Transmission of Hepatitis E Virus in Developing Countries
Abstract
:1. Introduction
2. Overview of Hepatitis E Virus (HEV)
3. Hepatitis E in Developing Countries
3.1. HEV-1 and HEV-2
3.2. HEV-3 and HEV-4
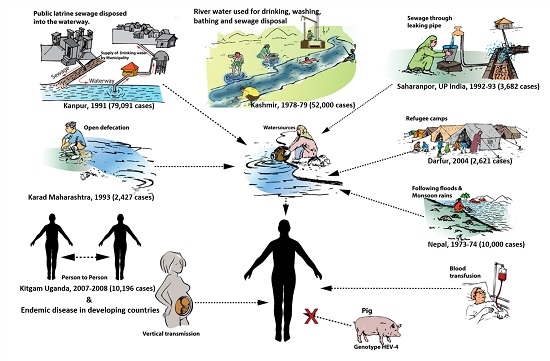
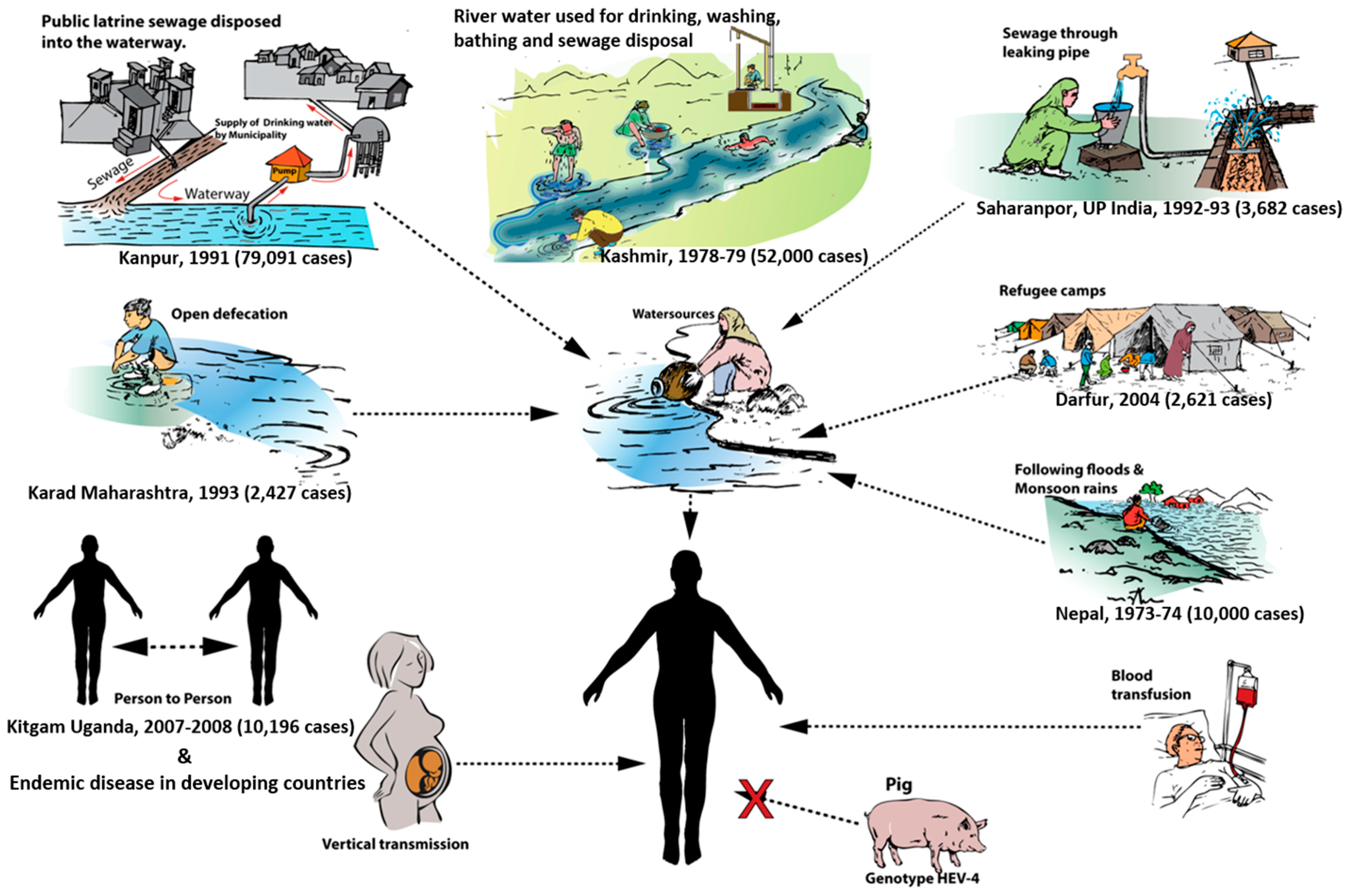
4. Modes of Transmission
4.1. Waterborne Transmission
4.2. Person-to-Person Transmission
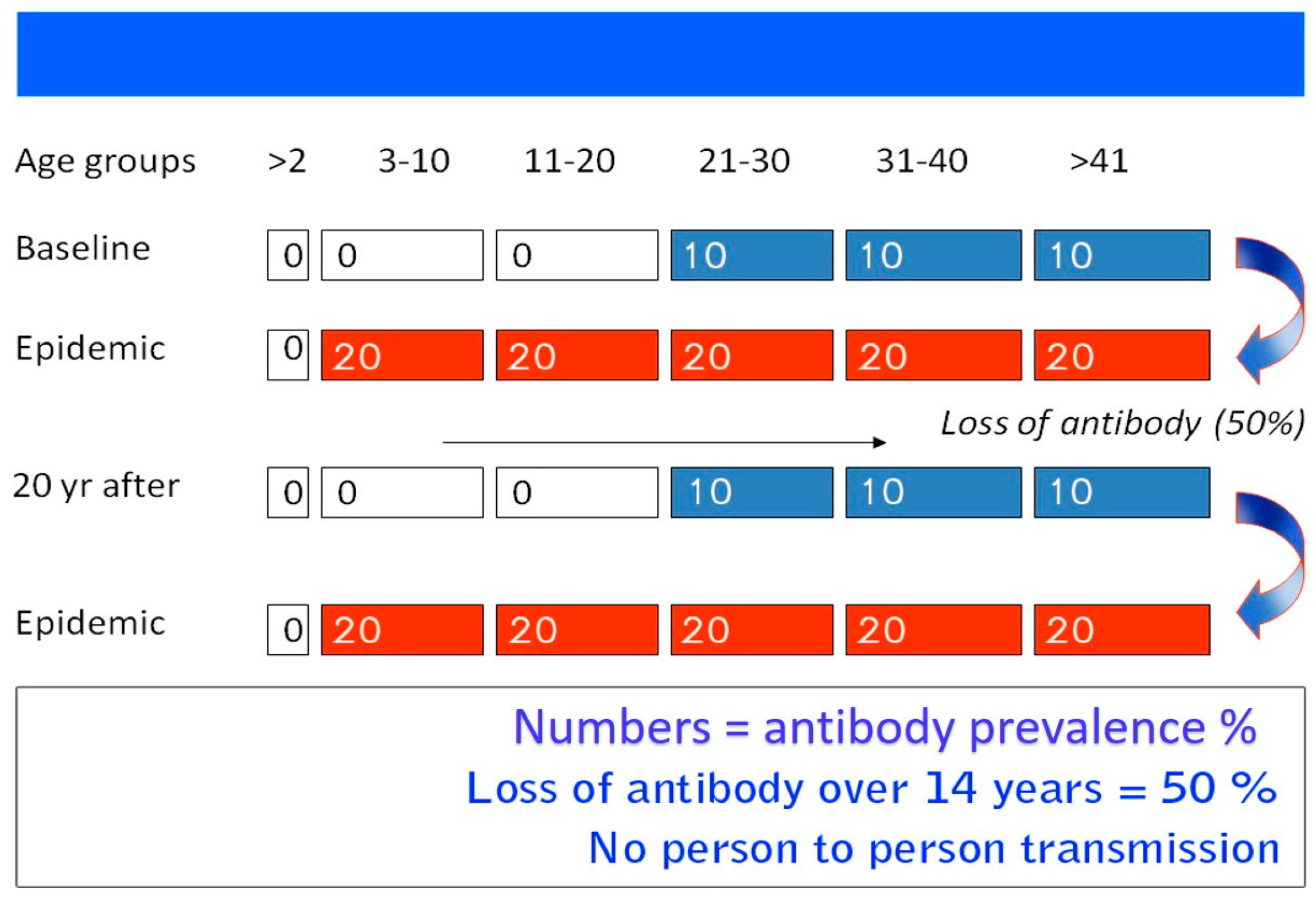
4.2.1. Epidemic Disease
4.2.2. Sporadic Disease
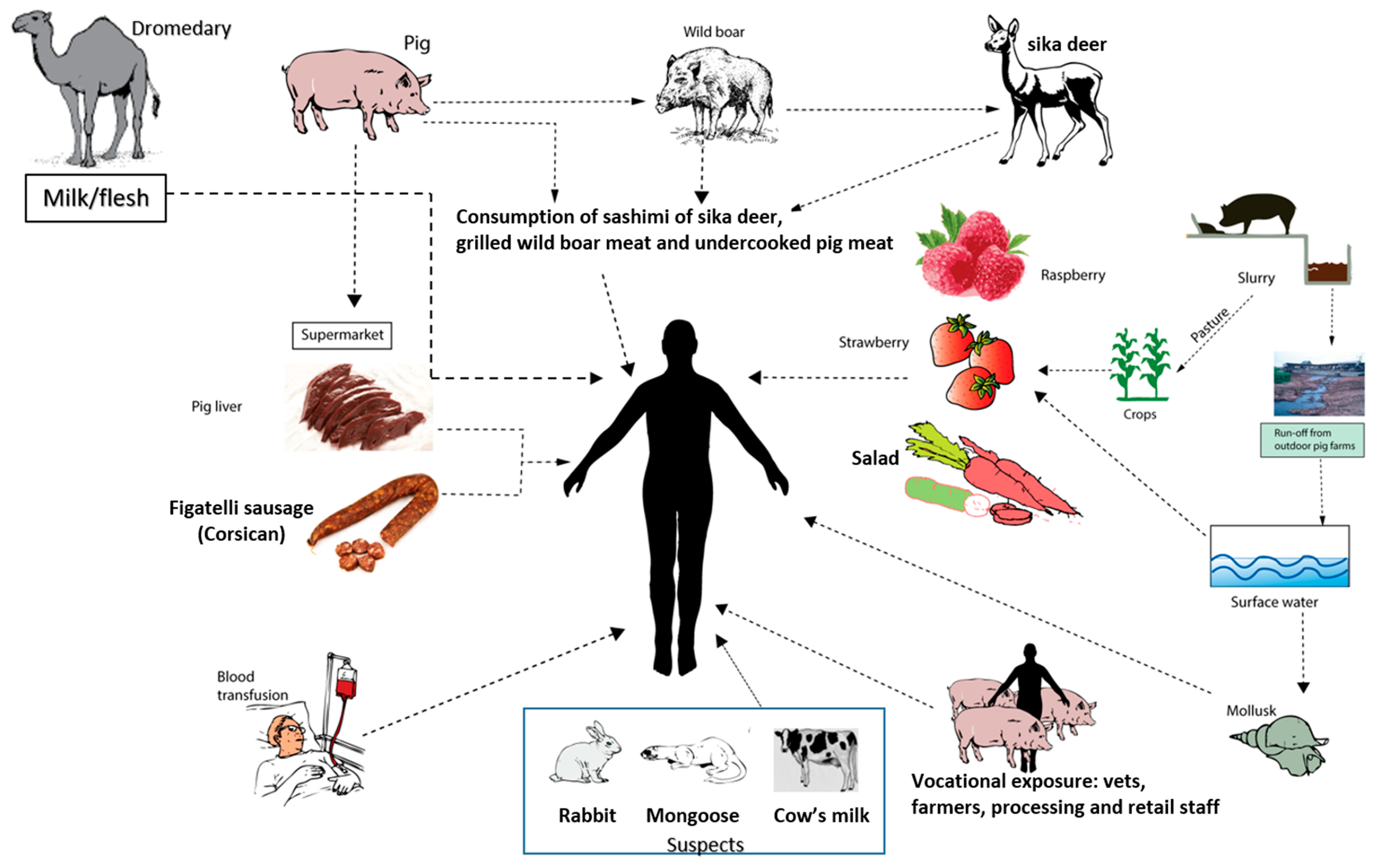
4.3. Zoonotic Transmission
4.4. Parenteral Transmission
4.5. Vertical Transmission
4.6. Nosocomial Transmission
4.7. Sexual Transmission
4.8. HEV and Milk
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Khuroo, M.S.; Khuroo, M.S. Hepatitis E: An emerging global disease—From discovery towards control and cure. J. Viral Hepat. 2016, 23, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Khuroo, M.S.; Khuroo, M.S. Sanitation and sewage disposal in India. JK-Practitioner 2015, 20, 43–46. [Google Scholar]
- Khuroo, M.S. Hepatitis E: The enterically transmitted non-a, non-b hepatitis. Indian J. Gastroenterol. 1991, 10, 96–100. [Google Scholar] [PubMed]
- Khuroo, M.S. Discovery of hepatitis E: The epidemic non-a, non-b hepatitis 30 years down the memory lane. Virus Res. 2011, 161, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Pavio, N.; Meng, X.J.; Renou, C. Zoonotic hepatitis E: Animal reservoirs and emerging risks. Vet. Res. 2010, 41, 46. [Google Scholar] [CrossRef] [PubMed]
- Thiry, D.; Mauroy, A.; Pavio, N.; Purdy, M.A.; Rose, N.; Thiry, E.; de Oliveira-Filho, E.F. Hepatitis E virus and related viruses in animals. Transbound. Emerg. Dis. 2015. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. List of Developing Countries. In Proceedings of the 26th General Assembly International Union Geodesy and Geophysics, Prague, Czech Republic, 22 June–2 July 2015; Available online: www.iugg2015prague.com (accessed on 30 June 2016).
- Khuroo, M.S. Study of an epidemic of non-a, non-b hepatitis. Possibility of another human hepatitis virus distinct from post-transfusion non-a, non-b type. Am. J. Med. 1980, 68, 818–824. [Google Scholar] [CrossRef]
- Khuroo, M.S. Discovery of Hepatitis E: The Untold Story. 1 September 2011. Available online: https://youtu.be/idoeaMbcbk0 (accessed on 30 June 2016).
- Khuroo, M.S.; Teli, M.R.; Skidmore, S.; Sofi, M.A.; Khuroo, M.I. Incidence and severity of viral hepatitis in pregnancy. Am. J. Med. 1981, 70, 252–255. [Google Scholar] [CrossRef]
- Khuroo, M.S.; Saleem, M.; Teli, M.R.; Sofi, M.A. Failure to detect chronic liver disease after epidemic non-A, non-B hepatitis. Lancet 1980, 2, 97–98. [Google Scholar] [CrossRef]
- Khuroo, M.S. Chronic liver disease after non-A, non-B hepatitis. Lancet 1980, 2, 860–861. [Google Scholar] [PubMed]
- Jameel, S.; Durgapal, H.; Habibullah, C.M.; Khuroo, M.S.; Panda, S.K. Enteric non-A, non-B hepatitis: Epidemics, animal transmission, and hepatitis E virus detection by the polymerase chain reaction. J. Med. Virol. 1992, 37, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Khuroo, M.S.; Kamili, S.; Jameel, S. Vertical transmission of hepatitis E virus. Lancet 1995, 345, 1025–1026. [Google Scholar]
- Khuroo, M.S.; Duermeyer, W.; Zargar, S.A.; Ahanger, M.A.; Shah, M.A. Acute sporadic non-A, non-B hepatitis in India. Am. J. Epidemiol. 1983, 118, 360–364. [Google Scholar] [PubMed]
