Tailoring Formulations for Intranasal Nose-to-Brain Delivery: A Review on Architecture, Physico-Chemical Characteristics and Mucociliary Clearance of the Nasal Olfactory Mucosa
Abstract
:1. CNS Drug Delivery
1.1. Chalenges in CNS Drug Delivery
1.2. Intranasal CNS Delivery and Some Clinical Evidences of Its Applicability
1.3. Importance of CNS Drug Delivery
2. Drug Delivery via the Nasal Cavity
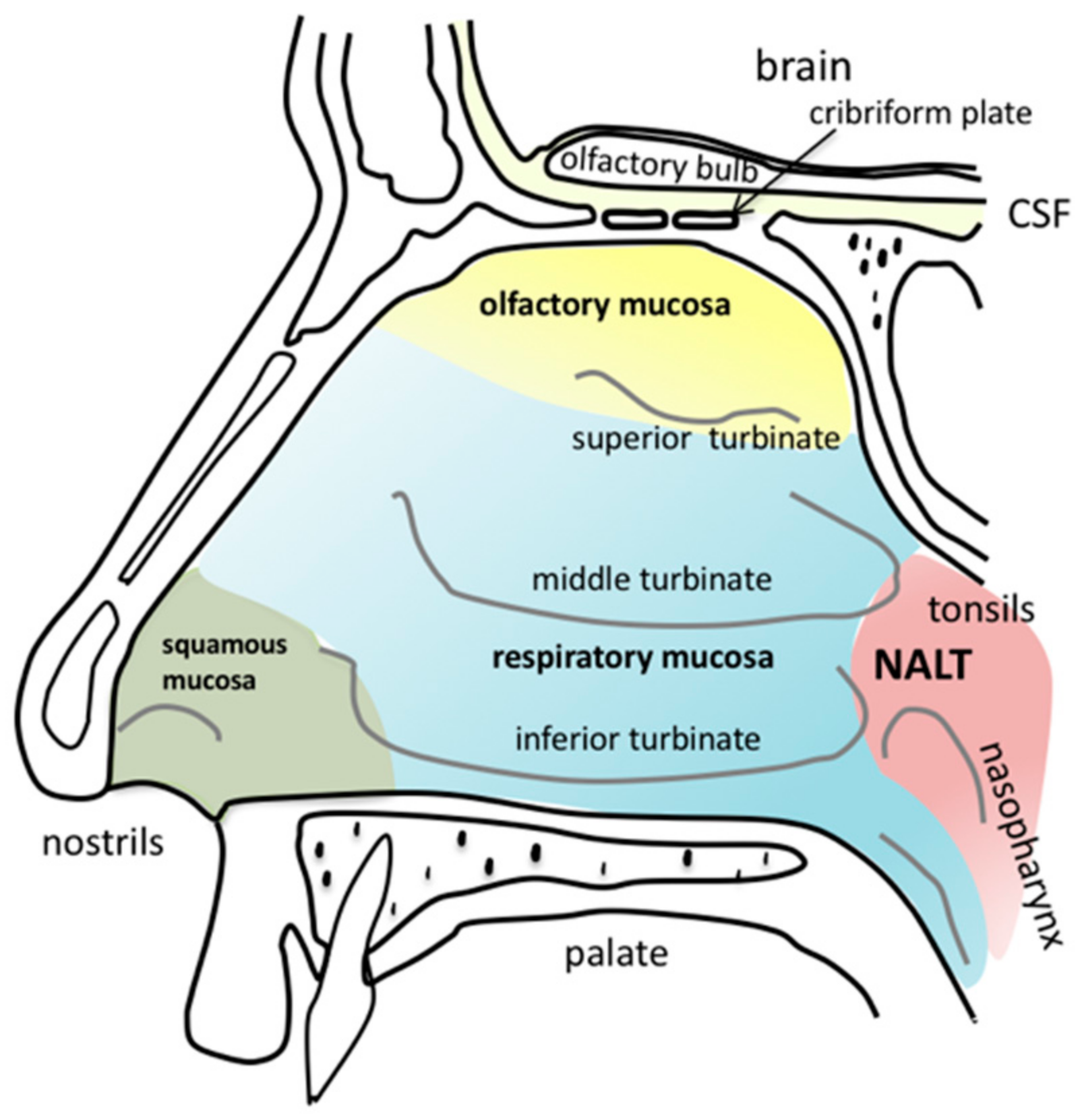
2.1. Anatomy of the Nasal Cavity
2.2. General Suitability of the Nasal Cavity for Drug Delivery
3. The Nasal Epithelia
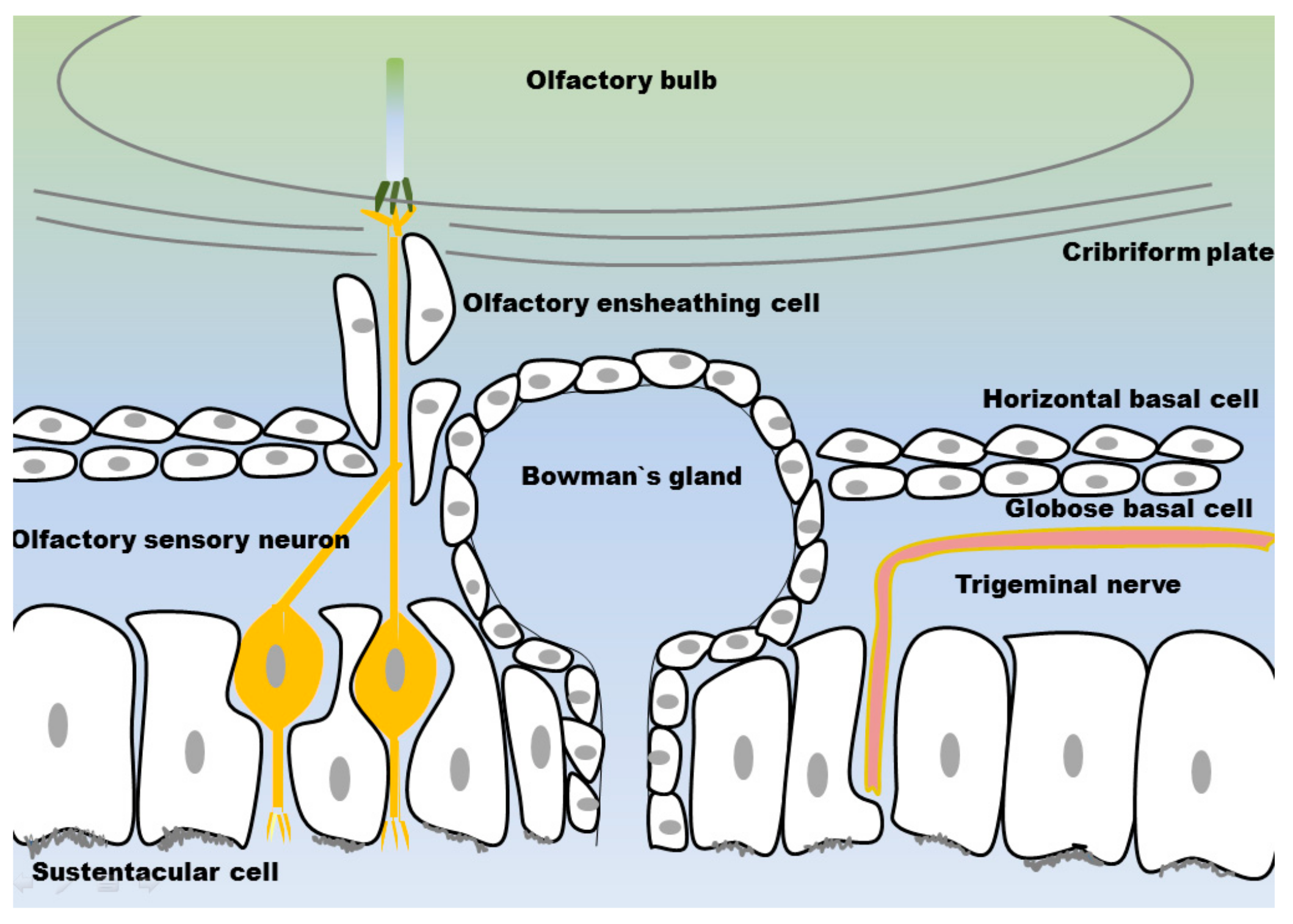
3.1. Olfactory Mucosa
3.1.1. Olfactory Sensory Neurons
3.1.2. Olfactory Ensheating Cells
3.1.3. Sustentacular Cells
3.1.4. Globose Basal Cells
3.1.5. Horizontal Basal Cells
3.1.6. Bowman’s Glands
3.2. Respiratory Mucosa
3.3. Nasopharynx-Associated Lymphatic Tissue
Microfold Cells
3.4. Trigeminal Nerve
3.5. Tight Junctions
4. Cilia, Nasal Mucus and Mucociliary Clearance
4.1. Cilia and Mucus Transport
4.2. Nasal Mucus and Clearance
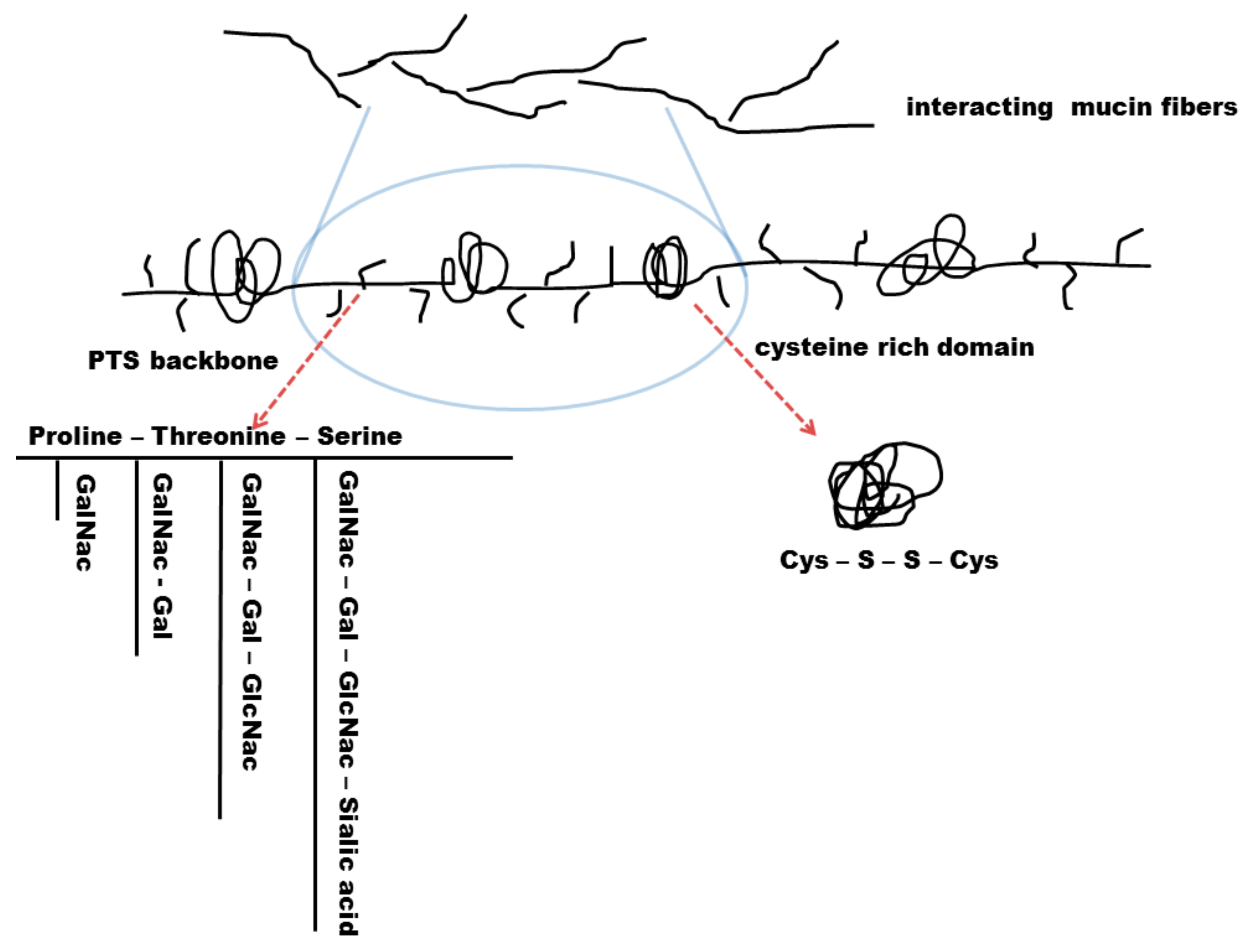
4.2.1. Biochemical Structure of the Mucus
4.2.2. Mucus Permeability and Turnover
4.2.3. Physico-Chemical Properties and Mucociliary Clearance
4.2.4. Aquaporins as a Mucosal Source of Water
5. Intranasal N2B Drug Delivery of CNS Active Substances
5.1. Mechanisms of Intranasal N2B Drug Delivery
5.2. Limitations of Nose-to-Brain Delivery
6. Formulations, Dosage Forms and Medical Devices for Intranasal Delivery
6.1. Excipients
6.1.1. Mucoadhesive Excipients
6.1.2. Adsorption Enhancers
6.1.3. Preservatives
6.2. Formulations and Dosage Forms
6.2.1. Semisolid Formulations
6.2.2. Particulate Formulations
6.2.3. Lipid-Based Formulations and Liposomes
6.2.4. Liquid Formulations and Formulations for Intranasal Vaccination
6.3. Intranasal Medical Drug Delivery Devices
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AQP | aquaporin |
| BG | Bowman’s gland |
| CNS | central nervous system |
| CSF | cerebrospinal fluid |
| GOB | globose basal cell |
| HBC | horizontal basal cell |
| HIV | human immunodeficiency virus |
| Ig | immunoglobulin |
| MALT | mucus-associated lymphatic tissue |
| M cell | microfold cell |
| MUC | mucin |
| N2B | nose-to-brain |
| NALT | nasopharynx-associated lymphatic tissue |
| OC | occludin |
| OE | olfactory epithelium/olfactory mucosa |
| OEC | olfactory ensheathing cell |
| OSN | olfactory sensory neuron |
| Pa·s | Pascal·second (unit of viscosity) |
| PEG | polyethylenglycol |
| PLA | poly lactic acid |
| PLGA | poly(lactid-co-glycolid) acid |
| RE | respiratory epithelium/respiratory mucosa |
| SUS | sustentacular cell |
| ZO | zonula occludens |
References
- Zhang, Y.; Chan, H.F.; Leong, K.W. Advanced materials and processing for drug delivery: The past and the future. Adv. Drug Deliv. Rev. 2013, 65, 104–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishan, M.; Gudelsky, G.A.; Desai, P.B.; Genter, M.B. Manipulation of olfactory tight junctions using papaverine to enhance intranasal delivery of gemcitabine to the brain. Drug Deliv. 2014, 21, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Ali, A.; Md, S.; Baboota, S.; Sahni, J.K.; Ali, J. Insights into direct nose to brain delivery: Current status and future perspective. Drug Deliv. 2014, 21, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, S.; Charalambides, C.; Matsota, P.; Tsangaris, I.; Kostopanagiotou, G. Malfunction of SynchroMed II Baclofen Pump Delivers a Near-Lethal Baclofen Overdose. Pediatr. Neurosurg. 2010, 46, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Maino, P.; Koetsier, E.; Perez, R.S. Fentanyl overdose caused by malfunction of SynchroMed II intrathecal pump: Two case reports. Reg. Anesth. Pain Med. 2014, 39, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Davanzo, J.R.; Rizk, E. Baclofen overdose from possible intrinsic malfunction of SynchroMed II pump. J. Neurosurg. Pediatr. 2015, 16, 232–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albright, A.L.; Awaad, Y.; Muhonen, M.; Boydston, W.R.; Gilmartin, R.; Krach, L.E.; Turner, M.; Zidek, K.A.; Wright, E.; Swift, D.; et al. Performance and complications associated with the Synchromed 10-mL infusion pump for intrathecal baclofen administration in children. J. Neurosurg. Pediatr. 2004, 101, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Awaad, Y.; Rizk, T.; Siddiqui, I.; Roosen, N.; Mcintosh, K.; Waines, G.M. Complications of Intrathecal Baclofen Pump: Prevention and Cure. ISRN Neurol. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lochhead, J.J.; Thorne, R.G. Drug Delivery to the Brain; Springer: Berlin/Heidelberg, Germany, 2014; Volume 10, ISBN 978-1-4614-9104-0. [Google Scholar]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Stützle, M.; Flamm, J.; Carle, S.; Schindowski, K. Nose-to-Brain delivery of insulin for Alzheimer’s disease. ADMET DMPK 2015, 3, 190–202. [Google Scholar] [CrossRef] [Green Version]
