MTase Domain of Dendrolimus punctatus cypovirus VP3 Mediates Virion Attachment and Interacts with Host ALP Protein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viruses, Larvae, and Antibodies
2.2. Recombinant Proteins
2.3. Purification of DpCPV Virions
2.4. Preparation of BBMVs
2.5. Binding Characteristics of S. exigua BBMVs and Competition Assays
2.6. Inhibition of Viral Binding by Antibodies
2.7. Far-Western Blotting
2.8. Mass Spectrometry Analysis
2.9. Co-Immunoprecipitation (Co-IP) Assays
2.10. Inhibition of Viral Binding
2.11. In Vivo Neutralization Tests
3. Results
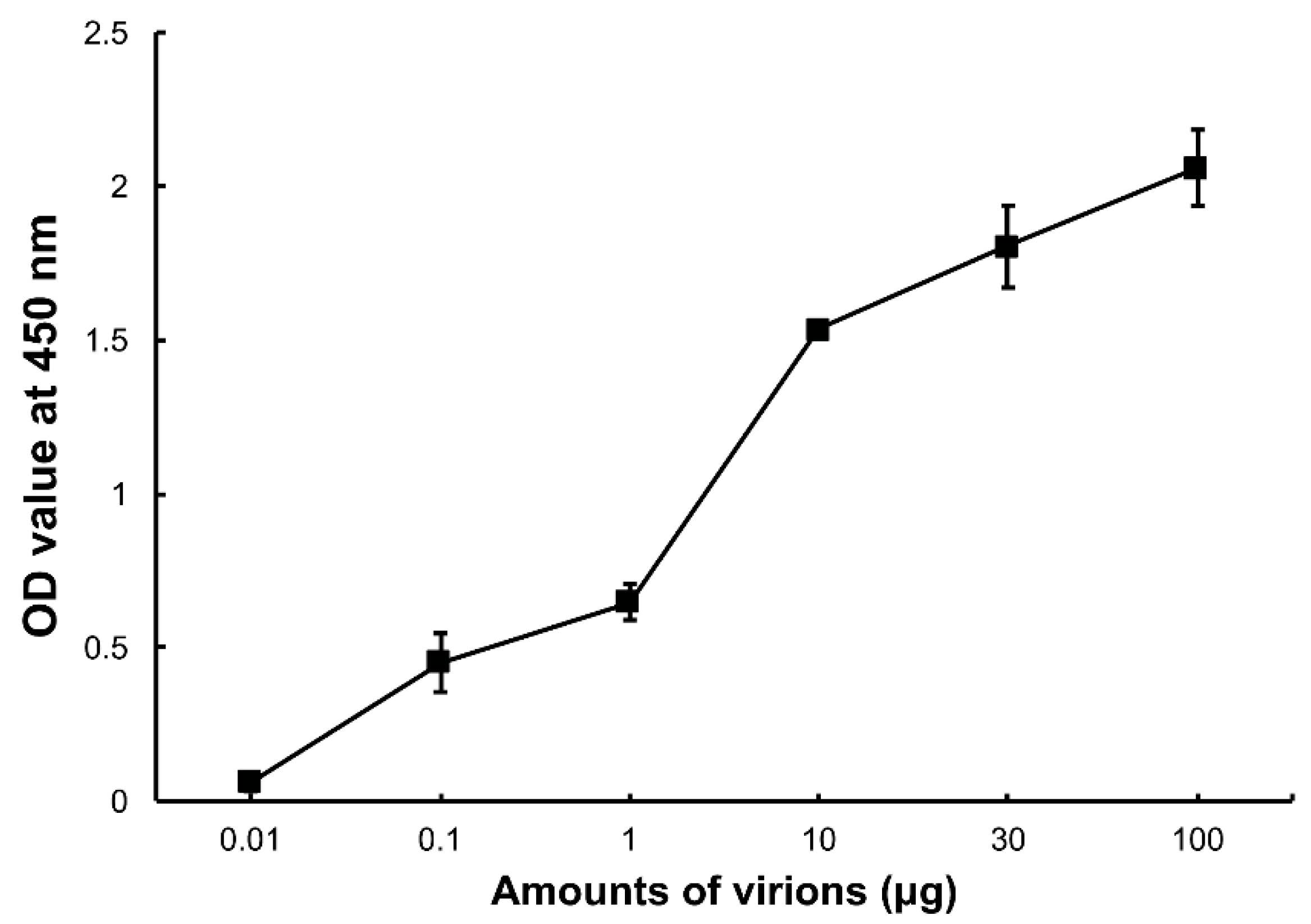
3.1. DpCPV Virions Binding to SeBBMVs
3.2. Binding Characteristics of the MBP Fusion Proteins to SeBBMVs
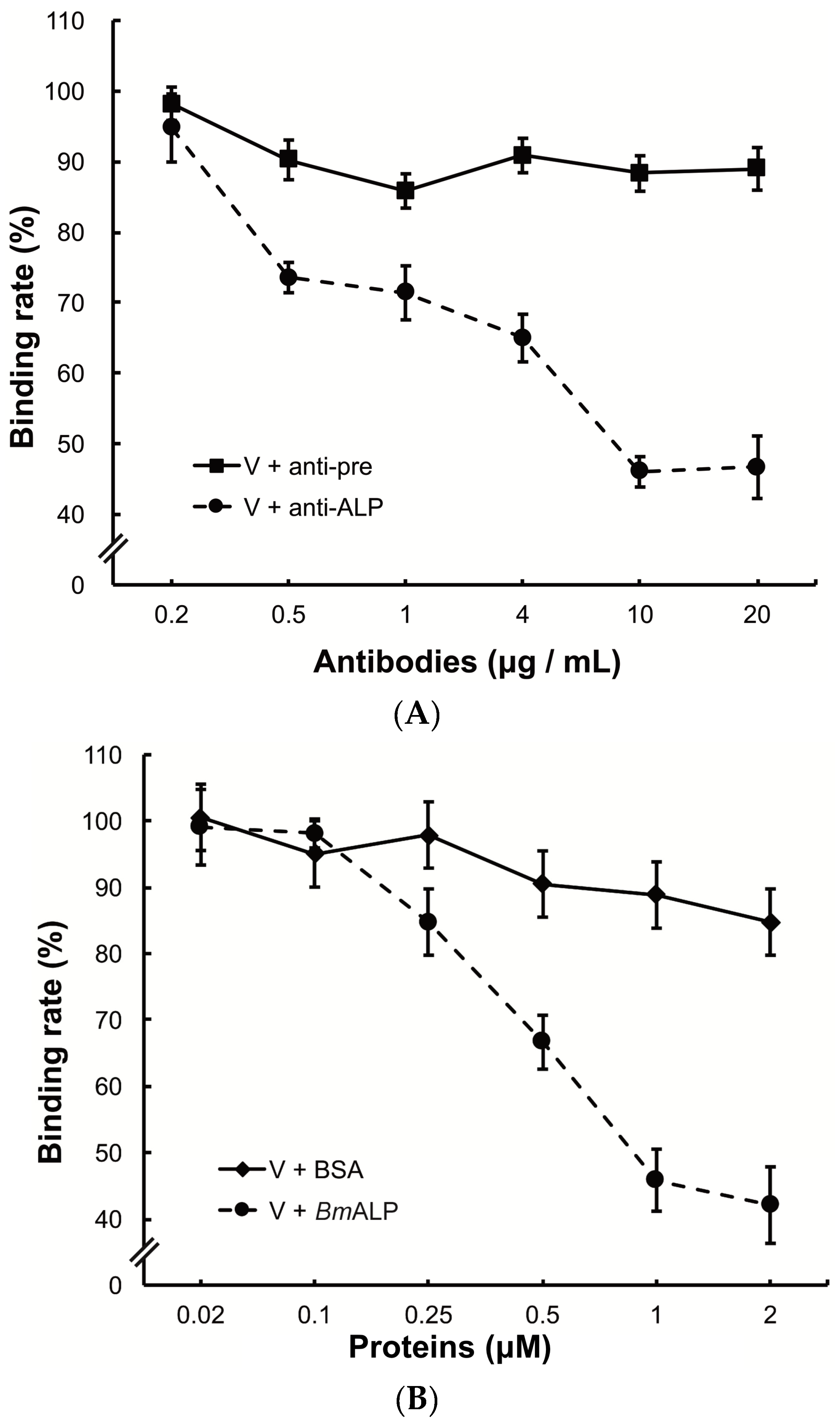
3.3. Inhibition of Viral Binding by Anti-MTase and Anti-VP4 Antibodies
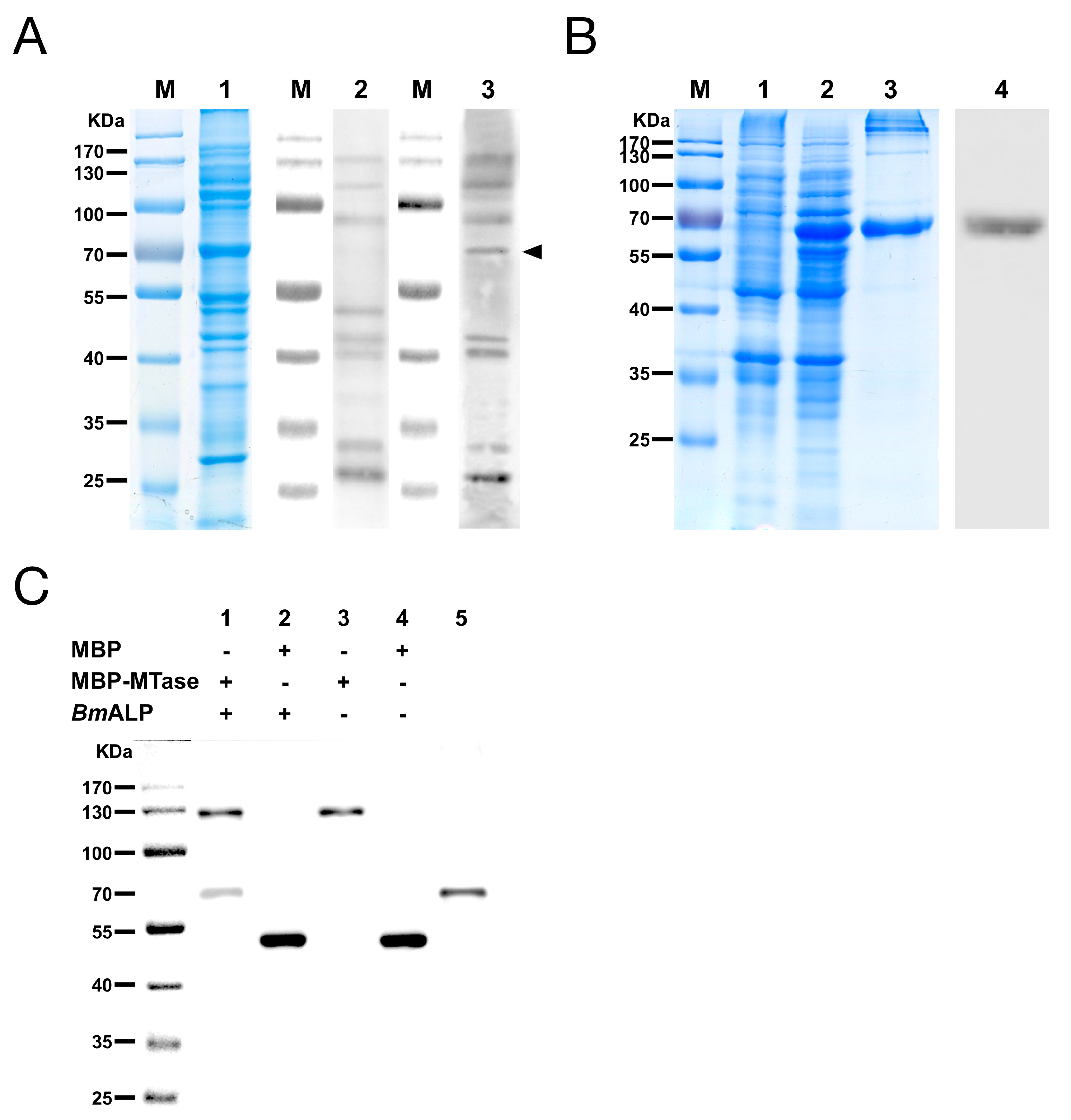
3.4. Identification of MTase Binding Proteins in Host BBMVs
3.5. Inhibition of Viral Binding to BmBBMVs
3.6. In Vivo Neutralization with ALP in S. exigua
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yu, X.; Jin, L.; Zhou, Z.H. 3.88 Å structure of cytoplasmic polyhedrosis virus by cryo-electron microscopy. Nature 2008, 453, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Metcalf, P. Cypoviruses. Insect Virology; Asgari, S., Johnson, K., Eds.; Caister Academic Press Publisher: Norfolk, UK, 2010; pp. 307–323. [Google Scholar]
