Alginate in Wound Dressings
Abstract
:1. Introduction
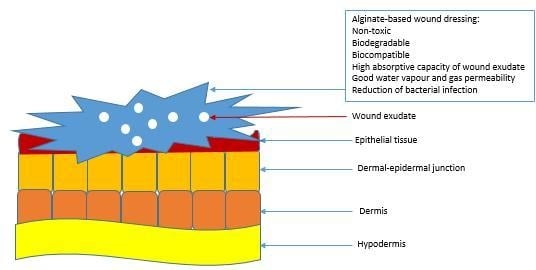
2. Wound Healing Process
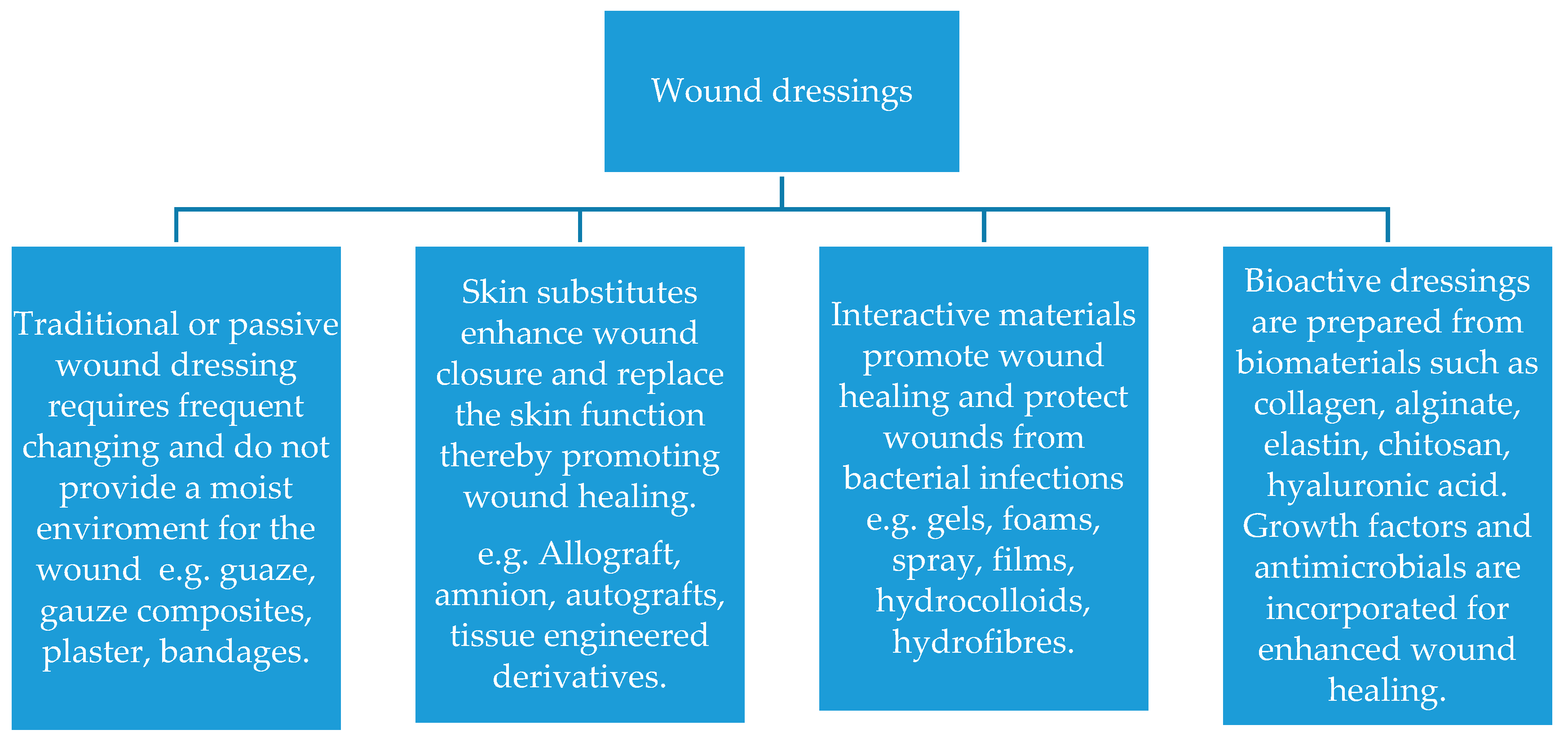
2.1. Wound Dressing Classification
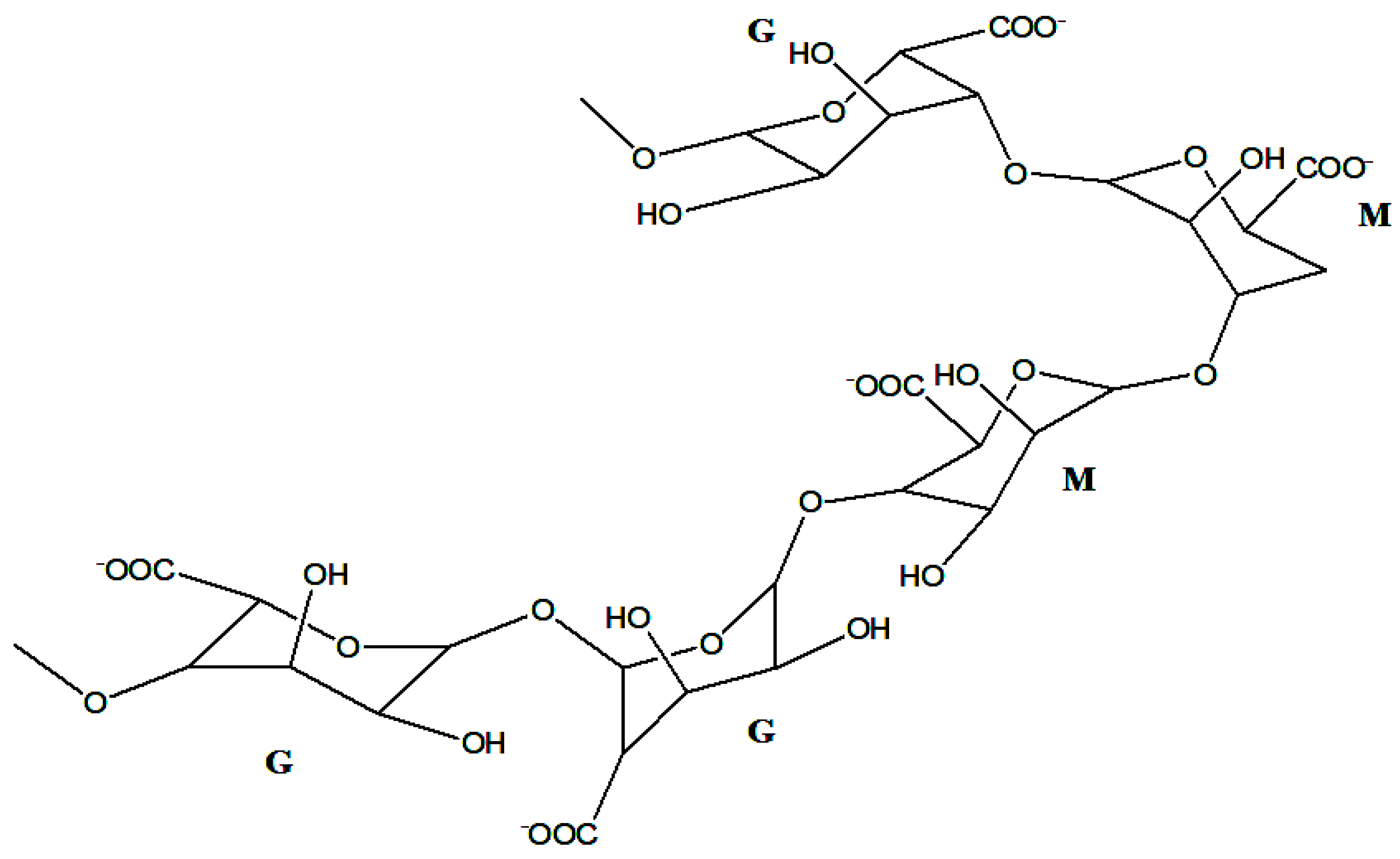
2.2. Alginate Properties and Structure
3. Alginate-Based Wound Dressing
3.1. Hydrogel
3.2. Films/Membranes
3.3. Nanofibres
3.4. Foams
3.5. Topical Formulation
3.6. Wafers
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wound Classification. Available online: http://www.clinimed.co.uk/Wound-Care/Education/Wound-Essentials/Wound-Classification.aspx (accessed on 13 December 2017).
