High-pressure homogenization | [39] | |||
| Hydroxycamptothecin | Antisolvent process | [107] | ||
| Docetaxel | High-pressure homogenizer + heat exchanger | [150] | ||
| Gemcitabine | Nanoprecipitation | [181] | ||
| Naproxen and paclitaxel | Nano-comminution | [182] | ||
| Camptothecin | Precipitation | [183] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gigliobianco, M.R.; Casadidio, C.; Censi, R.; Di Martino, P. Nanocrystals of Poorly Soluble Drugs: Drug Bioavailability and Physicochemical Stability. Pharmaceutics 2018, 10, 134. https://doi.org/10.3390/pharmaceutics10030134
Gigliobianco MR, Casadidio C, Censi R, Di Martino P. Nanocrystals of Poorly Soluble Drugs: Drug Bioavailability and Physicochemical Stability. Pharmaceutics. 2018; 10(3):134. https://doi.org/10.3390/pharmaceutics10030134
Chicago/Turabian StyleGigliobianco, Maria Rosa, Cristina Casadidio, Roberta Censi, and Piera Di Martino. 2018. "Nanocrystals of Poorly Soluble Drugs: Drug Bioavailability and Physicochemical Stability" Pharmaceutics 10, no. 3: 134. https://doi.org/10.3390/pharmaceutics10030134