- Zhang, Y.; Cao, G.; Zhu, L.; Chen, F.; Zar, M.S.; Wang, S.; Hu, X.; Wei, Y.; Xue, R.; Gong, C. Integrin beta and receptor for activated protein kinase C are involved in the cell entry of Bombyx mori cypovirus. Appl. Microbiol. Biotechnol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Sun, J.; Zhang, K.; Mou, Z.; Huang, X.; Ji, G.; Sun, F.; Zhang, J.; Zhu, P. Atomic model of a cypovirus built from cryo-EM structure provides insight into the mechanism of mRNA capping. Proc. Natl. Acad. Sci. USA 2011, 108, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.K.; Ge, P.; Jiang, J.S.; Atanasov, I.; Zhou, Z.H. Atomic model of CPV reveals the mechanism used by this single-shelled virus to economically carry out functions conserved in multishelled reoviruses. Structure 2011, 19, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.G.; Shao, Y.P.; Su, L.; Zhou, Y.; Sun, X.L. Interactions among dendrolimus punctatus cypovirus proteins and identification of the genomic segment encoding its A-spike. J. Gen. Virol. 2014, 95, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, J.; Atanasov, I.; Ryazantsev, S.; Zhou, Z.H. Electron tomography reveals polyhedrin binding and existence of both empty and full cytoplasmic polyhedrosis virus particles inside infectious polyhedra. J. Virol. 2011, 85, 6077–6081. [Google Scholar] [CrossRef] [PubMed]
- Nibert, M.L.; Baker, T.S. CPV, a stable and symmetrical machine for mRNA synthesis. Structure 2003, 11, 605–607. [Google Scholar] [CrossRef]
- Xu, G.; Wilson, W.; Mecham, J.; Murphy, K.; Zhou, E.M.; Tabachnick, W. VP7: An attachment protein of bluetongue virus for cellular receptors in Culicoides variipennis. J. Gen. Virol. 