- Baranoski, S.; Ayello, E.A.; Langemo, D.K. Wound assessment. In Wound Care Essentials: Practice Principles; Mills, J.E., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008; pp. 79–90. [Google Scholar]
- Cullum, N.; Buckley, H.; Dumville, J.; Hall, J.; Lamb, K.; Madden, M.; Morley, R.; O’Meara, S.; Goncalves, P.R.; Soares, M.; et al. Wounds research for patient benefit: A 5-year programme of research. 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK379923 (accessed on 13 December 2017).
- Frykberg, R.G.; Banks, J. Challenges in the treatment of chronic wounds. Adv Wound Care (New Rochelle) 2015, 4, 560–582. [Google Scholar] [CrossRef] [PubMed]
- Schmidtchen, A.; Pang, C.; Ni, G.; Sönnergren, H.; Car, J.; Järbrink, K.; Bajpai, R. The humanistic and economic burden of chronic wounds: A protocol for a systematic review. Systematic reviews. Syst. Rev. 2017, 6, 7. [Google Scholar] [CrossRef]
- Flanagan, M. Barriers to the Implementation of Best Practice in Wound Care. Available online: http://www.wounds-uk.com/pdf/content_9030.pdf (accessed on 13 December 2017).
- Yadav, P.; Yadav, H.; Shah, V.G.; Shah, G.; Dhaka, G. Biomedical biopolymers, their origin and evolution in biomedical sciences: A systematic review. J. Clin. Diagn. Res. 2015, 9, ZE21–ZE25. [Google Scholar] [CrossRef] [PubMed]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Pol. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [PubMed]
- Sudarsan, S.; Franklin, D.S.; Guhanathan, S. Imbibed salts and pH-responsive behaviours of sodium-alginate based eco-friendly biopolymeric hydrogels-A solventless approach. MMAIJ 2015, 11, 24–29. [Google Scholar]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound dressings and comparative effectiveness data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef] [PubMed]
- Sezer, A.D.; Cevher, E. Biopolymers as wound healing materials: Challenges and new strategies. In Biomaterials Applications for Nanomedicine; Pignatello, R., Ed.; InTech: Rijeka, Croatia, 2011; pp. 383–414. ISBN 978-953-307-661-4. [Google Scholar]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.O.; Jafari, S.H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Siddiqui, A.R.; Bernstein, J.M. Chronic wound infection: Facts and controversies. Clin. Dermatol. 2010, 28, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, Part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Adv. Skin Wound Care 2012, 25, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Cowan, L.; Stechmiller, J.; Phillips, P.; Schultz, G. Science of wound healing: Translation of bench science into advances for chronic wound care. In Chronic Wound Care: A Clinical Source Book for Healthcare Professionals, 5th ed.; Krasner, D.L., Rodeheaver, G.T., Sibbald, R.G., Woo, K.Y., Eds.; HMP Communications: Malvern, AL, USA, 2012; pp. 25–35. [Google Scholar]
- Benbow, M. Best practice in wound assessment. Nurs. Stand. 2016, 30, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.K.; Siprashvili, Z.; Khavari, P.A. Advances in skin grafting and treatment of cutaneous wounds. Science 2014, 346, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Aller, M.A.; Arias, J.I.; Arraez-Aybar, L.A.; Gilsanz, C.; Arias, J. Wound healing reaction: A switch from gestation to senescence. World J. Exp. Med. 2014, 4, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Zuliani-Alvarez, L.; Midwood, K.S. Fibrinogen-related proteins in tissue repair: How a unique domain with a common structure controls diverse aspects of wound healing. Adv. Wound Care 2015, 4, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.A.; DiPietro, L.A. Factors affecting wound healing. J. Dental Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Hamdan, N.; Ikeda, Y.; Grynpas, M.; Ganss, B.; Glogauer, M. Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: A review. Biomater. Res. 2017, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Oro, F.B.; Sikka, R.S.; Wolters, B.; Graver, R.; Boyd, J.L.; Nelson, B.; Swiontkowski, M.F. Autograft versus allograft: An economic cost comparison of anterior cruciate ligament reconstruction. Arthroscopy 2011, 27, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Fertah, M.; Belfkira, A.; Taourirte, M.; Brouillette, F. Extraction and characterization of sodium alginate from Moroccan Laminaria digitata brown seaweed. Arab. J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate gel particles—A review of production techniques and physical properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef] [PubMed]
- Donati, I.; Paoletti, S. Material properties of alginates. In Alginates: Biology and applications; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–53. [Google Scholar]
- Yang, C.H.; Wang, M.X.; Haider, H.; Yang, J.H.; Sun, J.-Y.; Chen, Y.M.; Zhou, J.; Suo, Z. Strengthening alginate/polyacrylamide hydrogels using various multivalent cations. ACS Appl. Mat. Interf. 2013, 5, 10418–10422. [Google Scholar] [CrossRef] [PubMed]
- Draget, K.I.; Taylor, C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Topuz, F.; Henke, A.; Richtering, W.; Groll, J. Magnesium ions and alginate do form hydrogels: A rheological study. Soft Matter 2012, 8, 4877–4881. [Google Scholar] [CrossRef]
- Mørch, Y.A.; Qi, M.; Gundersen, P.O.; Formo, K.; Lacik, I.; Skjåk-Bræk, G.; Oberholzer, J.; Strand, B.L. Binding and leakage of barium in alginate microbeads. J. Biomed. Mater. Res. A 2012, 100, 2939–2947. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Xu, F.; Ding, H.; Tan, F.; Song, F.; Wang, J. Incorporation of magnesium ions into photo-crosslinked alginate hydrogel enhanced cell adhesion ability. J. Tissue Eng. Regen. Med. 2015, 9, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci. 2016, 8, 1–17. [Google Scholar] [CrossRef]
- ALGICELL® Ag Antimicrobial Alginate Dressing. Available online: http://www.woundsource.com/product/algicell-ag-antimicrobial-alginate-dressing (accessed on 13 December 2017).
- AlgiSite M™. Available online: http://www.smith-nephew.com/professional/products/advanced-wound-management/algisite-m/ (accessed on 13 December 2017).
- SMTL Dressings Datacard. Available online: http://www.dressings.org/Dressings/comfeel-plus.html (accessed on 13 December 2017).
- KALTOSTAT Calcium Sodium Alginate Dressing. Available online: https://fsastore.com/KALTOSTAT-Calcium-Sodium-Alginate-Dressing-3-x-4-34-Box-of-10-P23356.aspx (accessed on 13 December 2017).
- Sorbsan Flat. Available online: http://www.aspenmedicaleurope.com/specialist_wound_car/sorbsan-flat/ (accessed on 13 December 2017).
- O’Meara, S.; Martyn-St James, M.; Adderley, U.J. Alginate Dressings for Venous Leg Ulcers. Available online: http://eprints.whiterose.ac.uk/92499/1/O%27Meara_et_al-2015-The_Cochrane_Library.pdf (accessed on 13 December 2017).
- Algivon. Available online: http://www.advancis.co.uk/products/activon-manuka-honey/algivon (accessed on 13 December 2017).
- FIBRACOL™ Plus Collagen Wound Dressing with Alginate. Available online: http://www.woundsource.com/product/fibracol-plus-collagen-wound-dressing-alginate (accessed on 13 December 2017).
- HYALOGRAN® Biodegradable Wound Dressing. Available online: http://www.anikatherapeutics.com/products/dermal/hyalogran/ (accessed on 13 December 2017).
- Tromboguard®. Available online: http://matopat.ro/wp-content/uploads/sites/2/2013/12/tromboguard-leaflet.pdf (accessed on 13 December 2017).