- Khuroo, M.S.; Kamili, S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J. Viral Hepat. 2003, 10, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Khuroo, M.S.; Rustgi, V.K.; Dawson, G.J.; Mushahwar, I.K.; Yattoo, G.N.; Kamili, S.; Khan, B.A. Spectrum of hepatitis E virus infection in India. J. Med. Virol. 1994, 43, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Khuroo, M.S.; Kamili, S. Aetiology and prognostic factors in acute liver failure in India. J. Viral Hepat. 2003, 10, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Khuroo, M.S. Acute liver failure in india. Hepatology 1997, 26, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Balayan, M.S.; Andjaparidze, A.G.; Savinskaya, S.S.; Ketiladze, E.S.; Braginsky, D.M.; Savinov, A.P.; Poleschuk, V.F. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology 1983, 20, 23–31. [Google Scholar] [PubMed]
- Bradley, D.W.; Purdy, M.A.; Reyes, G.R. Hepatitis E virus genome. Molecular features, expression of immunoreactive proteins and sequence divergence. J. Hepatol. 1991, 13 (Suppl. S4), S152–S154. [Google Scholar] [CrossRef]
- Reyes, G.R.; Purdy, M.A.; Kim, J.P.; Luk, K.C.; Young, L.M.; Fry, K.E.; Bradley, D.W. Isolation of a cDNA from the virus responsible for enterically transmitted non-A, non-B hepatitis. Science 1990, 247, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- Tam, A.W.; Smith, M.M.; Guerra, M.E.; Huang, C.C.; Bradley, D.W.; Fry, K.E.; Reyes, G.R. Hepatitis E virus (HEV): Molecular cloning and sequencing of the full-length viral genome. Virology 1991, 185, 120–131. [Google Scholar] [CrossRef]
- Yarbough, P.O.; Tam, A.W.; Fry, K.E.; Krawczynski, K.; McCaustland, K.A.; Bradley, D.W.; Reyes, G.R. Hepatitis E virus: Identification of type-common epitopes. J. Virol. 1991, 65, 5790–5797. [Google Scholar] [PubMed]
- Khuroo, M.S. Hepatitis E virus: Another addition to the existing alphabet of human hepatitis viruses. Ann. Saudi Med. 1996, 16, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Holla, R.P.; Ahmad, I.; Ahmad, Z.; Jameel, S. Molecular virology of hepatitis E virus. Semin. Liver Dis. 2013, 33, 3–14. [Google Scholar] [PubMed]
- Meng, X.J. From barnyard to food table: The omnipresence of hepatitis E virus and risk for zoonotic infection and food safety. Virus Res. 2011, 161, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.B.; Simmonds, P.; Jameel, S.; Emerson, S.U.; Harrison, T.J.; Meng, X.J.; Okamoto, H.; Van der Poel, W.H.; Purdy, M.A.; International Committee on the Taxonomy of Viruses Hepeviridae Study Group. Consensus proposals for classification of the family hepeviridae. J. Gen. Virol. 2014, 95, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Cossaboom, C.M.; Cordoba, L.; Dryman, B.A.; Meng, X.J. Hepatitis E virus in rabbits, Virginia, USA. Emerg. Infect. Dis. 2011, 17, 2047–2049. [Google Scholar] [CrossRef] [PubMed]