1997, 78, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Konopka-Anstadt, J.L.; Mainou, B.A.; Sutherland, D.M.; Sekine, Y.; Strittmatter, S.M.; Dermody, T.S. The Nogo receptor NgR1 mediates infection by mammalian reovirus. Cell Host Microbe 2014, 15, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Ludert, J.E.; Feng, N.; Yu, J.H.; Broome, R.L.; Hoshino, Y.; Greenberg, H.B. Genetic mapping indicates that VP4 is the rotavirus cell attachment protein in vitro and in vivo. J. Virol. 1996, 70, 487–493. [Google Scholar] [PubMed]
- Furlong, D.B.; Nibert, M.L.; Fields, B.N. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J. Virol. 1988, 62, 246–256. [Google Scholar] [PubMed]
- Kirchner, E.; Guglielmi, K.M.; Strauss, H.M.; Dermody, T.S.; Stehle, T. Structure of reovirus Sigma 1 in complex with its receptor junctional adhesion molecule-A. PLoS Pathog. 2008, 4, e1000235. [Google Scholar] [CrossRef] [PubMed]
- Schulz, W.L.; Haj, A.K.; Schiff, L.A. Reovirus uses multiple endocytic pathways for cell entry. J. Virol. 2012, 86, 12665–12675. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.F.; Isa, P.; Guerrero, C.A.; Méndez, E.; Zárate, S.; López, T.; Espinosa, R.; Romero, P.; López, S. Molecular biology of rotavirus cell entry. Arch. Med. Res. 2002, 33, 356–361. [Google Scholar] [CrossRef]
- Jin, L.; Wang, H.X.; Liu, J.J.; Wang, J.C.; Zhang, L.L.; Yang, G.; Zhang, X.H.; Zheng, G.H. Polyclonal antibody preparation and immune activity of structural proteins VP1, VP3 and VP5 of dendrolimus puntatus cytoplasmic polyhedrosis virus. Chin. J. Appl. Environ. Biol. 2014, 20, 345–350. (In Chinese) [Google Scholar]