- Almeida, J.F.; Ferreira, P.; Lopes, A.; Gil, M.H. Photocrosslinkable biodegradable responsive hydrogels as drug delivery systems. Int. J. Biol. Macromol. 2011, 49, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Fu, X. Naturally derived materials-based cell and drug delivery systems in skin regeneration. J. Control. Release 2010, 142, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Jagur-Grodzinski, J. Polymeric gels and hydrogels for biomedical and pharmaceutical applications. Polym. Adv. Technol. 2010, 21, 27–47. [Google Scholar] [CrossRef]
- Sikareepaisan, P.; Ruktanonchai, U.; Supaphol, P. Preparation and characterization of asiaticoside-loaded alginate films and their potential for use as effectual wound dressings. Carbohydr. Polym. 2011, 83, 1457–1469. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.R.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Hess, C.T. Clinical Guide: Wound Care, 4th ed.; Springhouse Corporation: Spring House, PA, USA, 2002; pp. 275–276. [Google Scholar]
- Saarai, A.; Sedlacek, T.; Kasparkova, V.; Kitano, T.; Saha, P. On the characterization of sodium alginate/gelatine-based hydrogels for wound dressing. J. Appl. Polym. Sci. 2012, 126, E79–E88. [Google Scholar] [CrossRef]
- Saarai, A.; Kasparkova, V.; Sedlacek, T.; Sáha, P. A comparative study of crosslinked sodium alginate/gelatin hydrogels for wound dressing. In Proceeding of the 4th WSEAS International Conference on Energy and Development, Corfu Island, Greece, 14–16 July 2011; pp. 384–389. [Google Scholar]
- Straccia, M.C.; d’Ayala, G.G.; Romano, I.; Oliva, A.; Laurienzo, P. Alginate hydrogels coated with chitosan for wound dressing. Mar. Drugs 2015, 13, 2890–2908. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Jiang, Y.Y.; Sun, T.W.; Qi, C.; Zhao, H.; Chen, F.; Shi, Z.; Zhu, Y.J.; Chen, D.; He, Y. Design of a novel wound dressing consisting of alginate hydrogel and simvastatin-incorporated mesoporous hydroxyapatite microspheres for cutaneous wound healing. RSC Adv. 2016, 6, 104375–104387. [Google Scholar] [CrossRef]
- Mohandas, A.; Sudheesh Kumar, P.T.; Raja, B.; Lakshmanan, V.K.; Jayakumar, R. Exploration of alginate hydrogel/nano zinc oxide composite bandages for infected wounds. Int. J. Nanomed. 2015, 10, 53–66. [Google Scholar]
- Singh, R.; Singh, D. Radiation synthesis of PVP/alginate hydrogel containing nanosilver as wound dressing. J. Mater. Sci. Mater. Med. 2012, 23, 2649–2658. [Google Scholar] [CrossRef] [PubMed]
- Nazeri, S.; Ardakani, E.M.; Babavalian, H.; Latifi, A.M. Evaluation of Effectiveness of Honey-Based Alginate Hyrogel on Wound Healing in a Mouse Model of Rat. J. Appl. Biotechnol. Rep. 2015, 2, 293–297. [Google Scholar]
- Murakami, K.; Aoki, H.; Nakamura, S.; Nakamura, S.I.; Takikawa, M.; Hanzawa, M.; Kishimoto, S.; Hattori, H.; Tanaka, Y.; Kiyosawa, T.; et al. Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials 2010, 31, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, E.A.; Kenawy, E.R.; Tamer, T.M.; El-Meligy, M.A.; Eldin, M.S. Poly (vinyl alcohol)-alginate physically crosslinked hydrogel membranes for wound dressing applications: Characterization and bio-evaluation. Arab. J. Chem. 2015, 8, 38–47. [Google Scholar] [CrossRef]
- Xing, N.; Tian, F.; Yang, J.; Li, Y.K., II. Characterizations of Alginate-Chitosan Hydrogel for Wound Dressing Application. Adv. Mater. Res. 2012, 490, 3124–3128. [Google Scholar] [CrossRef]
