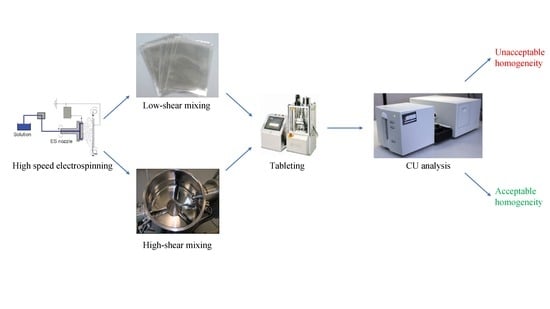
Homogenization of Amorphous Solid Dispersions Prepared by Electrospinning in Low-Dose Tablet Formulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Single-Needle Electrospinning (SNES)
2.3. High-Speed Electrospinning (HSES)
2.4. Low-Shear and High-Shear Homogenization
2.5. Tableting and in Process Control (IPC) Tests
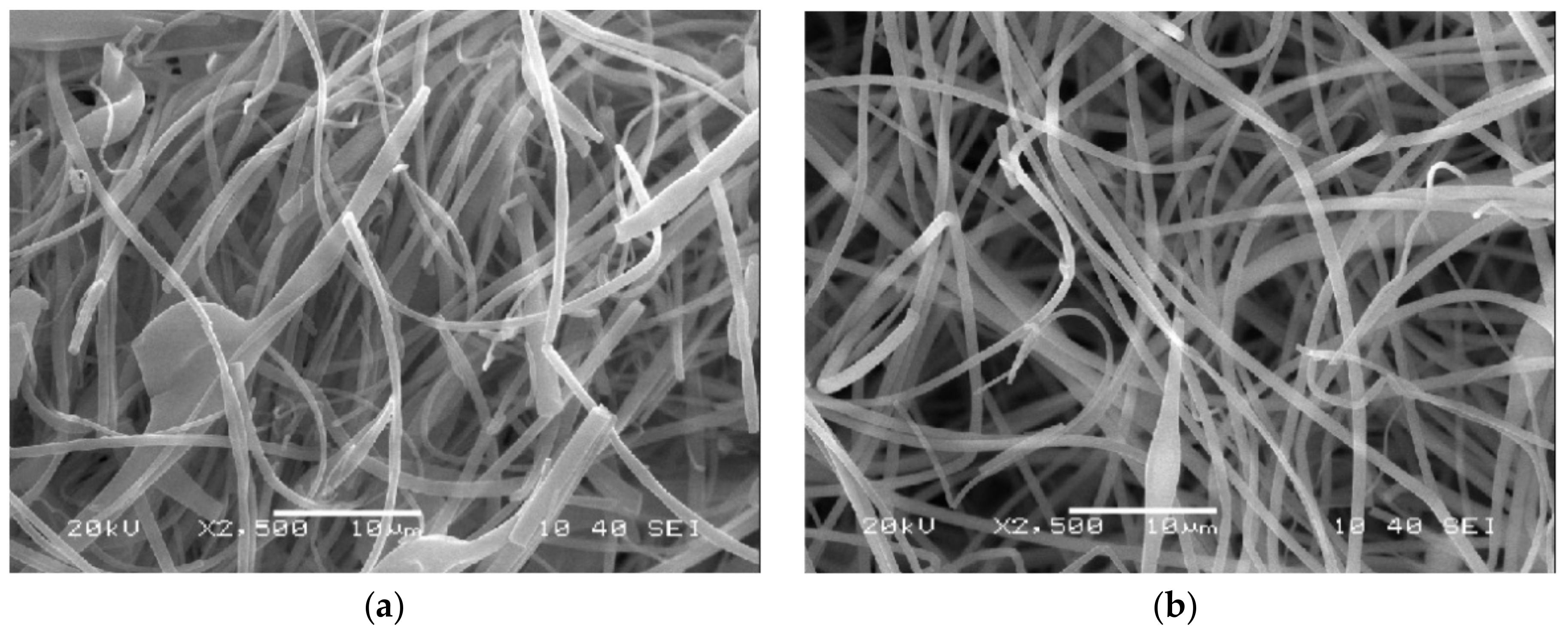
2.6. Scanning Electron Microscopy (SEM) and Fiber Diameter Analysis
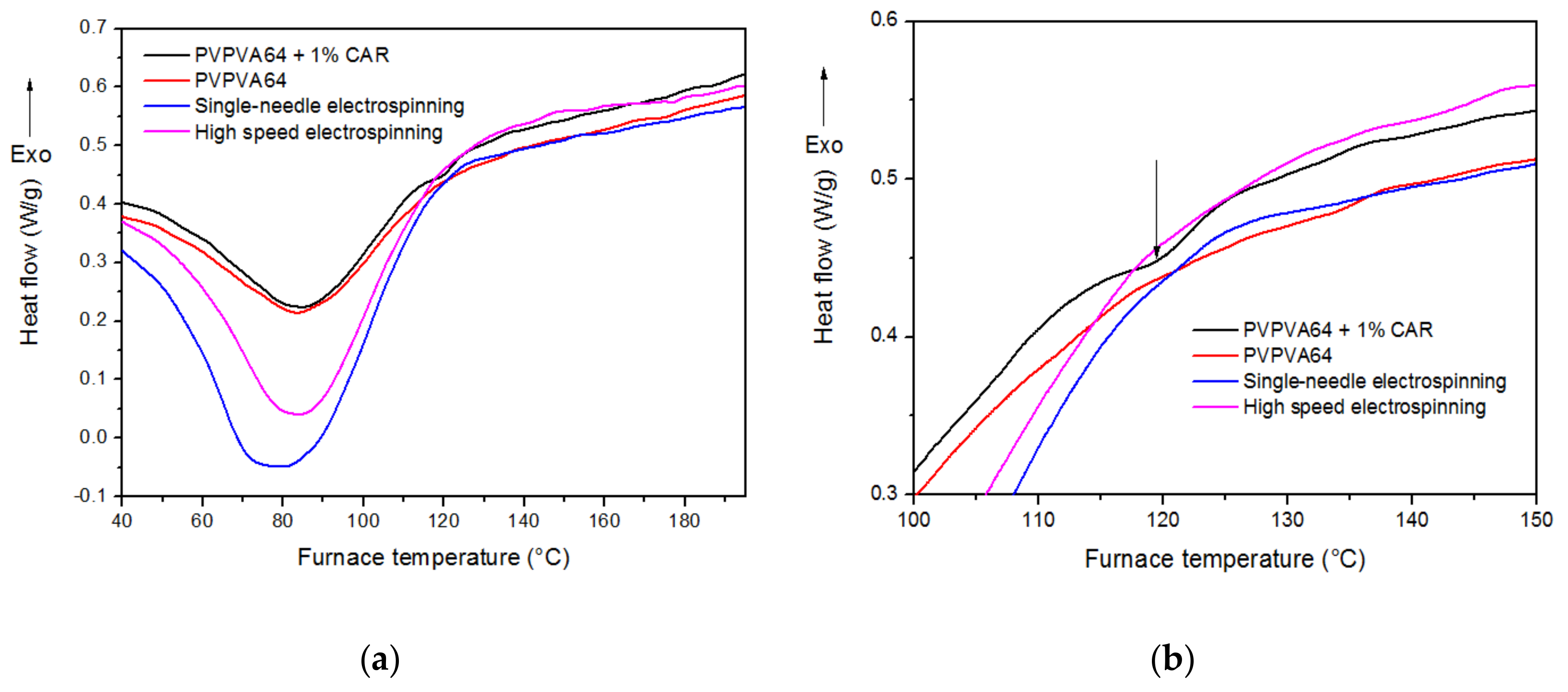
2.7. Differential Scanning Calorimetry (DSC)
2.8. Raman Mapping
2.9. Content Uniformity Analysis
2.10. Sieve Analysis
3. Results and Discussion
3.1. Electrospinning
3.2. Fiber Morphology
3.3. Differential Scanning Calorimetry
3.4. Tableting
3.5. Content Uniformity
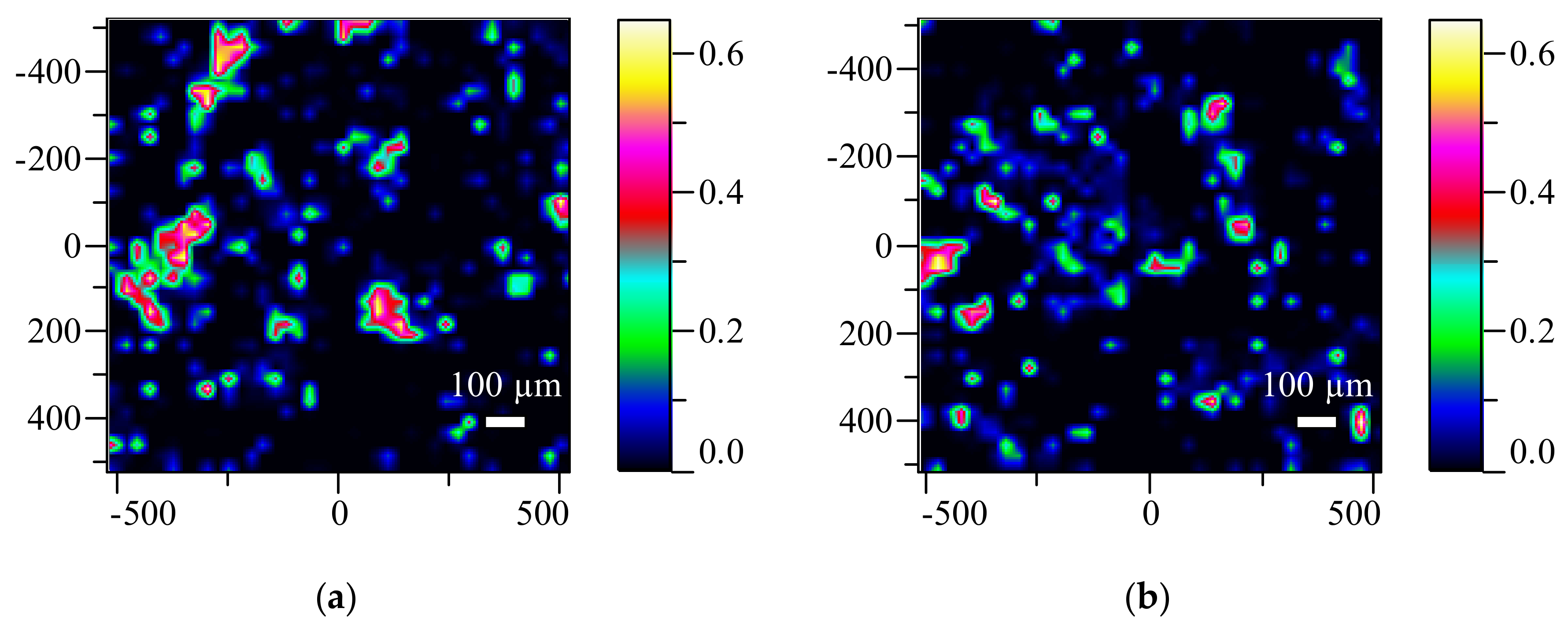
3.6. Raman Mapping Results