- Henkin, R.I. Intranasal insulin: From nose to brain. Nutrition 2010, 26, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Furubayashi, T.; Arai, M.; Inoue, D.; Kimura, S.; Kiriyama, A.; Kusamori, K.; Katsumi, H.; Yutani, R.; Sakane, T.; et al. Delivery of Oxytocin to the Brain for the Treatment of Autism Spectrum Disorder by Nasal Application. Mol. Pharm. 2018, 15, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Gao, H. Perspectives on Dual Targeting Delivery Systems for Brain Tumors. J. Neuroimmune Pharmacol. 2017, 12, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Dalpiaz, A.; Pavan, B. Nose-to-brain delivery of antiviral drugs: A way to overcome their active efflux? Pharmaceutics 2018, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L. Handbook of Olfaction and Gustation, 3rd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015; ISBN 9781118139226. [Google Scholar]
- Messerklinger, W. Über die Sekretströmung auf der Schleimhaut der oberen Luftwege. Z. Laryngol. Rhinol. Otol. 1951, 30, 302–308. [Google Scholar] [PubMed]
- Szefler, S.J. Pharmacokinetics of intranasal corticosteroids. J. Allergy Clin. Immunol. 2001, 108, S26–S31. [Google Scholar] [CrossRef] [PubMed]
- Hochhaus, G. Pharmacokinetic/pharmacodynamic profile of mometasone furoate nasal spray: Potential effects on clinical safety and efficacy. Clin. Ther. 2008, 30, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Price, D.; Bond, C.; Bouchard, J.; Costa, R.; Keenan, J.; Levy, M.L.; Orru, M.; Ryan, D.; Walker, S.; Watson, M. International Primary Care Respiratory Group (IPCRG) Guidelines: Management of allergic rhinitis. Prim. Care Respir. J. 2006, 15, 58–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardebo, J.E.; Dahlöf, C. Sumatriptan Nasal Spray (20 Mg/Dose) in the Acute Treatment of Cluster Headache. Cephalalgia 1998, 18, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.L.; Mottern, R.K.; Jabbour, J.T.; Fuseau, E. Pharmacokinetics of Sumatriptan Nasal Spray in Adolescents. J. Clin. Pharmacol. 2003, 43, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P. Potential of nasopharynx-associated lymphoid tissue for vaccine responses in the airways. Am. J. Respir. Crit. Care Med. 2011, 183, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Pabst, R. Mucosal vaccination by the intranasal route. Nose-associated lymphoid tissue (NALT)-Structure, function and species differences. Vaccine 2015, 33, 4406–4413. [Google Scholar] [CrossRef] [PubMed]
- Crespo, C.; Liberia, T.; Blasco-Ibáñez, J.M.; Nácher, J.; Varea, E. Cranial pair I: The olfactory nerve. Anat. Rec. 2018. [Google Scholar] [CrossRef] [PubMed]
- Schwob, J.E. Neural regeneration and the peripheral olfactory system. Anat. Rec. 2002, 269, 33–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacKay-Sim, A. Stem cells and their niche in the adult olfactory mucosa. Arch. Ital. Biol. 2010, 148, 47–58. [Google Scholar] [CrossRef] [PubMed]
- French, D.A.; Badamdorj, D.D.; Kleene, S.J. Spatial distribution of calcium-gated chloride channels in olfactory cilia. PLoS ONE 2010, 5, e15676. [Google Scholar] [CrossRef] [PubMed]
- Challis, R.C.; Tian, H.; Wang, J.; He, J.; Jiang, J.; Chen, X.; Yin, W.; Connelly, T.; Ma, L.; Yu, C.R.; et al. An Olfactory Cilia Pattern in the Mammalian Nose Ensures High Sensitivity to Odors. Curr. Biol. 2016, 25, 2503–2512. [Google Scholar] [CrossRef] [PubMed]
- Trimmer, C.; Snyder, L.L.; Mainland, J.D. High-throughput Analysis of Mammalian Olfactory Receptors: Measurement of Receptor Activation via Luciferase Activity. J. Vis. Exp. 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I. Singular expression of olfactory receptor genes. Cell 2013, 155, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, K.; Ando, M.; Yokouchi, K.; Sumitomo, N.; Karasawa, M.; Fukushima, N.; Moriizumi, T. Stereological quantification of olfactory receptor neurons in mice. Neuroscience 2014, 272, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Arneodo, E.M.; Penikis, K.B.; Rabinowitz, N.; Licata, A.; Cichy, A.; Zhang, J.; Bozza, T.; Rinberg, D. Stimulus dependent diversity and stereotypy in the output of an olfactory functional unit. Nat. Commun. 2018, 9, 1347. [Google Scholar] [CrossRef] [PubMed]