- Rudyardjo, D.I.; Wijayanto, S. The synthesis and characterization of hydrogel chitosan-alginate with the addition of plasticizer lauric acid for wound dressing application. J. Phys. Conf. Ser. 2017, 853. [Google Scholar] [CrossRef]
- Devi, M.P.; Sekar, M.; Chamundeswari, M.; Moorthy, A.; Krithiga, G.; Murugan, N.S.; Sastry, T.P. A novel wound dressing material-fibrin-chitosan-sodium alginate composite sheet. Bull. Mater. Sci. 2012, 35, 1157–1163. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, J.; Peng, C.; Huang, T.; Zhou, H.; Ou, B.; Chen, J.; Liu, Q.; He, S.; Cao, D.; et al. Fabrication and physical properties of gelatin/sodium alginate/hyaluronic acid composite wound dressing hydrogel. J. Macromol. Sci. Part A 2014, 51, 318–325. [Google Scholar] [CrossRef]
- Liakos, I.; Rizzello, L.; Scurr, D.J.; Pompa, P.P.; Bayer, I.S.; Athanassiou, A. All-natural composite wound dressing films of essential oils encapsulated in sodium alginate with antimicrobial properties. Int. J. Pharm. 2014, 463, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Carvalho, A.; Vaz, D.C.; Gil, M.H.; Mendes, A.; Bártolo, P. Development of novel alginate based hydrogel films for wound healing applications. Int. J. Biol. Macromol. 2013, 52, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Tojeira, A.; Vaz, D.C.; Mendes, A.; Bártolo, P. Preparation and characterization of films based on alginate and Aloe vera. Int. J. Polym. Anal. Charact. 2011, 16, 449–464. [Google Scholar] [CrossRef]
- George, M.; Joseph, L.; Francis, L.T. Development and evaluation of silver sulphadiazine loaded sodium alginate gelatin film for wound dressing applications. Eur. J. Pharm. Med. Res. 2017, 4, 420–423. [Google Scholar]
- Li, S.; Li, L.; Guo, C.; Qin, H.; Yu, X. A promising wound dressing material with excellent cytocompatibility and proangiogenesis action for wound healing: Strontium loaded Silk fibroin/Sodium alginate (SF/SA) blend films. Int. J. Biol. Macromol. 2017, 104, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Rezvanian, M.; Amin, M.C.; Ng, S.F. Development and physicochemical characterization of alginate composite film loaded with simvastatin as a potential wound dressing. Carbohydr. Polym. 2016, 137, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Thu, H.E.; Zulfakar, M.H.; Ng, S.F. Alginate based bilayer hydrocolloid films as potential slow-release modern wound dressing. Int. J. Pharm. 2012, 434, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Dantas, M.D.; Cavalcante, D.R.; Araújo, F.E.; Barretto, S.R.; Aciole, G.T.; Pinheiro, A.L.; Ribeiro, M.A.; Lima-Verde, I.B.; Melo, C.M.; Cardoso, J.C.; et al. Improvement of dermal burn healing by combining sodium alginate/chitosan-based films and low level laser therapy. J. Photochem. Photobiol. B Biol. 2011, 105, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sanpui, P.; Chattopadhyay, A.; Ghosh, S.S. Fabrication of antibacterial silver nanoparticle-sodium alginate-chitosan composite films. RSC. Adv. 2012, 2, 5837–5843. [Google Scholar] [CrossRef]
- Abrigo, M.; McArthur, S.L.; Kingshott, P. Electrospun nanofibers as dressings for chronic wound care: Advances, challenges, and future prospects. Macromol. Biosci. 2014, 14, 772–792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lim, C.T.; Ramakrishna, S.; Huang, Z.M. Recent development of polymer nanofibers for biomedical and biotechnological applications. J. Mater. Sci. Mater. Med. 2005, 16, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, R.L.; Kasper, F.K.; Mikos, A.G. Polymeric nanofibers in tissue engineering. Tissue Eng. Part B Rev. 2011, 17, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Andreu, V.; Mendoza, G.; Arruebo, M.; Irusta, S. Smart dressings based on nanostructured fibers containing natural origin antimicrobial, anti-inflammatory, and regenerative compounds. Materials 2015, 8, 5154–5193. [Google Scholar] [CrossRef] [PubMed]
