Hydrogels of Polycationic Acetohydrazone-Modified Phosphorus Dendrimers for Biomedical Applications: Gelation Studies and Nucleic Acid Loading
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
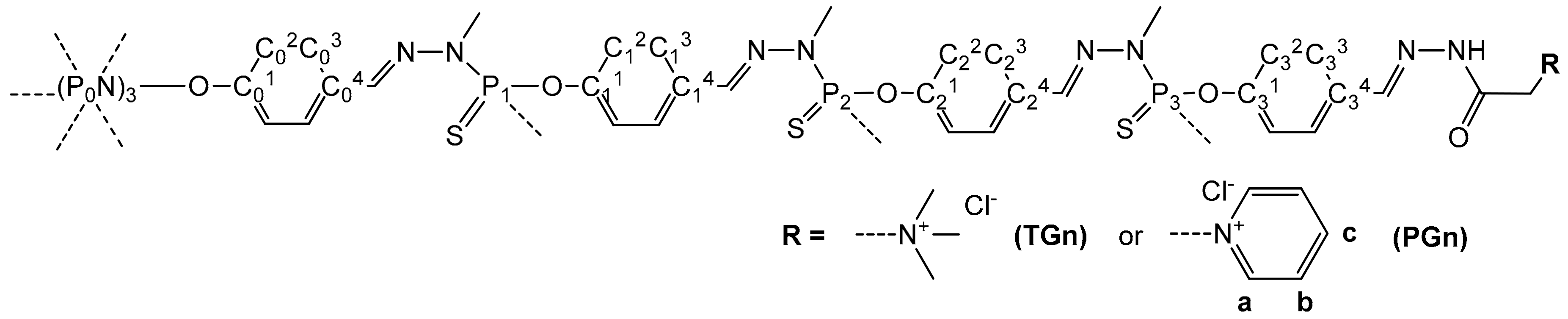
2.2. Synthesis of Girard T, P-Modified Phosphorus Dendrimers
Grafting of Girard Reagents onto the Periphery of Dendrimers: General Procedure
2.3. Preparation of Hydrogels
2.4. Transmission Electron Microscopy
2.5. Fluorescence Intensity Measurements
2.6. Oligonucleotide Binding by Hydrogels
2.7. Oligonucleotide Release from Hydrogels
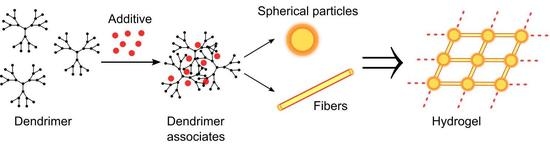
3. Results and Discussion
3.1. Dendrimer Synthesis
3.2. Gelation Studies
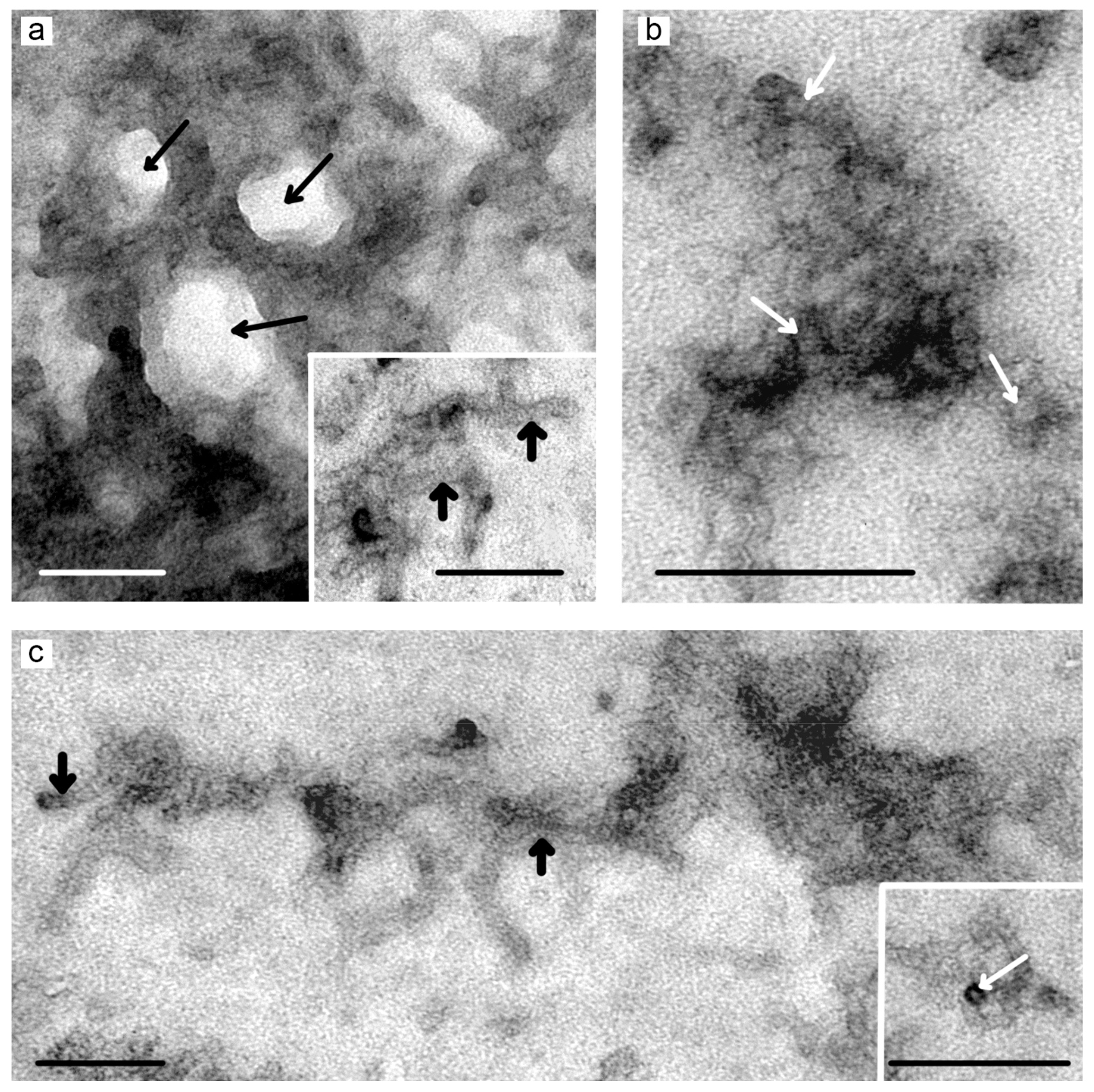