- Mackay-Sim, A.; St John, J.A. Olfactory ensheathing cells from the nose: Clinical application in human spinal cord injuries. Exp. Neurol. 2011, 229, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Au, E.; Roskams, A.J. Olfactory ensheathing cells of the lamina propria in vivo and in vitro. Glia 2003, 41, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Barnett, S.C.; Chang, L. Olfactory ensheathing cells and CNS repair: Going solo or in need of a friend? Trends Neurosci. 2004, 27, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Herbert, R.P.; Harris, J.; Chong, K.P.; Chapman, J.; West, A.K.; Chuah, M.I. Cytokines and olfactory bulb microglia in response to bacterial challenge in the compromised primary olfactory pathway. J. Neuroinflammation 2012, 9, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, J.A. Anti-Bacterial Properties of Olfactory Ensheathing Cells and the Primary Olfactory Pathway. Ph.D. Thesis, University of Tasmania, Hobart, Australia, 2013; pp. 1–16. [Google Scholar]
- Lalancette-Hébert, M.; Phaneuf, D.; Soucy, G.; Weng, Y.C.; Kriz, J. Live imaging of Toll-like receptor 2 response in cerebral ischaemia reveals a role of olfactory bulb microglia as modulators of inflammation. Brain 2009, 132, 940–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, F. Olfactory receptor neuronal dendrites become mostly intra-sustentacularly enwrapped upon maturity. J. Anat. 2018, 232, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Le Bourhis, M.; Rimbaud, S.; Grebert, D.; Congar, P.; Meunier, N. Endothelin uncouples gap junctions in sustentacular cells and olfactory ensheathing cells of the olfactory mucosa. Eur. J. Neurosci. 2014, 40, 2878–2887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cao, Q.; Guo, A.; Chu, H.; Chan, Y.G.; Buschdorf, J.P.; Low, B.C.; Ling, E.A.; Liang, F. Juxtanodin: An oligodendroglial protein that promotes cellular arborization and 2′,3′-cyclic nucleotide-3′-phosphodiesterase trafficking. Proc. Natl. Acad. Sci. USA 2005, 102, 11527–11532. [Google Scholar] [CrossRef] [PubMed]
- Joiner, A.M.; Green, W.W.; McIntyre, J.C.; Allen, B.L.; Schwob, J.E.; Martens, J.R. Primary Cilia on Horizontal Basal Cells Regulate Regeneration of the Olfactory Epithelium. J. Neurosci. 2015, 35, 13761–13772. [Google Scholar] [CrossRef] [PubMed]
- Iwai, N.; Zhou, Z.; Roop, D.R.; Behringer, R.R. Horizontal Basal Cells Are Multipotent Progenitors in Normal and Injured Adult Olfactory Epithelium. Stem Cells 2008, 26, 1298–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solbu, T.T.; Holen, T. Aquaporin pathways and mucin secretion of bowman’s glands might protect the olfactory mucosa. Chem. Senses 2012, 37, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Breipohl, W. Licht-und elektronenmikroskopische Befunde zur Struktur der Bowmanschen Drüsen im Riechepithel der weißen Maus. Zeitschrift für Zellforschung und Mikroskopische Anatomie 1972, 131, 329–346. [Google Scholar] [CrossRef] [PubMed]
- Frisch, D. Ultrastructure of mouse olfactory mucosa. Am. J. Anat. 1967, 121, 87–119. [Google Scholar] [CrossRef] [PubMed]
- Getchell, M.L.; Getchell, T.V. Fine structural aspects of secretion and extrinsic innervation in the olfactory mucosa. Microsc. Res. Tech. 1992, 23, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.C.; Zhang, H.; Zador, Z.; Verkman, A.S. Impaired olfaction in mice lacking aquaporin-4 water channels. FASEB J. 2008, 22, 3216–3223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ablimit, A.; Matsuzaki, T.; Suzuki, T.; Aoki, T.; Hagiwara, H.; Takata, K. Immunolocalization of water channel aquaporins in the olfactory mucosa. Arch. Histol. Cytol. 2006, 69, 1–12. [Google Scholar] [CrossRef]
- Katz, S.; Merzel, J. Distribution of epithelia and glands of the nasal septum mucosa in the rat. Cells Tissues Organs 1977, 99, 58–66. [Google Scholar] [CrossRef]
- Cuschieri, A.; Bannister, L.H. Some histochemical observations on the mucosubstances of the nasal glands of the mouse. Histochem. J. 1974, 6, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Verkman, A.S. Aquaporin-5 Dependent Fluid Secretion in Airway Submucosal Glands. J. Biol. Chem. 2001, 276, 41288–41292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoeckelhuber, M.; Olzowy, B.; Ihler, F.; Matthias, C.; Scherer, E.Q.; Babaryka, G.; Loeffelbein, D.J.; Rohleder, N.H.; Nieberler, M.; Kesting, M.R. Immunolocalization of antimicrobial and cytoskeletal components in the serous glands of human sinonasal mucosa. Histol. Histopathol. 2014, 29, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Ma, W.; Zhan, W.; Wang, X.; Dong, H.; Zhao, H.; Yang, L.; Ji, C.; Han, Q.; Ji, C.; et al. Internal biliary drainage superior to external biliary drainage in improving gut mucosa barrier because of goblet cells and mucin-2 upregulation. Biosci. Rep. 2018, BSR20171241. [Google Scholar] [CrossRef] [PubMed]