3.7. Sieve Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lieberman, H.A.; Lachman, L. Pharmaceutical Dosage Forms: Tablets; M. Dekker: New York, NY, USA, 1981. [Google Scholar]
- Muselik, J.; Franc, A.; Dolezel, P.; Gonec, R.; Krondlova, A.; Lukasova, I. Influence of process parameters on content uniformity of a low dose active pharmaceutical ingredient in a tablet formulation according to GMP. Acta Pharm. 2014, 64, 355–367. [Google Scholar] [CrossRef] [PubMed]
- US Pharmacopeia. Uniformity of Dosage Units/Content Uniformity. Available online: http://www.drugfuture.com/Pharmacopoeia/usp35/PDF/0420-0423%20[905]%20UNIFORMITY%20OF%20DOSAGE%20UNITS.pdf (accessed on 8 February 2017).
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Polymeric amorphous solid dispersions: A review of amorphization, crystallization, stabilization, solid-state characterization, and aqueous solubilization of biopharmaceutical classification system class II drugs. J. Pharm. Sci. 2016, 105, 2527–2544. [Google Scholar] [CrossRef] [PubMed]
- Paudel, A.; Worku, Z.A.; Meeus, J.; Guns, S.; Van den Mooter, G. Manufacturing of solid dispersions of poorly water soluble drugs by spray drying: Formulation and process considerations. Int. J. Pharm. 2013, 453, 253–284. [Google Scholar] [CrossRef] [PubMed]
- Patil, H.; Tiwari, R.V.; Repka, M.A. Hot-melt extrusion: From theory to application in pharmaceutical formulation. AAPS Pharm. Sci. Technol. 2016, 17, 20–42. [Google Scholar] [CrossRef] [PubMed]
- BioPharm. FDA Approves First Spray-Dried Biologic. Available online: http://www.biopharminternational.com/fda-approves-first-spray-dried-biologic (accessed on 7 May 2017).