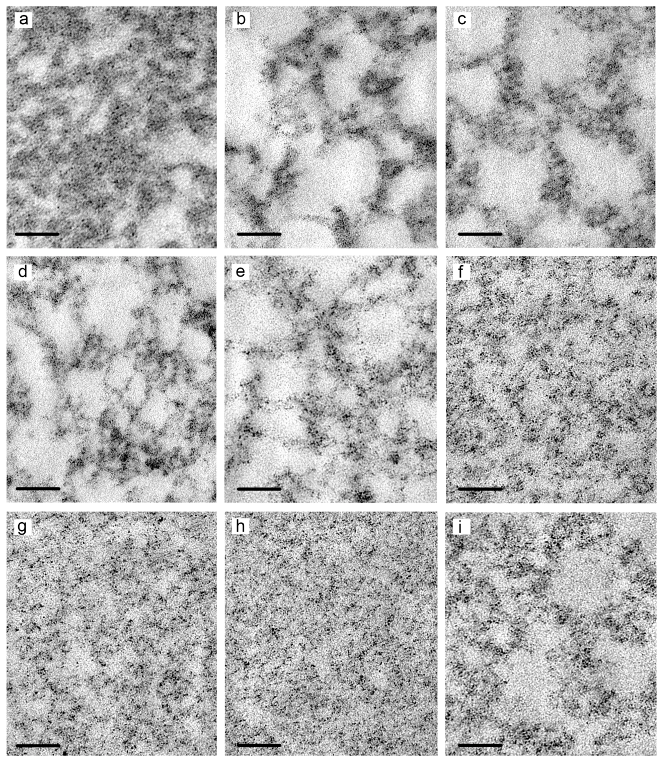
3.3. TEM Studies of Hydrogels
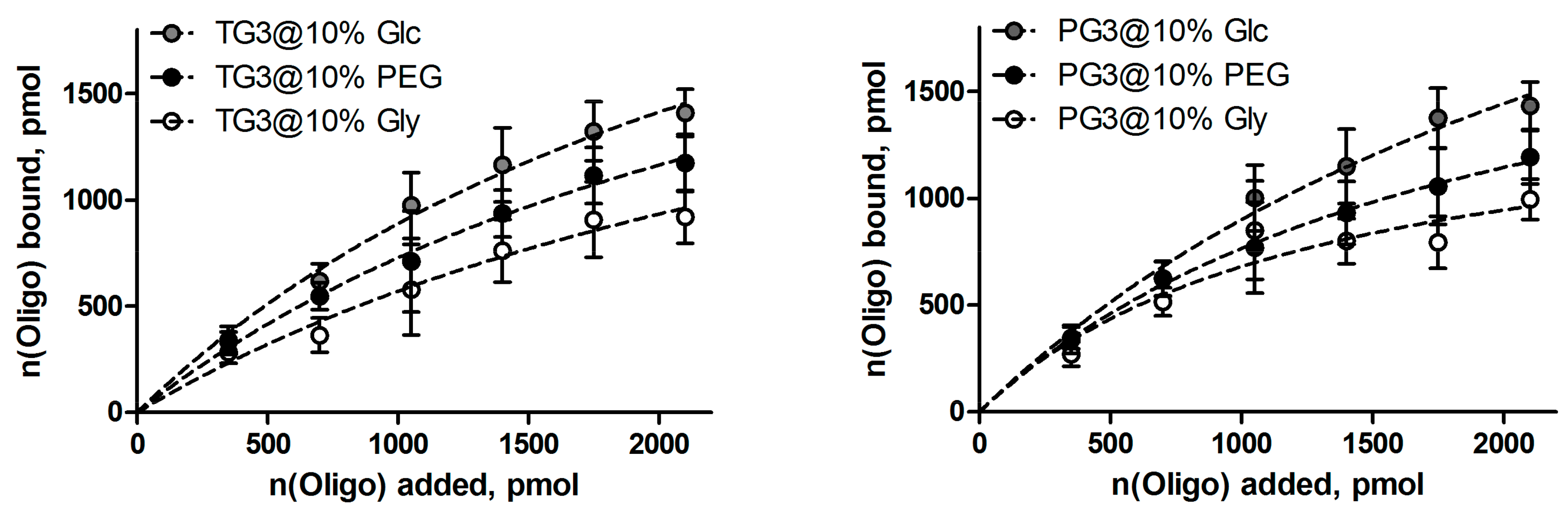
3.4. Oligonucleotide Binding and Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benson, H.A.E.; Watkinson, A.C. (Eds.) Topical and Transdermal Drug Delivery; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; ISBN 9781118140505. [Google Scholar]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. A mini review on hydrogels classification and recent developments in miscellaneous applications. Mater. Sci. Eng. C 2017, 79, 958–971. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Park, E.J.; Na, D.H. Recent progress in dendrimer-based nanomedicine development. Arch. Pharm. Res. 2018, 41, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Kaga, S.; Arslan, M.; Sanyal, R.; Sanyal, A. Dendrimers and dendrons as versatile building blocks for the fabrication of functional hydrogels. Molecules 2016, 21, 497. [Google Scholar] [CrossRef] [PubMed]
- Nummelin, S.; Liljeström, V.; Saarikoski, E.; Ropponen, J.; Nykänen, A.; Linko, V.; Seppälä, J.; Hirvonen, J.; Ikkala, O.; Bimbo, L.M.; Kostiainen, M.A. Self-assembly of amphiphilic Janus dendrimers into mechanically robust supramolecular hydrogels for sustained drug release. Chem. Eur. J. 2015, 21, 14433–14439. [Google Scholar] [CrossRef] [PubMed]
- Retention and Duration of Activity of SPL7013 (VivaGel®) after Vaginal Dosing. Available online: https://clinicaltrials.gov/ct2/show/NCT00740584 (accessed on 5 August 2018).