- Cesta, M.F. Normal Structure, Function, and Histology of Mucosa-Associated Lymphoid Tissue. Toxicol. Pathol. 2006, 34, 599–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corr, S.C.; Gahan, C.C.G.M.; Hill, C. M-cells: Origin, morphology and role in mucosal immunity and microbial pathogenesis. FEMS Immunol. Med. Microbiol. 2008, 52, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Zuercher, A.W.; Coffin, S.E.; Thurnheer, M.C.; Fundova, P.; Cebra, J.J. Nasal-Associated Lymphoid Tissue Is a Mucosal Inductive Site for Virus-Specific Humoral and Cellular Immune Responses. J. Immunol. 2002, 168, 1796–1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Gao, Z.; Zhang, Z.; Pan, L.; Zhang, Y. Roles of M cells in infection and mucosal vaccines. Hum. Vaccines Immunother. 2014, 10, 3544–3551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nochi, T.; Yuki, Y.; Matsumura, A.; Mejima, M.; Terahara, K.; Kim, D.-Y.; Fukuyama, S.; Iwatsuki-Horimoto, K.; Kawaoka, Y.; Kohda, T.; et al. A novel M cell–specific carbohydrate-targeted mucosal vaccine effectively induces antigen-specific immune responses. J. Exp. Med. 2007, 204, 2789–2796. [Google Scholar] [CrossRef] [PubMed]
- Mutoh, M.; Kimura, S.; Takahashi-Iwanaga, H.; Hisamoto, M.; Iwanaga, T.; Iida, J. RANKL regulates differentiation of microfold cells in mouse nasopharynx-associated lymphoid tissue (NALT). Cell Tissue Res. 2016, 364, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Lo, D.D. Vigilance or Subversion? Constitutive and Inducible M Cells in Mucosal Tissues. Trends Immunol. 2018, 39, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Mabbott, N.A.; Donaldson, D.S.; Ohno, H.; Williams, I.R.; Mahajan, A. Microfold (M) cells: Important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013, 6, 666–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumberg, R.S. Current Concepts in Mucosal Immunity. Am. J. Physiol. 1998, 274, 227–231. [Google Scholar]
- Pappo, J.; Ermak, T.H. Uptake and translocation of fluorescent latex particles by rabbit Peyer’s patch follicle epithelium: A quantitative model for M cell uptake. Clin. Exp. Immunol. 1989, 76, 144–148. [Google Scholar] [PubMed]
- Rochereau, N.; Pavot, V.; Verrier, B.; Jospin, F.; Ensinas, A.; Genin, C.; Corthésy, B.; Paul, S. Delivery of antigen to nasal-associated lymphoid tissue microfold cells through secretory IgA targeting local dendritic cells confers protective immunity. J. Allergy Clin. Immunol. 2016, 137, 214–222.e2. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, K.; Gowthamarajan, K.; Karri, V.V.S.R. Nose to brain transport pathways an overview: Potential of nanostructured lipid carriers in nose to brain targeting. Artif. Cells Nanomed. Biotechnol. 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Silver, W.L.; Finger, T.E. The anatomical and electrophysiological basis of peripheral nasal trigeminal chemoreception. Ann. N. Y. Acad. Sci. 2009, 1170, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Stone, H.; Williams, B.; Carregal, E.J.A. The role of the trigeminal nerve in olfaction. Exp. Neurol. 1968, 21, 11–19. [Google Scholar] [CrossRef]
- Frasnelli, J.; Schuster, B.; Hummel, T. Interactions between Olfaction and the Trigeminal System: What Can Be Learned from Olfactory Loss. Cereb. Cortex 2007, 17, 2268–2275. [Google Scholar] [CrossRef] [PubMed]
- St. John, J.A.; Walkden, H.; Nazareth, L.; Beagley, K.W.; Ulett, G.C.; Batzloff, M.R.; Beacham, I.R.; Ekberg, J.A.K. Burkholderia pseudomallei Rapidly Infects the Brain Stem and Spinal Cord via the Trigeminal Nerve after Intranasal Inoculation. Infect. Immun. 2016, 84, 2681–2688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolburg, H.; Wolburg-Buchholz, K.; Sam, H.; Horvát, S.; Deli, M.A.; Mack, A.F. Epithelial and endothelial barriers in the olfactory region of the nasal cavity of the rat. Histochem. Cell Biol. 2008, 130, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Hussar, P.; Tserentsoodol, N.; Koyama, H.; Yokoo-Sugawara, M.; Matsuzaki, T.; Takami, S.; Takata, K. The glucose transporter GLUT1 and the tight junction protein occludin in nasal olfactory mucosa. Chem. Senses 2002, 27, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Steinke, A.; Meier-Stiegen, S.; Drenckhahn, D.; Asan, E. Molecular composition of tight and adherens junctions in the rat olfactory epithelium and fila. Histochem. Cell Biol. 2008, 130, 339–361. [Google Scholar] [CrossRef] [PubMed]
- Escada, P.A.; Lima, C.; Da Silva, J.M. The human olfactory mucosa. Eur. Arch. Oto-Rhino-Laryngol. 2009, 266, 1675–1680. [Google Scholar] [CrossRef] [PubMed]