- Shalumon, K.T.; Anulekha, K.H.; Nair, S.V.; Nair, S.V.; Chennazhi, K.P.; Jayakumar, R. Sodium alginate/poly (vinyl alcohol)/nano ZnO composite nanofibers for antibacterial wound dressings. Int. J. Biol. Macromol. 2011, 49, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Gong, R.H.; Zhou, F.L. Electrospun sodium alginate/polyethylene oxide fibers and nanocoated yarns. Int. J. Polym. Sci. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Hajiali, H.; Summa, M.; Russo, D.; Armirotti, A.; Brunetti, V.; Bertorelli, R.; Athanassiou, A.; Mele, E. Alginate–lavender nanofibers with antibacterial and anti-inflammatory activity to effectively promote burn healing. J. Mater. Chem. B 2016, 4, 1686–1695. [Google Scholar] [CrossRef] [Green Version]
- Üstündağ, G.C.; Özbek, S.; Karaca, E.; Çavuşoğlu, İ. In vivo evaluation of electrospun poly (vinyl alcohol)/sodium alginate nanofibrous mat as wound dressing. Tekstil ve Konfeksiyon 2010, 20, 290–298. [Google Scholar]
- Coşkun, G.; Karaca, E.; Ozyurtlu, M.; Özbek, S.; Yermezler, A.; Çavuşoğlu, İ. Histological evaluation of wound healing performance of electrospun poly (vinyl alcohol)/sodium alginate as wound dressing in vivo. BioMed. Mater. Eng. 2014, 24, 1527–1536. [Google Scholar] [PubMed]
- Park, S.A.; Park, K.E.; Kim, W. Preparation of sodium alginate/poly (ethylene oxide) blend nanofibers with lecithin. Macromol. Res. 2010, 18, 891–896. [Google Scholar] [CrossRef]
- Fu, R.; Li, C.; Yu, C.; Xie, H.; Shi, S.; Li, Z.; Wang, Q.; Lu, L. A novel electrospun membrane based on moxifloxacin hydrochloride/poly (vinyl alcohol)/sodium alginate for antibacterial wound dressings in practical application. Drug Deliv. 2016, 23, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Park, I.K.; Kim, Y.S.; Kim, H.J.; Moon, H.; Mueller, S.; Jeong, Y.I. Physical, morphological, and wound healing properties of a polyurethane foam-film dressing. Biomater. Res. 2016, 20, 11. [Google Scholar]
- Foam Dressings. Available online: https://woundeducators.com/foam-dressings (accessed on 12 December 2017).
- Hegge, A.B.; Andersen, T.; Melvik, J.E.; Bruzell, E.; Kristensen, S.; Tønnesen, H.H. Formulation and bacterial phototoxicity of curcumin loaded alginate foams for wound treatment applications: Studies on curcumin and curcuminoides XLII. J. Pharm. Sci. 2011, 100, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Valerón Bergh, V.J.; Johannessen, E.; Andersen, T.; Tønnesen, H.H. Evaluation of porphyrin loaded dry alginate foams containing poloxamer 407 and β-cyclodextrin-derivatives intended for wound treatment. Pharm. Dev. Technol. 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Topical Semi-Solid Dosage Forms. Available online: https://www.malvern.com/en/industry-applications/sample-type-form/topicals-creams-and-gels (accessed on 12 December 2017).
- Topical Delivery—The Importance of the Right Formulation in Topical Drug Development. Drug Development and Delivery. 1 August 2015. Available online: http://www.drug-dev.com/Main/Back-Issues/TOPICAL-DELIVERY-The-Importance-of-the-Right-Formu-833 (accessed on 12 December 2017).