- Nandy, B.; Saurabh, S.; Sahoo, A.K.; Dixit, N.M.; Maiti, P.K. The SPL7013 dendrimer destabilizes the HIV-1 gp120–CD4 complex. Nanoscale 2015, 7, 18628–18641. [Google Scholar] [CrossRef] [PubMed]
- Pellett Madan, R.; Dezzutti, C.S.; Rabe, L.; Hillier, S.L.; Marrazzo, J.; McGowan, I.; Richardson, B.A.; Herold, B.C. Soluble immune mediators and vaginal bacteria impact innate genital mucosal antimicrobial activity in young women. Am. J. Reprod. Immunol. 2015, 74, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Efficacy and Safety Study of SPL7013 Gel to Prevent the Recurrence of Bacterial Vaginosis (BV). Available online: https://clinicaltrials.gov/ct2/show/NCT02237950 (accessed on 6 August 2018).
- Caminade, A.-M.; Turrin, C.-O.; Majoral, J.-P. Phosphorous Dendrimers in Biology and Nanomedicine: Syntheses, Characterization, and Properties; Pan Stanford Publishing: Singapore, 2018; ISBN 9789814774338. [Google Scholar]
- El Brahmi, N.; El Kazzouli, S.; Mignani, S.M.; Essassi, E.M.; Aubert, G.; Laurent, R.; Caminade, A.-M.; Bousmina, M.M.; Cresteil, T.; Majoral, J.-P. Original multivalent copper(II)-conjugated phosphorus dendrimers and corresponding mononuclear copper(II) complexes with antitumoral activities. Mol. Pharm. 2013, 10, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.M.; El Brahmi, N.; El Kazzouli, S.; Laurent, R.; Ladeira, S.; Caminade, A.-M.; Pedziwiatr-Werbicka, E.; Szewczyk, E.M.; Bryszewska, M.; Bousmina, M.M.; et al. Original multivalent Gold(III) and dual gold(III)–copper(II) conjugated phosphorus dendrimers as potent antitumoral and antimicrobial agents. Mol. Pharm. 2017, 14, 4087–4097. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.-M.; Fruchon, S.; Turrin, C.-O.; Poupot, M.; Ouali, A.; Maraval, A.; Garzoni, M.; Maly, M.; Furer, V.; Kovalenko, V.; et al. The key role of the scaffold on the efficiency of dendrimer nanodrugs. Nat. Commun. 2015, 6, 7722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solassol, J.; Crozet, C.; Perrier, V.; Leclaire, J.; Béranger, F.; Caminade, A.-M.; Meunier, B.; Dormont, D.; Majoral, J.-P.; Lehmann, S. Cationic phosphorus-containing dendrimers reduce prion replication both in cell culture and in mice infected with scrapie. J. Gen. Virol. 2004, 85, 1791–1799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loup, C.; Zanta, M.-A.; Caminade, A.-M.; Majoral, J.-P.; Meunier, B. Preparation of water-soluble cationic phosphorus-containing dendrimers as dna transfecting agents. Chem. Eur. J. 1999, 5, 3644–3650. [Google Scholar] [CrossRef]
- Padié, C.; Maszewska, M.; Majchrzak, K.; Nawrot, B.; Caminade, A.-M.; Majoral, J.-P. Polycationic phosphorus dendrimers: Synthesis, characterization, study of cytotoxicity, complexation of DNA, and transfection experiments. New J. Chem. 2009, 33, 318–326. [Google Scholar] [CrossRef]
- Ionov, M.; Lazniewska, J.; Dzmitruk, V.; Halets, I.; Loznikova, S.; Novopashina, D.; Apartsin, E.; Krasheninina, O.; Venyaminova, A.; Milowska, K.; et al. Anticancer siRNA cocktails as a novel tool to treat cancer cells. Part (A). Mechanisms of interaction. Int. J. Pharm. 2015, 485, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Dzmitruk, V.; Szulc, A.; Shcharbin, D.; Janaszewska, A.; Shcharbina, N.; Lazniewska, J.; Novopashina, D.; Buyanova, M.; Ionov, M.; Klajnert-Maculewicz, B.; et al. Anticancer siRNA cocktails as a novel tool to treat cancer cells. Part (B). Efficiency of pharmacological action. Int. J. Pharm. 