- Review, A.P. Recent Innovations in Pharmaceutical Hot Melt Extrusion. Available online: https://www.americanpharmaceuticalreview.com/Featured-Articles/179317-Recent-Innovations-in-Pharmaceutical-Hot-Melt-Extrusion/ (accessed on 4 June 2017).
- Sosnik, A.; Seremeta, K.P. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Crowley, M.M.; Zhang, F.; Repka, M.A.; Thumma, S.; Upadhye, S.B.; Kumar Battu, S.; McGinity, J.W.; Martin, C. Pharmaceutical applications of hot-melt extrusion: Part I. Drug Dev. Ind. Pharm. 2007, 33, 909–926. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.L.; O’Connor, T.F.; Yang, X.; Cruz, C.N.; Chatterjee, S.; Madurawe, R.D.; Moore, C.M.V.; Yu, L.X.; Woodcock, J. Modernizing pharmaceutical manufacturing: From batch to continuous production. J. Pharm. Innov. 2015, 10, 191–199. [Google Scholar] [CrossRef]
- Poechlauer, P.; Manley, J.; Broxterman, R.; Gregertsen, B.; Ridemark, M. Continuous processing in the manufacture of active pharmaceutical ingredients and finished dosage forms: An industry perspective. Org. Process Res. Dev. 2012, 16, 1586–1590. [Google Scholar] [CrossRef]
- Vertex. FDA Approves Orkambi™ (Lumacaftor/Ivacaftor)—The First Medicine to Treat the Underlying Cause of Cystic Fibrosis for People Ages 12 and Older with Two Copies of the F508del Mutation. Available online: http://investors.vrtx.com/releasedetail.cfm?releaseid=920512 (accessed on 8 June 2017).
- Pharmtech. EMA Approves Janssen Drug Made via Continuous Manufacturing. Available online: http://www.pharmtech.com/ema-approves-janssen-drug-made-continuous-manufacturing (accessed on 7 July 2017).
- Pharmtech. Vertex Receives FDA Approval for Continuously Manufactured Drug Product. Available online: http://www.pharmtech.com/vertex-receives-fda-approval-continuously-manufactured-drug-product (accessed on 4 July 2017).
- Lukáš, D.; Sarkar, A.; Martinová, L.; Vodsed’álková, K.; Lubasová, D.; Chaloupek, J.; Pokorný, P.; Mikeš, P.; Chvojka, J.; Komárek, M. Physical principles of electrospinning (electrospinning as a nano-scale technology of the twenty-first century). Text. Prog. 2009, 41, 59–140. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Dalton, P.D. Melt electrospinning. Chem. Asian J. 2011, 6, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, D.-G.; Zhang, L.-L.; Liu, X.-K.; Deng, Y.-C.; Zhao, M. Electrospun hypromellose-based hydrophilic composites for rapid dissolution of poorly water-soluble drug. Carbohydr. Polym. 2017, 174, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Vuddanda, P.R.; Mathew, A.P.; Velaga, S. Electrospun nanofiber mats for ultrafast release of ondansetron. React. Funct. Polym. 2016, 99, 65–72. [Google Scholar] [CrossRef]
- Illangakoon, U.E.; Gill, H.; Shearman, G.C.; Parhizkar, M.; Mahalingam, S.; Chatterton, N.P.; Williams, G.R. Fast dissolving paracetamol/caffeine nanofibers prepared by electrospinning. Int. J. Pharm. 2014, 477, 369–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, F.L.; Shearman, G.C.; Gaisford, S.; Williams, G.R. Amorphous formulations of indomethacin and griseofulvin prepared by electrospinning. Mol. Pharm. 2014, 11, 4327–4338. [Google Scholar] [CrossRef] [PubMed]
- Balogh, A.; Farkas, B.; Domokos, A.; Farkas, A.; Démuth, B.; Borbás, E.; Nagy, B.; Marosi, G.; Nagy, Z.K. Controlled-release solid dispersions of eudragit® FS 100 and poorly soluble spironolactone prepared by electrospinning and melt extrusion. Eur. Polym. J. 2017, 95, 406–417. [Google Scholar] [CrossRef]
- Hamori, M.; Yoshimatsu, S.; Hukuchi, Y.; Shimizu, Y.; Fukushima, K.; Sugioka, N.; Nishimura, A.; Shibata, N. Preparation and pharmaceutical evaluation of nano-fiber matrix supported drug delivery system using the solvent-based electrospinning method. Int. J. Pharm. 2014, 464, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Yu, D.; Zhu, L.; Branford-White, C.; White, K.; Chatterton, N.P. Electrospun diclofenac sodium loaded eudragit® l 100-55 nanofibers for colon-targeted drug delivery. Int. J. Pharm. 2011, 408, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, K.; Guhathakarta, S.; Rajaram, R.; Korrapati, P.S. Electrospun zein/eudragit nanofibers based dual drug delivery system for the simultaneous delivery of aceclofenac and pantoprazole. Int. J. Pharm. 2012, 438, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun nanofibers: New concepts, materials, and applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef] [Green Version]