- Jin, L.; Dai, C.W.; Qin, T.C.; Sun, X.L. Molecular characterization of protein p50 of dendrolimus punctatus cytoplasmic polyhedrosis virus. J. Basic Microbiol. 2013, 53, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Wolfersberger, M.; Luthy, P.; Maurer, A.; Parenti, P.; Sacchi, V.F.; Giordana, B.; Hanozet, G.M. Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (pieris brassicae). Comp. Biochem. Physiol. 1987, 86, 301–308. [Google Scholar] [CrossRef]
- Zhou, Y.; Qin, T.C.; Xiao, Y.Z.; Qin, F.J.; Lei, C.F.; Sun, X.L. Genomic and biological characterization of a new cypovirus isolated from dendrolimus punctatus. PLoS ONE 2014, 9, e113201. [Google Scholar] [CrossRef] [PubMed]
- Kalbfleisch, J.D.; Prentice, R.D. The Statistical Analysis of Failure Time Data; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Zhu, B.; Yang, C.W.; Liu, H.R.; Cheng, L.P.; Song, F.; Zeng, S.J.; Huang, X.J.; Ji, G.; Zhu, P. Identification of the active sites in the methyltransferases of a transcribing dsRNA virus. J. Mol. Biol. 2014, 426, 2167–2174. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, F.; Chiu, E.; Ikeda, K.; Gutmann, S.; Haebel, P.W.; Schulze-Briese, C.; Mori, H.; Metcalf, P. The molecular organization of cypovirus polyhedra. Nature 2007, 446, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cheng, Z.; Zhang, S.; Xiong, W.; Xia, H.; Qiu, Y.; Wang, Z.; Wu, F.; Qin, C.F.; Yin, L.; et al. A cypovirus VP5 displays the RNA chaperone-like activity that destabilizes RNA helices and accelerates strand annealing. Nucleic Acids Res. 2014, 42, 2538–2554. [Google Scholar] [CrossRef] [PubMed]