- Izopet, J.; Dubois, M.; Bertagnoli, S.; Lhomme, S.; Marchandeau, S.; Boucher, S.; Kamar, N.; Abravanel, F.; Guerin, J.L. Hepatitis E virus strains in rabbits and evidence of a closely related strain in humans, France. Emerg. Infect. Dis 2012, 18, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.; Lau, S.K.; Teng, J.L.; Tsang, A.K.; Joseph, M.; Wong, E.Y.; Tang, Y.; Sivakumar, S.; Xie, J.; Bai, R.; et al. New hepatitis E virus genotype in camels, the Middle East. Emerg. Infect. Dis. 2014, 20, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Tan, B.H.; Chi-Yuan Teo, E.; Lim, S.G.; Dan, Y.Y.; Wee, A.; Kim Aw, P.P.; Zhu, Y.; Hibberd, M.L.; Tan, C.K.; et al. Chronic infection with camelid hepatitis E virus in a liver transplant recipient who regularly consumes camel meat and milk. Gastroenterology 2016, 150, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Yugo, D.M.; Cossaboom, C.M.; Meng, X.J. Naturally occurring animal models of human hepatitis E virus infection. ILAR J. 2014, 55, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Teshale, E.H.; Hu, D.J.; Holmberg, S.D. The two faces of hepatitis E virus. Clin. Infect. Dis. 2010, 51, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Pischke, S.; Wedemeyer, H. Hepatitis E virus infection: Multiple faces of an underestimated problem. J. Hepatol. 2013, 58, 1045–1046. [Google Scholar] [CrossRef] [PubMed]
- Riveiro-Barciela, M.; Rodriguez-Frias, F.; Buti, M. Hepatitis E virus: New faces of an old infection. Ann. Hepatol. 2013, 12, 861–870. [Google Scholar] [PubMed]
- Rein, D.B.; Stevens, G.A.; Theaker, J.; Wittenborn, J.S.; Wiersma, S.T. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology 2012, 55, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Khuroo, M.S.; Kamili, S.; Dar, M.Y.; Moecklii, R.; Jameel, S. Hepatitis E and long-term antibody status. Lancet 1993, 341, 1355. [Google Scholar] [PubMed]
- Khuroo, M.S.; Khuroo, M.S. Seroepidemiology of a second epidemic of hepatitis E in a population that had recorded first epidemic 30 years before and has been under surveillance since then. Hepatol. Int. 2010, 4, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Labrique, A.B.; Zaman, K.; Hossain, Z.; Saha, P.; Yunus, M.; Hossain, A.; Ticehurst, J.R.; Nelson, K.E. Epidemiology and risk factors of incident hepatitis E virus infections in rural Bangladesh. Am. J. Epidemiol. 2010, 172, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Thakral, D.; Rehman, S. Hepatitis E virus. Rev. Med. Virol. 2007, 17, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Fierro, N.A.; Realpe, M.; Meraz-Medina, T.; Roman, S.; Panduro, A. Hepatitis E virus: An ancient hidden enemy in Latin America. World J. Gastroenterol. 2016, 22, 2271–2283. [Google Scholar] [PubMed]
- Huang, R.T.; Li, D.R.; Wei, J.; Huang, X.R.; Yuan, X.T.; Tian, X. Isolation and identification of hepatitis E virus in Xinjiang, China. J. Gen. Virol. 1992, 73, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Hamid, S.S.; Atiq, M.; Shehzad, F.; Yasmeen, A.; Nissa, T.; Salam, A.; Siddiqui, A.; Jafri, W. Hepatitis E virus superinfection in patients with chronic liver disease. Hepatology 2002, 36, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Aggarwal, R.; Naik, S.R.; Saraswat, V.; Ghoshal, U.C.; Naik, S. Hepatitis E virus is responsible for decompensation of chronic liver disease in an endemic region. Indian J. Gastroenterol. 2004, 23, 59–62. [Google Scholar] [PubMed]
- Arif, M. Enterically transmitted hepatitis in Saudi Arabia: An epidemiological study. Ann. Trop. Med. Parasitol. 1996, 90, 197–201. [Google Scholar] [PubMed]