- Nagy, Z.K.; Balogh, A.; Demuth, B.; Pataki, H.; Vigh, T.; Szabo, B.; Molnar, K.; Schmidt, B.T.; Horak, P.; Marosi, G.; et al. High speed electrospinning for scaled-up production of amorphous solid dispersion of itraconazole. Int. J. Pharm. 2015, 480, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R.; Chatterton, N.P.; Nazir, T.; Yu, D.G.; Zhu, L.M.; Branford-White, C.J. Electrospun nanofibers in drug delivery: Recent developments and perspectives. Ther. Deliv. 2012, 3, 515–533. [Google Scholar] [CrossRef] [PubMed]
- Ignatious, F.; Sun, L.; Lee, C.P.; Baldoni, J. Electrospun nanofibers in oral drug delivery. Pharm. Res. 2010, 27, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Lukas, D.; Sarkar, A.; Pokorny, P. Self-organization of jets in electrospinning from free liquid surface: A generalized approach. J. Appl. Phys. 2008, 103, 084309. [Google Scholar] [CrossRef]
- Sebe, I.; Szabó, B.; Nagy, Z.K.; Szabó, D.; Zsidai, L.; Kocsis, B.; Zelkó, R. Polymer structure and antimicrobial activity of polyvinylpyrrolidone-based iodine nanofibers prepared with high-speed rotary spinning technique. Int. J. Pharm. 2013, 458, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Raimi-Abraham, B.T.; Mahalingam, S.; Davies, P.J.; Edirisinghe, M.; Craig, D.Q.M. Development and characterization of amorphous nanofiber drug dispersions prepared using pressurized gyration. Mol. Pharm. 2015, 12, 3851–3861. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Karaca, B.; Vanoosten, S.; Yuca, E.; Mahalingam, S.; Edirisinghe, M.; Tamerler, C. Coupling infusion and gyration for the nanoscale assembly of functional polymer nanofibers integrated with genetically engineered proteins. Macromol. Rapid Commun. 1322, 36, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Mahalingam, S.; Edirisinghe, M. Simultaneous application of pressure-infusion-gyration to generate polymeric nanofibers. Macromol. Mater. Eng. 2017, 302, 1600564. [Google Scholar] [CrossRef]
- Heseltine Phoebe, L.; Ahmed, J.; Edirisinghe, M. Developments in pressurized gyration for the mass production of polymeric fibers. Macromol. Mater. Eng. 2018, 1800218. [Google Scholar] [CrossRef]
- Hamori, M.; Nagano, K.; Kakimoto, S.; Naruhashi, K.; Kiriyama, A.; Nishimura, A.; Shibata, N. Preparation and pharmaceutical evaluation of acetaminophen nano-fiber tablets: Application of a solvent-based electrospinning method for tableting. Biomed. Pharmacother. 2016, 78, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Demuth, B.; Farkas, A.; Balogh, A.; Bartosiewicz, K.; Kallai-Szabo, B.; Bertels, J.; Vigh, T.; Mensch, J.; Verreck, G.; Van Assche, I.; et al. Lubricant-induced crystallization of itraconazole from tablets made of electrospun amorphous solid dispersion. J. Pharm. Sci. 2016, 105, 2982–2988. [Google Scholar] [CrossRef] [PubMed]
- Poller, B.; Strachan, C.; Broadbent, R.; Walker, G.F. A minitablet formulation made from electrospun nanofibers. Eur. J. Pharm. Biopharm. 2017, 114, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Demuth, B.; Nagy, Z.K.; Balogh, A.; Vigh, T.; Marosi, G.; Verreck, G.; Van Assche, I.; Brewster, M.E. Downstream processing of polymer-based amorphous solid dispersions to generate tablet formulations. Int. J. Pharm. 2015, 486, 268–286. [Google Scholar] [CrossRef] [PubMed]
- Vigh, T.; Démuth, B.; Balogh, A.; Galata, D.L.; Van Assche, I.; Mackie, C.; Vialpando, M.; Van Hove, B.; Psathas, P.; Borbás, E.; et al. Oral bioavailability enhancement of flubendazole by developing nanofibrous solid dosage forms. Drug Dev. Ind. Pharm. 2017, 43, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- US Pharmacopeia. Tablet Friability. Available online: https://www.usp.org/sites/default/files/usp/document/harmonization/gen-chapter/g06_pf_ira_32_2_2006.pdf (accessed on 7 August 2017).