- Mitchison, H.M.; Valente, E.M. Motile and non-motile cilia in human pathology: From function to phenotypes. J. Pathol. 2017, 241, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Gueron, S.; Levit-Gurevich, K. Energetic considerations of ciliary beating and the advantage of metachronal coordination. Proc. Natl. Acad. Sci. USA 1999, 96, 12240–12245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romanelli, M.; Gelardi, M.; Fiorella, M. Nasal ciliary motility: A new tool in estimating the time of death. Int. J. Leg. Med. 2012, 126, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Williams, O.W.; Sharafkhaneh, A.; Kim, V.; Dickey, B.F.; Evans, C.M. Airway Mucus. Am. J. Respir. Cell Mol. Biol. 2006, 34, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.J.; Wreschner, D.H.; Tran, M.; Eyre, H.J.; Sutherland, G.R.; McGuckin, M.A. MUC13, a Novel Human Cell Surface Mucin Expressed by Epithelial and Hemopoietic Cells. J. Biol. Chem. 2001, 276, 18327–18336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leal, J.; Smyth, H.D.C.; Ghosh, D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int. J. Pharm. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, S.; Sheehan, J.K.; Knight, D.; Richardson, P.S.; Thornton, D.J. Heterogeneity of airways mucus: Variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem. J. 2002, 361, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.G.; Livraghi-butrico, A.; Fletcher, A.A.; Melissa, M.; Evans, S.E.; Boerner, R.M.; Alexander, S.N.; Lindsey, K.; Song, A.S.; Petrova, Y.M.; et al. Muc5b is required for airway defense. Nature 2014, 505, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Shankar, V.; Gilmore, M.S.; Elkins, R.C.; Sachdev, G.P. A novel human airway mucin cDNA encodes a protein with unique tandem-repeat organization. Biochem. J. 1994, 300 Pt 2, 295–298. [Google Scholar] [CrossRef]
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Verdugo, P. Goblet Cells Secretion and Mucogenesis. Annu. Rev. Physiol. 1990, 52, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Verdugo, P. Hydration kinetics of exocytosed mucins in cultured secretory cells of the rabbit trachea: A new model. Ciba Found. Symp. 1984, 109, 212–225. [Google Scholar] [PubMed]
- Shankar, V.; Virmani, A.K.; Naziruddin, B.; Sachdev, G.P. Macromolecular properties and polymeric structure of canine tracheal mucins. Biochem. J. 1991, 276 Pt 2, 525–532. [Google Scholar] [CrossRef]
- Sheehan, J.K.; Thornton, D.J.; Somerville, M.; Carlstedt, I. The Structure and Heterogeneity of Respiratory Mucus Glycoproteins. Am. Rev. Respir. Dis. 1991, 144, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, J.K.; Oates, K.; Carlstedt, I. Electron microscopy of cervical, gastric and bronchial mucus glycoproteins. Biochem. J. 1986, 239, 147–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escande, F.; Porchet, N.; Aubert, J.-P.; Buisine, M.-P. The mouse Muc5b mucin gene: CDNA and genomic structures, chromosomal localization and expression. Biochem. J. 2002, 363, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Marttin, E.; Schipper, N.G.M.; Coos Verhoef, J.; Merkus, F.W.H.M. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv. Drug Deliv. Rev. 1998, 29, 13–38. [Google Scholar] [CrossRef]
- Olmsted, S.S.; Padgett, J.L.; Yudin, A.I.; Whaley, K.J.; Moench, T.R.; Cone, R.A. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophys. J. 2001, 81, 1930–1937. [Google Scholar] [CrossRef]
- Lai, S.K.; O’Hanlon, D.E.; Harrold, S.; Man, S.T.; Wang, Y.-Y.; Cone, R.; Hanes, J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc. Natl. Acad. Sci. USA 2007, 104, 1482–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigurdsson, H.H.; Kirch, J.; Lehr, C.M. Mucus as a barrier to lipophilic drugs. Int. J. Pharm. 2013, 453, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Frey, A.; Giannasca, K.T.; Weltzin, R.; Giannasca, P.J.; Reggio, H.; Lencer, W.I.; Neutra, M.R. Role of the glycocalyx in regulating access of microparticles to apical plasma membranes of intestinal epithelial cells: Implications for microbial attachment and oral vaccine targeting. J. Exp. Med. 1996, 184, 1045–1059. [Google Scholar] [CrossRef] [PubMed]
- Matsui, H.; Verghese, M.W.; Kesimer, M.; Schwab, U.E.; Randell, S.H.; Sheehan, J.K.; Grubb, B.R.; Boucher, R.C. Reduced Three-Dimensional Motility in Dehydrated Airway Mucus Prevents Neutrophil Capture and Killing Bacteria on Airway Epithelial Surfaces. J. Immunol. 2005, 175, 1090–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lale, A.M.; Mason, J.D.T.; Jones, N.S. Mucociliary transport and its assessment: A review. Clin. Otolaryngol. Allied Sci. 1998, 23, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Boegh, M.; Nielsen, H.M. Mucus as a barrier to drug delivery—Understanding and mimicking the barrier properties. Basic Clin. Pharmacol. Toxicol. 2015, 116, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M.; Ukai, K.; Majima, Y.; Sakakura, Y. Effects of Nasal Allergen Challenge on Dynamic Viscoelasticity of Nasal Mucus. Ann. Otol. Rhinol. Laryngol. 1993, 102, 314–317. [Google Scholar] [CrossRef] [PubMed]