- Saffar, M.J.; Farhadi, R.; Ajami, A.; Khalilian, A.R.; Babamahmodi, F.; Saffar, H. Seroepidemiology of hepatitis E virus infection in 2–25-year-olds in Sari district, Islamic Republic of Iran. East. Mediterr. Health J. 2009, 15, 136–142. [Google Scholar] [PubMed]
- Coursaget, P.; Depril, N.; Yenen, O.S.; Cavuslu, S.; Badur, S. Hepatitis E virus infection in Turkey. Lancet 1993, 342, 810–811. [Google Scholar] [CrossRef]
- Chow, W.C.; Lee, A.S.; Lim, G.K.; Cheong, W.K.; Chong, R.; Tan, C.K.; Yap, C.K.; Oon, C.J.; Ng, H.S. Acute viral hepatitis E: Clinical and serologic studies in Singapore. J. Clin. Gastroenterol. 1997, 24, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.M.; Uduman, S.; Rana, S.; Kochiyil, J.K.; Usmani, A.; Thomas, L. Sero-prevalence and mother-to-infant transmission of hepatitis E virus among pregnant women in the United Arab Emirates. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 100, 9–15. [Google Scholar] [CrossRef]
- Blackard, J.T.; Rouster, S.D.; Nady, S.; Galal, G.; Marzuuk, N.; Rafaat, M.M.; Daef, E.; El Din, S.S.; Purcell, R.H.; Emerson, S.U.; et al. Genotypic characterization of symptomatic hepatitis E virus (HEV) infections in Egypt. J. Clin. Virol. 2009, 46, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Aboulata, A.A.; Ahmad, M.S.; Shaban, M.M.; Zayd, K.M.; Abd El-Moktader, A.M. Prevalence of hepatitis E virus in Egyptian children presented with minor hepatic disorders. Egypt J. Immunol. 2005, 12, 71–76. [Google Scholar] [PubMed]
- Abdel, H.S.; El-Din, M.S.; El-Din, M.E. A high hepatitis E virus (HEV) seroprevalence among unpaid blood donors and haemodialysis patients in Egypt. J. Egypt Public Health Assoc. 1998, 73, 165–179. [Google Scholar]
- Meng, X.J. Zoonotic and foodborne transmission of hepatitis E virus. Semin. Liver Dis. 2013, 33, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Selves, J.; Mansuy, J.M.; Ouezzani, L.; Peron, J.M.; Guitard, J.; Cointault, O.; Esposito, L.; Abravanel, F.; Danjoux, M.; et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N. Engl. J. Med. 2008, 358, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Izopet, J.; Kamar, N. Hepatitis E: From zoonotic transmission to chronic infection in immunosuppressed patients. Med. Sci. 2008, 24, 1023–1025. [Google Scholar]
- Vishwanathan, R. Infectious hepatitis in Delhi (1955–1956). A critical study: Epidemiology. Indian J. Med. Res. 1957, 45, 49–58. [Google Scholar]
- Naik, S.R.; Aggarwal, R.; Salunke, P.N.; Mehrotra, N.N. A large waterborne viral hepatitis E epidemic in Kanpur, India. Bull. World Health Organ. 1992, 70, 597–604. [Google Scholar] [PubMed]
- Kane, M.A.; Bradley, D.W.; Shrestha, S.M.; Maynard, J.E.; Cook, E.H.; Mishra, R.P.; Joshi, D.D. Epidemic non-A, non-B hepatitis in Nepal. Recovery of a possible etiologic agent and transmission studies in marmosets. JAMA 1984, 252, 3140–3145. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, S.R.; Tilekar, B.N.; Walimbe, A.M.; Arankalle, V.A. Increased risk of hepatitis E in sewage workers from India. J. Occup. Environ. Med. 2003, 45, 1167–1170. [Google Scholar] [CrossRef] [PubMed]
- Ippagunta, S.K.; Naik, S.; Sharma, B.; Aggarwal, R. Presence of hepatitis E virus in sewage in Northern India: Frequency and seasonal pattern. J. Med. Virol. 2007, 79, 1827–1831. [Google Scholar] [CrossRef] [PubMed]