- Balogh, A.; Farkas, B.; Farago, K.; Farkas, A.; Wagner, I.; Van Assche, I.; Verreck, G.; Nagy, Z.K.; Marosi, G. Melt-blown and electrospun drug-loaded polymer fiber mats for dissolution enhancement: A comparative study. J. Pharm. Sci. 2015, 104, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Pharmtech. Direct Compression Versus Granulation. Available online: http://www.pharmtech.com/direct-compression-versus-granulation (accessed on 7 September 2017).
- Démuth, B.; Farkas, A.; Szabó, B.; Balogh, A.; Nagy, B.; Vágó, E.; Vigh, T.; Tinke, A.P.; Kazsu, Z.; Demeter, Á.; et al. Development and tableting of directly compressible powder from electrospun nanofibrous amorphous solid dispersion. Adv. Powder Technol. 2017, 28, 1554–1563. [Google Scholar] [CrossRef]
- Battu, S.K.; Repka, M.A.; Majumdar, S.; Rao, Y.M. Formulation and evaluation of rapidly disintegrating fenoverine tablets: Effect of superdisintegrants. Drug Dev. Ind. Pharm. 2007, 33, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.H.; Stiel, D.; Weiss, M.; Infeld, M.H.; Malick, A.W. Evaluation of two new tablet lubricants-sodium stearyl fumarate and glyceryl behenate. Measurement of physical parameters (compaction, ejection and residual forces) in the tableting process and the effect on the dissolution rate. Drug Dev. Ind. Pharm. 1986, 12, 1329–1346. [Google Scholar] [CrossRef]
- Pharmaapproach. High-Shear Granulator. Available online: http://pharmapproach.com/high-shear-granulator/ (accessed on 1 August 2017).
- EMA. Ich Topic q4b Annex 5 Disintegration Test General Chapter. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003292.pdf (accessed on 2 September 2017).