- King, M. Experimental models for studying mucociliary clearance. Eur. Respir. J. 1998, 11, 222–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chilvers, M.A. Analysis of ciliary beat pattern and beat frequency using digital high speed imaging: Comparison with the photomultiplier and photodiode methods. Thorax 2000, 55, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, W.M.; Radomsky, M.L.; Whaley, K.J.; Cone, R.A. Antibody diffusion in human cervical mucus. Biophys. J. 1994, 66, 508–515. [Google Scholar] [CrossRef] [Green Version]
- Eichner, H.; Behbehani, A.A.; Hochstrasser, K. Diagnostic value of nasal secretions, current state: Normal values. 1. Laryngol. Rhinol. Otol. 1983, 62, 561–565. [Google Scholar] [CrossRef]
- Majima, Y.; Harada, T.; Shimizu, T.; Takeuchi, K.; Sakakura, Y.; Yasuoka, S.; Yoshinaga, S. Effect of biochemical components on rheologic properties of nasal mucus in chronic sinusitis. Am. J. Respir. Crit. Care Med. 1999, 160, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Beule, A.G. Physiology and pathophysiology of respiratory mucosa of the nose and the paranasal sinuses. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2010, 9, Doc07. [Google Scholar] [CrossRef] [PubMed]
- Washington, N.; Steele, R.J.C.; Jackson, S.J.; Bush, D.; Mason, J.; Gill, D.A.; Pitt, K.; Rawlins, D.A. Determination of baseline human nasal pH and the effect of intranasally administered buffers. Int. J. Pharm. 2000, 198, 139–146. [Google Scholar] [CrossRef]
- Schuhl, J.F. Nasal mucociliary clearance in perennial rhinitis. J. Investig. Allergol. Clin. Immunol. 1995, 5, 333–336. [Google Scholar] [PubMed]
- Brinker, T.; Stopa, E.; Morrison, J.; Klinge, P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 2014, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Shields, S.D.; Moore, K.D.; Phelps, P.E.; Basbaum, A.I. Olfactory ensheathing glia express aquaporin 1. J. Comp. Neurol. 2010, 518, 4329–4341. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, J.J.; Wolak, D.J.; Pizzo, M.E.; Thorne, R.G. Rapid transport within cerebral perivascular spaces underlies widespread tracer distribution in the brain after intranasal administration. J. Cereb. Blood Flow Metab. 2015, 35, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Buchner, K.; Seitz-Tutter, D.; Schönitzer, K.; Weiss, D.G. A quantitative study of anterograde and retrograde axonal transport of exogenous proteins in olfactory nerve C-fibers. Neuroscience 1987, 22, 697–707. [Google Scholar] [CrossRef]
- Wu, H.; Hu, K.; Jiang, X. From nose to brain: Understanding transport capacity and transport rate of drugs. Expert Opin. Drug Deliv. 2008, 5, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, C.V.; Belgamwar, V.S. Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood-brain barrier: An excellent platform for brain targeting. Expert Opin. Drug Deliv. 2013, 10, 957–972. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, S.; Pathak, K. Physicochemical and physiological considerations for efficient nose-to-brain targeting. Expert Opin. Drug Deliv. 2012, 9, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Bourganis, V.; Kammona, O.; Alexopoulos, A.; Kiparissides, C. Recent advances in carrier mediated nose-to-brain delivery of pharmaceutics. Eur. J. Pharm. Biopharm. 2018, 128, 337–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ugwoke, M.; Agu, R.; Verbeke, N.; Kinget, R. Nasal mucoadhesive drug delivery: Background, applications, trends and future perspectives. Adv. Drug Deliv. Rev. 2005, 57, 1640–1665. [Google Scholar] [CrossRef] [PubMed]
- Netsomboon, K.; Bernkop-Schnürch, A. Mucoadhesive vs. mucopenetrating particulate drug delivery. Eur. J. Pharm. Biopharm. 2016, 98, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S.; Burke, P.A.; Langer, R. Overcoming the challenges in administering biopharmaceuticals: Formulation and delivery strategies. Nat. Rev. Drug Discov. 2014, 13, 655–672. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, P.C.; Cattoz, B.; Ibrahim, M.S.; Anuonye, J.C. Probing the interaction of nanoparticles with mucin for drug delivery applications using dynamic light scattering. Eur. J. Pharm. Biopharm. 2015, 97, 218–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batts, A.H.; Mariott, C.; Martin, G.P.; Wood, C.F.; Bond, S.W. The Effect of Some Preservatives Used in Nasal Preparations on the Mucus and Ciliary Components of Mucociliary Clearance. J. Pharm. Pharmacol. 1990, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Drug, T. Ciliotoxicity of methyl- and propyl-p-hydroxybenzoates: A dose-response and surface-response study. J. Pharm. Pharmacol. 1993, 45, 925–927. [Google Scholar]