- Ishida, S.; Yoshizumi, S.; Ikeda, T.; Miyoshi, M.; Goto, A.; Matsubayashi, K.; Ikeda, H. Detection and molecular characterization of hepatitis E virus in clinical, environmental and putative animal sources. Arch. Virol. 2012, 157, 2363–2368. [Google Scholar] [CrossRef] [PubMed]
- Masclaux, F.G.; Hotz, P.; Friedli, D.; Savova-Bianchi, D.; Oppliger, A. High occurrence of hepatitis E virus in samples from wastewater treatment plants in Switzerland and comparison with other enteric viruses. Water Res. 2013, 47, 5101–5109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corwin, A.; Jarot, K.; Lubis, I.; Nasution, K.; Suparmawo, S.; Sumardiati, A.; Widodo, S.; Nazir, S.; Orndorff, G.; Choi, Y.; et al. Two years’ investigation of epidemic hepatitis E virus transmission in West Kalimantan (Borneo), Indonesia. Trans. R. Soc. Trop. Med. Hyg. 1995, 89, 262–265. [Google Scholar] [CrossRef]
- Gurav, Y.K.; Kakade, S.V.; Kakade, R.V.; Kadam, Y.R.; Durgawale, P.M. A study of hepatitis E outbreak in rural area of Western Maharashtra. Indian J. Community Med. 2007, 32, 182–184. [Google Scholar] [CrossRef]
- Clayson, E.T.; Vaughn, D.W.; Innis, B.L.; Shrestha, M.P.; Pandey, R.; Malla, D.B. Association of hepatitis E virus with an outbreak of hepatitis at a military training camp in Nepal. J. Med. Virol. 1998, 54, 178–182. [Google Scholar] [CrossRef]
- Singh, V.; Singh, V.; Raje, M.; Nain, C.K.; Singh, K. Routes of transmission in the hepatitis E epidemic of Saharanpur. Trop. Gastroenterol. 1998, 19, 107–109. [Google Scholar] [PubMed]
- Sailaja, B.; Murhekar, M.V.; Hutin, Y.J.; Kuruva, S.; Murthy, S.P.; Reddy, K.S.; Rao, G.M.; Gupte, M.D. Outbreak of waterborne hepatitis E in Hyderabad, India, 2005. Epidemiol. Infect. 2009, 137, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.T.; Prajapati, P.; Trivedi, A.V.; Bhagyalaxmi, A. Epidemic investigation of the jaundice outbreak in Girdharnagar, Ahmedabad, Gujarat, India, 2008. Indian J. Community Med. 2010, 35, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Bali, S.; Kar, S.S.; Kumar, S.; Ratho, R.K.; Dhiman, R.K.; Kumar, R. Hepatitis E epidemic with bimodal peak in a town of North India. Indian J. Public Health 2008, 52, 189–193, 199. [Google Scholar] [PubMed]
- Goldsmith, R.; Yarbough, P.O.; Reyes, G.R.; Fry, K.E.; Gabor, K.A.; Kamel, M.; Zakaria, S.; Amer, S.; Gaffar, Y. Enzyme-linked immunosorbent assay for diagnosis of acute sporadic hepatitis E in Egyptian children. Lancet 1992, 339, 328–331. [Google Scholar] [CrossRef]
- Aggarwal, R. Hepatitis E virus and person-to-person transmission. Clin. Infect. Dis. 2010, 51, 477–478; author reply 478–479. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Naik, S.R. Hepatitis E: Does person-to-person spread occur? Indian J. Gastroenterol. 1992, 11, 109–112. [Google Scholar] [PubMed]
- Khuroo, M.S.; Dar, M.Y. Hepatitis E: Evidence for person-to-person transmission and inability of low dose immune serum globulin from an Indian source to prevent it. Indian J. Gastroenterol. 1992, 11, 113–116. [Google Scholar] [PubMed]
- Arankalle, V.A.; Chadha, M.S.; Mehendale, S.M.; Tungatkar, S.P. Epidemic hepatitis E: Serological evidence for lack of intrafamilial spread. Indian J. Gastroenterol. 2000, 19, 24–28. [Google Scholar] [PubMed]