- Kolliopoulou, A.; Nieuwerburgh, V.F.; Stravopodis, D.J.; Deforce, D.; Swevers, L.; Smagghe, G. Transcriptome analysis of bombyx mori larval midgut during persistent and pathogenic cytoplasmic polyhedrosis virus infection. PLoS ONE 2015, 10, e0121447. [Google Scholar] [CrossRef] [PubMed]
- Pigott, C.R.; Ellar, D.J. Role of receptors in bacillus thuringiensis crystal toxin activity. Microbiol. Mol. Biol. Rev. 2007, 71, 255–281. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, L.E.; Aimanova, K.G.; Gill, S.S.; Bravo, A.; Soberón, M. A GPI-anchored alkaline phosphatase is a functional midgut receptor of cry11Aa toxin in Aedes aegypti larvae. Biochem. J. 2006, 394, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Arenas, I.; Bravo, A.; Soberón, M.; Gómez, I. Role of alkaline phosphatase from manduca sexta in the mechanism of action of bacillus thuringiensis Cry1Ab toxin. J. Biol. Chem. 2010, 285, 12497–12503. [Google Scholar] [CrossRef] [PubMed]
- Yamayoshi, S.; Yamashita, Y.; Li, J.; Hanagata, N.; Minowa, T.; Takemura, T.; Koike, S. Scavenger receptor B2 is a cellular receptor for enterovirus 71. Nat. Med. 2009, 15, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Shimojima, M.; Tano, Y.; Miyamura, T.; Wakita, T.; Shimizu, H. Human P-selectin glycoprotein ligand-1 is a functional receptor for enterovirus 71. Nat. Med. 2009, 15, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.L.; Chou, Y.T.; Wu, C.N.; Ho, M.S. Annexin II binds to capsid protein VP1 of enterovirus 71 and enhances viral infectivity. J. Virol. 2011, 85, 11809–11820. [Google Scholar] [CrossRef] [PubMed]


| Primer | Primer Sequence (5′→3′) |
|---|---|
| gtase-F | CGGGATCCATGTGGCATTATACGAGTATCAAC (BamHI Site underlined) |