- Braat, J.P.; Ainge, G.; Bowles, J.A.; Richards, D.H.; Van Riessen, D.; Visser, W.J.; Rijntjes, E. The lack of effect of benzalkonium chloride on the cilia of the nasal mucosa in patients with perennial allergic rhinitis: A combined functional, light, scanning and transmission electron microscopy study. Clin. Exp. Allergy 1995, 25, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Su, X.-Y.; Po, A.L.W.; Millership, J.S. Ciliotoxicity of intranasal formulations: Menthol enantiomers. Chirality 1993, 5, 58–60. [Google Scholar] [CrossRef]
- Kushnir, N. Rhinitis Medicamentosa. Medscape 2013, 25, 1–7. [Google Scholar]
- Romeijn, S.G.; Verhoef, J.C.; Marttin, E.; Merkus, F.W.H.M. The effect of nasal drug formulations on ciliary beating in vitro. Int. J. Pharm. 1996, 135, 137–145. [Google Scholar] [CrossRef]
- D’Souza, R.; Mutalik, S.; Venkatesh, M.; Vidyasagar, S.; Udupa, N. Nasal insulin gel as an alternate to parenteral insulin: Formulation, preclinical, and clinical studies. AAPS PharmSciTech 2005, 6, E184–E189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, J.H.; Robinson, J.R. Bioadhesive-Based Dosage Forms: The Next Generation. J. Pharm. Sci. 2000, 89, 850–866. [Google Scholar] [CrossRef]
- Vyas, T.K.; Babbar, A.K.; Sharma, R.K.; Singh, S.; Misra, A. Intranasal Mucoadhesive Microemulsions of Clonazepam: Preliminary Studies on Brain Targeting. J. Pharm. Sci. 2006, 95, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Vyas, T.K.; Babbar, A.K.; Sharma, R.K.; Singh, S.; Misra, A. Preliminary Brain-targeting Studies on Intranasal Mucoadhesive Microemulsions of Sumatriptan. AAPS PharmSciTech 2006, 7, E1–E9. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.D.; Vanjari, Y.H.; Sancheti, K.H.; Belgamwar, V.S.; Surana, S.J. Nanotechnology-mediated nose to brain drug delivery for Parkinson’s disease: A mini review. J. Drug Target. 2015, 23, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Gao, H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm. Sin. B 2016, 6, 268–286. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, T.; Pellitteri, R.; Spatuzza, M.; Puglisi, G. Nose-to-Brain Delivery: Evaluation of Polymeric Nanoparticles on Olfactory Ensheathing Cells Uptake. J. Pharm. Sci. 2014, 103, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, T.; Niedergall, K.; Wojciukiewicz, D.; Gose, T.; Gruber-Traub, C.; Weber, A.; Hirth, T.; Tovar, G. NANOCYTES-Technology—Biomimetic nanoparticles for molecular recognition by molecular imprinting. In Bio Sensors, Instruments, Medical, Environment and Energy: An Interdisciplinary Integrative Forum on Nanotechnology, Biotechnology and Mic (Volume 3), Proceedings of the 2010 NSTI Nanotechnology Conference and Expo-Nanotech Conference & Expo 2010, Berlin, Germany, 20–22 October 2010; CRC Press: Boca Raton, FL, USA, 2010; pp. 242–245. [Google Scholar]
- Alpar, H.; Somavarapu, S.; Atuah, K.; Bramwell, V. Biodegradable mucoadhesive particulates for nasal and pulmonary antigen and DNA delivery. Adv. Drug Deliv. Rev. 2005, 57, 411–430. [Google Scholar] [CrossRef] [PubMed]
- Bari, N.K.; Fazil, M.; Hassan, M.Q.; Haider, M.R.; Gaba, B.; Narang, J.K.; Baboota, S.; Ali, J. Brain delivery of buspirone hydrochloride chitosan nanoparticles for the treatment of general anxiety disorder. Int. J. Biol. Macromol. 2015, 81, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Türeli, N.G.; Türeli, A.E. Good Manufacturing Practices (GMP) of Magnetic nanoparticles. In Clinical Applications of Magnetic Nanoparticles; Thanh, N.T.K., Ed.; CRC Press: Boca Raton, FL, USA; Taylor and Francis: London, UK; New York, NY, USA, 2018; pp. 473–482. [Google Scholar]
- Wacker, M. Nanocarriers for intravenous injection—The long hard road to the market. Int. J. Pharm. 2013, 457, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Günday Türeli, N.; Torge, A.; Juntke, J.; Schwarz, B.C.; Schneider-Daum, N.; Türeli, A.E.; Lehr, C.-M.; Schneider, M. Ciprofloxacin-loaded PLGA nanoparticles against cystic fibrosis P. aeruginosa lung infections. Eur. J. Pharm. Biopharm. 2017, 117, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Günday Türeli, N.; Türeli, A.E.; Schneider, M. Optimization of ciprofloxacin complex loaded PLGA nanoparticles for pulmonary treatment of cystic fibrosis infections: Design of experiments approach. Int. J. Pharm. 2016, 515, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Shadab, M.D.; Khan, R.A.; Mustafa, G.; Chuttani, K.; Baboota, S.; Sahni, J.K.; Ali, J. Bromocriptine loaded chitosan nanoparticles intended for direct nose to brain delivery: Pharmacodynamic, Pharmacokinetic and Scintigraphy study in mice model. Eur. J. Pharm. Sci. 2013, 48, 393–405. [Google Scholar] [CrossRef]