- Ahmed, M.M.; Jahangir, M.A.; Saleem, M.A.; Kazmi, I.; Bhavani, P.D.; Muheem, A. Formulation and Evaluation of Fucidin Topical Gel Containing Wound Healing Modifiers. Am. J. PharmTech Res. 2015, 5, 232–242. [Google Scholar]
- Fredric, S.; Gowda, D.V.; Yashashwini, M. Wafers for wound healing. J. Chem. Pharm. Res. 2015, 7, 450–468. [Google Scholar]
- Lipsky, B.A.; Hoey, C. Topical antimicrobial therapy for treating chronic wounds. Clin. Infect. Dis. 2009, 49, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.; Burgos-Amador, R.; Okeke, O.; Pawar, H. Composite alginate and gelatin based bio-polymeric wafers containing silver sulfadiazine for wound healing. Int. J. Biol. Macromol. 2015, 79, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.H.; Stevens, H.N.; Auffret, A.D.; Humphrey, M.J.; Eccleston, G.M. Lyophilised wafers as a drug delivery system for wound healing containing methylcellulose as a viscosity modifier. Int. J. Pharm. 2005, 289, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Pawar, H.V.; Boateng, J.S.; Ayensu, I.; Tetteh, J. Multifunctional medicated lyophilised wafer dressing for effective chronic wound healing. J. Pharm. Sci. 2014, 103, 1720–1733. [Google Scholar] [CrossRef] [PubMed]
- Gowda, D.V.; Fredric, S.; Srivastava, A.; Osmani, R.A. Design and development of antimicrobial wafers for chronic wound healing. Pharm. Lett. 2016, 8, 70–79. [Google Scholar]


| Barriers | Examples |
|---|---|
| Educational factor | Poor quality of research, lack of appropriate training, ritualistic practice and lack of appropriate skills. |
| Organizational factor | Lack of standardisation of practice that is acceptable, lack of expert opinion, instability in the health services. |
| Clinical factor | Bacterial infection, hypersensitivity, malnutrition, poor tissue perfusion, copious exudate, too much or too little information on wound management. |
| Psychosocial factor | Social isolation resulting in depression and reduced motivation with treatment, pain resulting in loss of sufficient sleep and lack of self-care. |
| Commercially Available Alginate-Based Wound Dressings | Composition | Applications | References |
|---|---|---|---|
| Algicell™ | Sodium alginate, 1.4% silver | Diabetic foot ulcer, leg ulcers, pressure ulcers, donor sites, and traumatic and surgical wounds. | [25,35] |
| AlgiSite M™ | Calcium alginate | Leg ulcers, pressure ulcers, diabetic foot ulcers and surgical wounds. | [36] |
| Comfeel Plus™ | Sodium carboxymethylcellulose and calcium alginate | Ulcers such as venous leg ulcers, pressure ulcers; burns, donor sites, postoperative wounds and necrotic wounds. | [37] |
| Kaltostat™ | Sodium alginate | Pressure ulcers, venous ulcers, diabetic ulcers, donor sites, and traumatic wounds. | [38] |
| Sorbsan™ | Calcium alginate | Arterial, venous, and diabetic leg ulcers Pressure ulcers, post-operative wounds, donor and graft sites and traumatic wounds. | [39] |
| Tegagen™ | Sodium alginate | Diabetic and infected wounds. | [40] |
| Guardix-SG® | Sodium alginate and poloxamer | To avoid post-operative adhesions in thyroid and spine surgeries. | [34] |
| SeaSorb® | Calcium alginate | Good for high exuding wounds e.g., ulcers such as diabetic and leg pressure ulcers. | [34] |
| Algivon® | Calcium alginate and Manuka honey | It eliminates odour and ideal for necrotic wounds and wounds with odours. | [41] |
| FibracolTMPlus | Calcium alginate and collagen | Full and partial-thickness wounds, for ulcers such as pressure ulcers, venous ulcers, diabetic ulcers and second-degree burns. | [42] |
| Hyalogran® | An ester of hyaluronic acid and sodium alginate | Used for ulcers, diabetic wounds, pressure sores, ischemic, necrotic wounds. | [43] |
| Tromboguard® | Sodium alginate, calcium alginate, chitosan, polyurethane and silver cations | Used to stop bleeding in postoperative wounds, traumatic wounds, gun shots, skin graft donor sites, bleeding from accidents. | [44] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. https://doi.org/10.3390/pharmaceutics10020042
Aderibigbe BA, Buyana B. Alginate in Wound Dressings. Pharmaceutics. 2018; 10(2):42. https://doi.org/10.3390/pharmaceutics10020042
Chicago/Turabian StyleAderibigbe, Blessing Atim, and Buhle Buyana. 2018. "Alginate in Wound Dressings" Pharmaceutics 10, no. 2: 42. https://doi.org/10.3390/pharmaceutics10020042