| gtase-R | CCCAAGCTTTTAGGAAATATAATTCGCGGTGAT (HindIII Site underlined) |
| mtase-F | CGGAATTCGAGCCAATGAGCATAGCT (EcoRI Site underlined) |
| mtase-R | CGGGATCCTTACGAGCGTACAAGTTTGATC (BamHI Site underlined) |
| vp4-F | CGGAATTCATGTTCGCAATCGATCCACT (EcoRI Site underlined) |
| vp4-R | CGGAATTCATGTTCGCAATCGATCCACT (BamHI Site underlined) |
| vp5-F | CGGAATTCATGTTACAACAACCAGCAGGA (EcoRI Site underlined) |
| vp5-R | CGGGATCCTCATAGGATGTCATCTGAGTGC (BamHI Site underlined) |
| Accession | Similar Protein (Protein Name/Organism) | MASCOT Score | Nominal Mass (Da) | Sequence Coverage (%) |
|---|---|---|---|---|
| gi|3721840 | aminopeptidase N/Bombyx mori | 41 | 109,624 | 16 |
| gi|227072221 | troponin I variant D/Bombyx mandarina | 38 | 23,711 | 7 |
| gi|356582739 | TSSK/Bombyx mori | 33 | 39,640 | 9 |
| gi|189332880 | alkaline phosphatase/Bombyx mori | 32 | 60,682 | 9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, L.; Xu, C.; Cheng, C.; Lei, C.; Sun, X. MTase Domain of Dendrolimus punctatus cypovirus VP3 Mediates Virion Attachment and Interacts with Host ALP Protein. Viruses 2017, 9, 66. https://doi.org/10.3390/v9040066
Su L, Xu C, Cheng C, Lei C, Sun X. MTase Domain of Dendrolimus punctatus cypovirus VP3 Mediates Virion Attachment and Interacts with Host ALP Protein. Viruses. 2017; 9(4):66. https://doi.org/10.3390/v9040066
Chicago/Turabian StyleSu, Lan, Congrui Xu, Chuangang Cheng, Chengfeng Lei, and Xiulian Sun. 2017. "MTase Domain of Dendrolimus punctatus cypovirus VP3 Mediates Virion Attachment and Interacts with Host ALP Protein" Viruses 9, no. 4: 66. https://doi.org/10.3390/v9040066