2015, 485, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Ihnatsyeu-Kachan, A.; Dzmitruk, V.; Apartsin, E.; Krasheninina, O.; Ionov, M.; Loznikova, S.; Venyaminova, A.; Miłowska, K.; Shcharbin, D.; Mignani, S.; et al. Multi-target inhibition of cancer cell growth by siRNA cocktails and 5-fluorouracil using effective piperidine-terminated phosphorus dendrimers. Colloids Interfaces 2017, 1, 6. [Google Scholar] [CrossRef]
- Marmillon, C.; Gauffre, F.; Gulik-Krzywicki, T.; Loup, C.; Caminade, A.-M.; Majoral, J.-P.; Vors, J.-P.; Rump, E. Organophosphorus dendrimers as new gelators for hydrogels. Angew. Chem. Int. Ed. 2001, 40, 2626–2629. [Google Scholar] [CrossRef]
- Launay, N.; Caminade, A.-M.; Lahana, R.; Majoral, J.-P. A General synthetic strategy for neutral phosphorus-containing dendrimers. Angew. Chem. Int. Ed. 1994, 33, 1589–1592. [Google Scholar] [CrossRef]
- Slany, M.; Bardaji, M.; Casanove, M.-J.; Caminade, A.-M.; Majoral, J.-P.; Chaudret, B. Dendrimer surface chemistry. facile route to polyphosphines and their gold complexes. J. Am. Chem. Soc. 1995, 117, 9764–9765. [Google Scholar] [CrossRef]
- Blais, J.-C.; Turrin, C.-O.; Caminade, A.-M.; Majoral, J.-P. MALDI TOF mass spectrometry for the characterization of phosphorus-containing dendrimers. Scope and limitations. Anal. Chem. 2000, 72, 5097–5105. [Google Scholar] [CrossRef] [PubMed]
- Apartsin, E.K.; Venyaminova, A.G.; Mignani, S.; Caminade, A.-M.; Majoral, J.-P. Synthesis of dissymmetric phosphorus dendrimers using an unusual protecting group. New J. Chem. 2018. [Google Scholar] [CrossRef]
- Gutierrez-Ulloa, C.E.; Buyanova, M.Y.; Apartsin, E.K.; Venyaminova, A.G.; de la Mata, F.J.; Gómez, R. Carbon nanotubes decorated with cationic carbosilane dendrons and their hybrids with nucleic acids. ChemNanoMat 2018, 4, 220–230. [Google Scholar] [CrossRef]
- El Ghzaoui, A.; Gauffre, F.; Caminade, A.-M.; Majoral, J.-P.; Lannibois-Drean, H. Self-assembly of water-soluble dendrimers into thermoreversible hydrogels and macroscopic fibers. Langmuir 2004, 20, 9348–9353. [Google Scholar] [CrossRef] [PubMed]
- Gulrez, S.K.H.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of preparation, characterisation and applications. In Progress in Molecular and Environmental Bioengineering—From Analysis and Modeling to Technology Applications; Capri, A., Ed.; InTech Open: London, UK, 2011; pp. 117–150. [Google Scholar]
- Hernandez-Lopez, J.-L.; Khor, H.L.; Caminade, A.-M.; Majoral, J.-P.; Mittler, S.; Knoll, W.; Kim, D.H. Bioactive multilayer thin films of charged N,N-disubstituted hydrazine phosphorus dendrimers fabricated by layer-by-layer self-assembly. Thin Solid Films 2008, 516, 1256–1264. [Google Scholar] [CrossRef]


| Composition (Dendrimer, Dispersant) 1 | Gelation Time, h | Dendrimer Content, wt % | Hydration (103 Water Molecules Per Dendrimer Molecule) | Appearance and Properties |
|---|---|---|---|---|
| TG1 | ||||
| 10% PEG in water | 350 | Incomplete gelation | White rigid gel | |
| 10% Glc in PBS | 260 | 6.7 | 3.3 | White rigid gel |
| 10% Gly in PBS | 260 | 4.0 | 5.6 | White rigid gel |
| TG2 | ||||
| 10% Glc in water | 60 | 2.3 | 22.3 | White rigid gel |
| 10% Gly in water | 350 | Partial gelation | Light yellow transparent gel | |
| 10% PEG in water | 350 | Incomplete gelation | White rigid gel | |