- Sanchez-Ramos, J.; Song, S.; Kong, X.; Foroutan, P.; Martinez, G.; Dominguez-Viqueria, W.; Mohapatra, S.; Mohapatra, S.; Haraszti, R.A.; Khvorova, A.; et al. Chitosan-Mangafodipir nanoparticles designed for intranasal delivery of siRNA and DNA to brain. J. Drug Deliv. Sci. Technol. 2018, 43, 453–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, E.; Boschi, A.; Ravani, L.; Cortesi, R.; Drechsler, M.; Mariani, P.; Moscatelli, S.; Contado, C.; Di Domenico, G.; Nastruzzi, C.; et al. Biodistribution of nanostructured lipid carriers: A tomographic study. Eur. J. Pharm. Biopharm. 2015, 89, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Corthésy, B.; Bioley, G. Lipid-based particles: Versatile delivery systems for mucosal vaccination against infection. Front. Immunol. 2018, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- Kuper, C.F.; Arts, J.H.E.; Feron, V.J. Toxicity to nasal-associated lymphoid tissue. Toxicol. Lett. 2003, 140–141, 281–285. [Google Scholar] [CrossRef]
- Scheibe, M.; Bethge, C.; Witt, M.; Hummel, T.; Article, O. Intranasal administration of drugs. Arch. Otolaryngol. Head. Neck Surg. 2008, 134, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Damm, M.; Vent, J.; Schmidt, M.; Theissen, P.; Eckel, H.E.; Lötsch, J.; Hummel, T. Intranasal volume and olfactory function. Chem. Senses 2002, 27, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Schriever, V.A.; Hummel, T.; Lundström, J.N.; Freiherr, J. Size of nostril opening as a measure of intranasal volume. Physiol. Behav. 2013, 110–111, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, L.; Röhm, M.; Mavoungou, C.; Schindowski, K.; Schafmeister, A.; Simon, U. First Steps to Develop and Validate a CFPD Model in Order to Support the Design of Nose-to-Brain Delivered Biopharmaceuticals. Pharm. Res. 2016, 33, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Born, J.; Lange, T.; Kern, W.; McGregor, G.P.; Bickel, U.; Fehm, H.L. Sniffing neuropeptides: A transnasal approach to the human brain. Nat. Neurosci. 2002, 5, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Albu, S. Novel drug-delivery systems for patients with chronic rhinosinusitis. Drug Des. Devel. Ther. 2012, 6, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djupesland, P.G. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology 2008, 71, 864. [Google Scholar] [CrossRef] [PubMed]
- Djupesland, P.G.; Skretting, A.; Winderen, M.; Holand, T. Breath actuated device improves delivery to target sites beyond the nasal valve. Laryngoscope 2006, 116, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Djupesland, P.G.; Skretting, A.; Winderen, M.; Holand, T. Bi-directional nasal delivery of aerosols can prevent lung deposition. J. Aerosol Med. 2004, 17, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Hoekman, J.D.; Ho, R.J.Y. Enhanced analgesic responses after preferential delivery of morphine and fentanyl to the olfactory epithelium in rats. Anesth. Analg. 2011, 113, 641. [Google Scholar] [CrossRef] [PubMed]




| Drug Delivery Route Related to Different Nasal Mucosa | Examples with Supporting Clinical Data | |
|---|---|---|
| local administration | predominantly squamous and RE | decongestants, local anaesthetics, glucocorticoide [19,20] |
| systemic delivery | predominantly RE | calcitonin, sumatriptan, desmopressin [21,22,23] |
| intranasal vaccination | NALT and immune cells in all mucosal types | seasonal flu vaccine [24,25] |
| CNS delivery (N2B) | OE: olfactory neuronal bundles; RE/OE: trigeminal nerve endings | oxytocin, insulin [13,14] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gänger, S.; Schindowski, K. Tailoring Formulations for Intranasal Nose-to-Brain Delivery: A Review on Architecture, Physico-Chemical Characteristics and Mucociliary Clearance of the Nasal Olfactory Mucosa. Pharmaceutics 2018, 10, 116. https://doi.org/10.3390/pharmaceutics10030116
Gänger S, Schindowski K. Tailoring Formulations for Intranasal Nose-to-Brain Delivery: A Review on Architecture, Physico-Chemical Characteristics and Mucociliary Clearance of the Nasal Olfactory Mucosa. Pharmaceutics. 2018; 10(3):116. https://doi.org/10.3390/pharmaceutics10030116
Chicago/Turabian StyleGänger, Stella, and Katharina Schindowski. 2018. "Tailoring Formulations for Intranasal Nose-to-Brain Delivery: A Review on Architecture, Physico-Chemical Characteristics and Mucociliary Clearance of the Nasal Olfactory Mucosa" Pharmaceutics 10, no. 3: 116. https://doi.org/10.3390/pharmaceutics10030116