- Teshale, E.H.; Grytdal, S.P.; Howard, C.; Barry, V.; Kamili, S.; Drobeniuc, J.; Hill, V.R.; Okware, S.; Hu, D.J.; Holmberg, S.D. Evidence of person-to-person transmission of hepatitis E virus during a large outbreak in Northern Uganda. Clin. Infect. Dis. 2010, 50, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Teshale, E.H.; Howard, C.M.; Grytdal, S.P.; Handzel, T.R.; Barry, V.; Kamili, S.; Drobeniuc, J.; Okware, S.; Downing, R.; Tappero, J.W.; et al. Hepatitis E epidemic, Uganda. Emerg. Infect. Dis. 2010, 16, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Somani, S.K.; Aggarwal, R.; Naik, S.R.; Srivastava, S.; Naik, S. A serological study of intrafamilial spread from patients with sporadic hepatitis E virus infection. J. Viral Hepat. 2003, 10, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Jothikumar, N.; Aparna, K.; Kamatchiammal, S.; Paulmurugan, R.; Saravanadevi, S.; Khanna, P. Detection of hepatitis E virus in raw and treated wastewater with the polymerase chain reaction. Appl. Environ. Microbiol. 1993, 59, 2558–2562. [Google Scholar] [PubMed]
- Vaidya, S.R.; Chitambar, S.D.; Arankalle, V.A. Polymerase chain reaction-based prevalence of hepatitis A, hepatitis E and TT viruses in sewage from an endemic area. J. Hepatol. 2002, 37, 131–136. [Google Scholar] [CrossRef]
- Pavio, N.; Meng, X.J.; Doceul, V. Zoonotic origin of hepatitis E. Curr. Opin. Virol. 2015, 10, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Yugo, D.M.; Meng, X.J. Hepatitis E virus: Foodborne, waterborne and zoonotic transmission. Int. J. Environ. Res. Public Health 2013, 10, 4507–4533. [Google Scholar] [CrossRef] [PubMed]
- Khuroo, M.S.; Khuroo, M.S. Hepatitis E virus. Curr. Opin. Infect. Dis. 2008, 21, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K.; Kang, J.H.; Saga, A.; Takahashi, K.; Shimamura, T.; Yasumoto, A.; Fukushima, H.; Sogabe, S.; Konishi, K.; Uchida, T.; et al. Three cases of acute or fulminant hepatitis E caused by ingestion of pork meat and entrails in Hokkaido, Japan: Zoonotic food-borne transmission of hepatitis E virus and public health concerns. Hepatol. Res. 2012, 42, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Colson, P.; Borentain, P.; Queyriaux, B.; Kaba, M.; Moal, V.; Gallian, P.; Heyries, L.; Raoult, D.; Gerolami, R. Pig liver sausage as a source of hepatitis E virus transmission to humans. J. Infect. Dis. 2010, 202, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Garbuglia, A.R.; Alessandrini, A.I.; Pavio, N.; Tesse, S.; Grignolo, S.; Viscoli, C.; Lapa, D.; Capobianchi, M.R. Male patient with acute hepatitis E in Genoa, Italy: Figatelli (pork liver sausage) as probable source of the infection. Clin. Microbiol. Infect. 2015, 21, e4–e6. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Okada, K.; Takahashi, K.; Mishiro, S. Severe hepatitis E virus infection after ingestion of uncooked liver from a wild boar. J. Infect. Dis. 2003, 188, 944. [Google Scholar] [CrossRef] [PubMed]
- Bouwknegt, M.; Frankena, K.; Rutjes, S.A.; Wellenberg, G.J.; de Roda Husman, A.M.; van der Poel, W.H.; de Jong, M.C. Estimation of hepatitis E virus transmission among pigs due to contact-exposure. Vet. Res. 2008, 39, 40. [Google Scholar] [CrossRef] [PubMed]