| 10% Glc in PBS | 60 | 2.6 | 19.2 | White rigid gel |
| 10% Gly in PBS | 350 | Partial gelation | Light yellow gel | |
| TG3 | ||||
| 10% Glc in water | 60 | 2.4 | 44.4 | White rigid gel |
| 10% Gly in water | 350 | Partial gelation | Light yellow gel | |
| 10% PEG in water | 330 | 5.9 | 17.6 | Light yellow transparent rigid gel |
| PBS | 350 | Partial gelation | Light yellow gel | |
| 10% Glc in PBS | 60 | 2.8 | 38.9 | Milky white rigid gel |
| 10% Gly in PBS | 105 | 5.8 | 18.2 | White rigid gel |
| 10% PEG in PBS | 350 | Incomplete gelation | White rigid gel | |
| PG1 | ||||
| Water | 350 | Partial gelation | Light yellow transparent gel | |
| 10% Glc in water | 350 | Incomplete gelation | Yellow rigid gel | |
| 10% Gly in water | 105 | 4.2 | 5.2 | Light yellow transparent gel; semifluid, sticky |
| 10% PEG in water | 260 | 4.2 | 5.6 | Light yellow transparent gel |
| PBS | 350 | Incomplete gelation | White rigid gel | |
| 10% Glc in PBS | 60 | 2.6 | 9.0 | Milky white rigid gel |
| 10% Gly in PBS | 60 | 2.7 | 8.8 | Light yellow transparent gel; incoherent |
| 10% PEG in PBS | 230 | 6.7 | 3.4 | Milky white rigid gel |
| PG2 | ||||
| Water | 350 | Partial gelation | Colourless gel | |
| 10% Glc in water | 60 | 2.5 | 21.6 | Milky white rigid gel |
| 10% Gly in water | 135 | 4.8 | 11.0 | Light yellow transparent rigid gel |
| 10% PEG in water | 230 | 3.1 | 17.6 | Light yellow rigid gel |
| PBS | 60 | 2.8 | 21.5 | Milky white rigid gel |
| 10% Glc in PBS | 60 | 2.9 | 18.8 | Milky white rigid gel |
| 10% Gly in PBS | 60 | 2.7 | 20.0 | Light yellow transparent gel; incoherent |
| 10% PEG in PBS | 350 | Incomplete gelation | White rigid gel | |
| PG3 | ||||
| Water | 350 | Partial gelation | Colourless gel | |
| 10% Glc in water | 60 | 2.5 | 45.6 | White rigid gel |
| 10% Gly in water | 90 | 3.4 | 33.3 | Light yellow transparent rigid gel |
| 10% PEG in water | 350 | Incomplete gelation | Colourless gel | |
| PBS | 60 | 2.8 | 40.4 | Milky white rigid gel |
| 10% Glc in PBS | 70 | 2.7 | 42.7 | Milky white rigid gel |
| 10% Gly in PBS | 60 | 2.8 | 40.4 | Light yellow transparent rigid gel |
| 10% PEG in PBS | 280 | 2.2 | 51.9 | Milky white rigid gel |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apartsin, E.K.; Grigoryeva, A.E.; Malrin-Fournol, A.; Ryabchikova, E.I.; Venyaminova, A.G.; Mignani, S.; Caminade, A.-M.; Majoral, J.-P. Hydrogels of Polycationic Acetohydrazone-Modified Phosphorus Dendrimers for Biomedical Applications: Gelation Studies and Nucleic Acid Loading. Pharmaceutics 2018, 10, 120. https://doi.org/10.3390/pharmaceutics10030120
Apartsin EK, Grigoryeva AE, Malrin-Fournol A, Ryabchikova EI, Venyaminova AG, Mignani S, Caminade A-M, Majoral J-P. Hydrogels of Polycationic Acetohydrazone-Modified Phosphorus Dendrimers for Biomedical Applications: Gelation Studies and Nucleic Acid Loading. Pharmaceutics. 2018; 10(3):120. https://doi.org/10.3390/pharmaceutics10030120
Chicago/Turabian StyleApartsin, Evgeny K., Alina E. Grigoryeva, Audrey Malrin-Fournol, Elena I. Ryabchikova, Alya G. Venyaminova, Serge Mignani, Anne-Marie Caminade, and Jean-Pierre Majoral. 2018. "Hydrogels of Polycationic Acetohydrazone-Modified Phosphorus Dendrimers for Biomedical Applications: Gelation Studies and Nucleic Acid Loading" Pharmaceutics 10, no. 3: 120. https://doi.org/10.3390/pharmaceutics10030120