- Gordon, K.C.; McGoverin, C.M. Raman mapping of pharmaceuticals. Int. J. Pharm. 2011, 417, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Vo, C.L.-N.; Park, C.; Lee, B.-J. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2013, 85, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.C. The segregation of particulate materials. A review. Powder Technol. 1976, 15, 245–251. [Google Scholar] [CrossRef]

| Applied Tableting Ingredients | Amount (mg)/Tablet | Amount (%)/Tablet | Total Amount of Materials (Batch Size 5000 Tablets) |
|---|---|---|---|
| Electrospun/Crys. Carvedilol | 0.05 | 0.05 | 0.25 g |
| poly (vinylpyrrolidone-co-vinyl acetate) (PVPVA64) | 4.95 | 4.95 | 24.75 g |
| Flowlac 100 mesh (amorphous lactose) | 65.00 | 65.00 | 325 g |
| Microcrystalline cellulose (MCC) 112 | 25.50 | 25.50 | 127.5 g |
| Croscarmellose sodium (CCS) | 3.00 | 3.00 | 15.0 g |
| Sodium stearyl fumarate (Pruv) | 1.5 | 1.50 | 7.5 g |
| ∑ | 100.0 | 100.0 | 500.00 g |
| Sample | Preparation Method | Applied Solvent | Dissolved PVPVA64 and CAR (99:1) in 10 mL of Solvent (g) | Flow Rate (mL/h) | Productivity for Dried Material (g/h) |
|---|---|---|---|---|---|
| PVPVA64 +1% CAR SNES | Single-needle electrospinning | 96% EtOH | 4.00 | 6 | 1.8 |
| PVPVA64 +1% CAR HSES | High-speed electrospinning | 96% EtOH | 4.00 | 750 | 225 |
| Characteristics | LSM Reference | HSM Reference | LSM HSES Batch A | LSM HSES Batch B | HSM HSES Batch A | HSM HSES Batch B |
|---|---|---|---|---|---|---|
| API type | Crystalline CAR | Crystalline CAR | Electrospun CAR | Electrospun CAR | Electrospun CAR | Electrospun CAR |
| Method of homogenization | Manual (LSM) | High-shear (HSM) | Manual (LSM) | Manual (LSM) | High-shear (HSM) | High-shear (HSM) |
| Weight (mg) | 99.9 ± 0.5 | 99.2 ± 0.7 | 99.2 ± 0.8 | 100.2 ± 0.7 | 100.9 ± 1.0 | 99.8 ± 1.1 |
| Thickness (mm) | 3.08 ± 0.44 | 3.02 ± 0.54 | 3.06 ± 0.69 | 3.04 ± 0.57 | 3.05 ± 0.60 | 3.03 ± 0.52 |
| Hardness (N) | 64.3 ± 7.62 | 69.2 ± 6.22 | 60.6 ± 5.67 | 63.8 ± 7.51 | 68.6 ± 5.11 | 62.7 ± 6.71 |
| Diameter (mm) | 5.97 ± 0.19 | 5.95 ± 0.22 | 5.97 ± 0.27 | 5.98 ± 0.17 | 5.99 ± 0.29 | 5.97 ± 0.21 |
| Friability (%) | 0.21 | 0.31 | 0.39 | 0.25 | 0.20 | 0.27 |
| Disintegration (s) | 84 | 95 | 103 | 119 | 138 | 125 |
| Sample Number | Carvedilol % | |||||
|---|---|---|---|---|---|---|
| LSM Reference | HSM Reference | LSM ES A | LSM ES B | HSM ES A | HSM ES B | |
| Average | 98.76 | 97.68 | 94.19 | 93.21 | 97.63 | 96.71 |
| SD (%) | 7.43 | 4.65 | 9.50 | 6.80 | 2.04 | 2.49 |
| RSD | 7.52 | 5.13 | 10.08 | 7.30 | 2.09 | 2.58 |
| Sieve Size (µm) | Pure ES Material (g) | LSM Final Powder Mixture (g) | HSM Final Powder Mixture (g) |
|---|---|---|---|
| 355 | 13.80 | 0.22 | 0.02 |
| 180 | 1.61 | 5.02 | 5.41 |
| 90 | 1.03 | 9.22 | 9.35 |
| sub 90 | 8.56 | 10.54 | 10.22 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fülöp, G.; Balogh, A.; Farkas, B.; Farkas, A.; Szabó, B.; Démuth, B.; Borbás, E.; Nagy, Z.K.; Marosi, G. Homogenization of Amorphous Solid Dispersions Prepared by Electrospinning in Low-Dose Tablet Formulation. Pharmaceutics 2018, 10, 114. https://doi.org/10.3390/pharmaceutics10030114
Fülöp G, Balogh A, Farkas B, Farkas A, Szabó B, Démuth B, Borbás E, Nagy ZK, Marosi G. Homogenization of Amorphous Solid Dispersions Prepared by Electrospinning in Low-Dose Tablet Formulation. Pharmaceutics. 2018; 10(3):114. https://doi.org/10.3390/pharmaceutics10030114
Chicago/Turabian StyleFülöp, Gergő, Attila Balogh, Balazs Farkas, Attila Farkas, Bence Szabó, Balázs Démuth, Enikő Borbás, Zsombor Kristóf Nagy, and György Marosi. 2018. "Homogenization of Amorphous Solid Dispersions Prepared by Electrospinning in Low-Dose Tablet Formulation" Pharmaceutics 10, no. 3: 114. https://doi.org/10.3390/pharmaceutics10030114