- Rutjes, S.A.; Lodder, W.J.; Lodder-Verschoor, F.; van den Berg, H.H.; Vennema, H.; Duizer, E.; Koopmans, M.; de Roda Husman, A.M. Sources of hepatitis E virus genotype 3 in The Netherlands. Emerg. Infect. Dis. 2009, 15, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Perez-Gracia, M.T.; Mateos, M.L.; Galiana, C.; Fernandez-Barredo, S.; Garcia, A.; Gomez, M.T.; Moreira, V. Autochthonous hepatitis E infection in a slaughterhouse worker. Am. J. Trop. Med. Hyg. 2007, 77, 893–896. [Google Scholar] [PubMed]
- Galiana, C.; Fernandez-Barredo, S.; Garcia, A.; Gomez, M.T.; Perez-Gracia, M.T. Occupational exposure to hepatitis E virus (HEV) in swine workers. Am. J. Trop. Med. Hyg. 2008, 78, 1012–1015. [Google Scholar] [PubMed]
- Bouwknegt, M.; Engel, B.; Herremans, M.M.; Widdowson, M.A.; Worm, H.C.; Koopmans, M.P.; Frankena, K.; de Roda Husman, A.M.; De Jong, M.C.; Van Der Poel, W.H. Bayesian estimation of hepatitis E virus seroprevalence for populations with different exposure levels to swine in The Netherlands. Epidemiol. Infect. 2008, 136, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Vulcano, A.; Angelucci, M.; Candelori, E.; Martini, V.; Patti, A.M.; Mancini, C.; Santi, A.L.; Calvani, A.; Casagni, L.; Lamberti, A. prevalence in the general population and among workers at zoonotic risk in Latium region. Ann. Ig. 2007, 19, 181–186. [Google Scholar] [PubMed]
- Olsen, B.; Axelsson-Olsson, D.; Thelin, A.; Weiland, O. Unexpected high prevalence of igg-antibodies to hepatitis E virus in swedish pig farmers and controls. Scand. J. Infect. Dis. 2006, 38, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.; Muller, P.; Letellier, A.; Quessy, S.; Simard, C.; Trottier, Y.L.; Houde, A.; Brassard, J. Molecular characterization of hepatitis E virus detected in swine farms in the province of Quebec. Can. J. Vet. Res. 2008, 72, 27–31. [Google Scholar] [PubMed]
- Brassard, J.; Gagne, M.J.; Genreux, M.; Cote, C. Detection of huamn food-borne and zoonotic viruses on irrigated, field-grown strwberries. Appl. Environ. Microbiol. 2012, 78, 3763–3766. [Google Scholar] [CrossRef] [PubMed]
- Steyer, A.; Naglic, T.; Mocilnik, T.; Poljsak-Prijatelj, M.; Poljak, M. Hepatitis E virus in domestic pigs and surface waters in Slovenia: Prevalence and molecular characterization of a novel genotype 3 lineage. Infect. Genet. Evol. 2011, 11, 1732–1737. [Google Scholar] [CrossRef] [PubMed]
- Tyrrel, S.F.; Quinton, J.N. Overland flow transport of pathogens from agricultural land receiving faecal wastes. J. Appl. Microbiol. 2003, 94, 87S–93S. [Google Scholar] [CrossRef] [PubMed]
- Namsai, A.; Louisirirotchanakul, S.; Wongchinda, N.; Siripanyaphinyo, U.; Virulhakul, P.; Puthavathana, P.; Myint, K.S.; Gannarong, M.; Ittapong, R. Surveillance of hepatitis A and E viruses contamination in shellfish in Thailand. Lett. Appl. Microbiol. 2011, 53, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Diez-Valcarce, M.; Kokkinos, P.; Soderberg, K.; Bouwknegt, M.; Willems, K.; de Roda-Husman, A.M.; Von Bonsdorff, C.H.; Bellou, M.; Hernandez, M.; Maunula, L. Occurrence of human enteric viruses in commercial mussels at retail level in three European countries. Food Environ. Virol. 2012, 4, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Crossan, C.; Baker, P.J.; Craft, J.; Takeuchi, Y.; Dalton, H.R.; Scobie, L. Hepatitis E virus genotype 3 in shellfish, United Kingdom. Emerg. Infect. Dis. 2012, 18, 2085–2087. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.J.; Jeong, H.J.; Kim, Y.J.; Lee, S.W.; Lee, J.B.; Park, S.Y.; Song, C.S.; Park, H.M.; Choi, I.S. Analysis of complete genome sequences of swine hepatitis E virus and possible risk factors for transmission of to humans in Korea. J. Med. Virol. 2010, 82, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Li, T.C.; Miyamura, T.; Takeda, N. Detection of hepatitis E virus RNA from the bivalve Yamato-Shijimi (Corbicula japonica) in Japan. Am. J. Trop. Med. Hyg. 2007, 76, 170–172. [Google Scholar] [PubMed]
- Ijaz, S.; Arnold, E.; Banks, M.; Bendall, R.P.; Cramp, M.E.; Cunningham, R.; Dalton, H.R.; Harrison, T.J.; Hill, S.F.; Macfarlane, L.; et al. Non-travel-associated hepatitis E in England and Wales: Demographic, clinical, and molecular epidemiological characteristics. J. Infect. Dis. 2005, 192, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Cacopardo, B.; Russo, R.; Preiser, W.; Benanti, F.; Brancati, G.; Nunnari, A. Acute hepatitis E in Catania (Eastern Sicily) 1980–1994. The role of hepatitis E virus. Infection 1997, 25, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Renou, C.; Moreau, X.; Pariente, A.; Cadranel, J.F.; Maringe, E.; Morin, T.; Causse, X.; Payen, J.L.; Izopet, J.; Nicand, E.; et al. A national survey of acute hepatitis E in France. Aliment. Pharmacol. Ther. 2008, 27, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Said, B.; Ijaz, S.; Kafatos, G.; Booth, L.; Thomas, H.L.; Walsh, A.; Ramsay, M.; Morgan, D.; Hepatitis, E.I.I.T. Hepatitis E outbreak on cruise ship. Emerg. Infect. Dis. 2009, 15, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.; Chauhan, U.K.; Naik, S.; Anderson, D.; Aggarwal, R. Hepatitis E virus infection among animals in Northern India: An unlikely source of human disease. J. Viral Hepat. 2007, 14, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Arankalle, V.A.; Chobe, L.P.; Joshi, M.V.; Chadha, M.S.; Kundu, B.; Walimbe, A.M. Human and swine hepatitis E viruses from Western India belong to different genotypes. J. Hepatol. 2002, 36, 417–425. [Google Scholar] [CrossRef]
- Rasche, A.; Saqib, M.; Liljander, A.M.; Bornstein, S.; Zohaib, A.; Renneker, S.; Steinhagen, K.; Wernery, R.; Younan, M.; Gluecks, I.; et al. Hepatitis E virus infection in dromedaries, North and East Africa, United Arab Emirates, and Pakistan, 1983–2015. Emerg. Infect. Dis. 2016, 22, 1249–1252. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Cao, X.Y.; Liu, C.B.; Wang, G.M. Epidemiology of hepatitis E in China. Gastroenterol. Jpn. 1991, 26 (Suppl. S3), 135–138. [Google Scholar] [PubMed]
- Aye, T.T.; Uchida, T.; Ma, X.Z.; Iida, F.; Shikata, T.; Zhuang, H.; Win, K.M. Complete nucleotide sequence of a hepatitis E virus isolated from the Xinjiang Epidemic (1986–1988) of China. Nucleic Acids Res. 1992, 20, 3512. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Dong, C.; Zhou, Z.; Liang, J.; Dong, M.; Yang, Y.; Fu, J.; Tian, H.; Wang, S.; Fan, J.; et al. Hepatitis E virus genotype 4, Nanjing, China, 2001–2011. Emerg. Infect. Dis. 2013, 19, 1528–1530. [Google Scholar] [CrossRef] [PubMed]
- Khuroo, M.S.; Kamili, S.; Yattoo, G.N. Hepatitis E virus infection may be transmitted through blood transfusions in an endemic area. J. Gastroenterol. Hepatol. 2004, 19, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, K.; Nagaoka, Y.; Sakata, H.; Sato, S.; Fukai, K.; Kato, T.; Takahashi, K.; Mishiro, S.; Imai, M.; Takeda, N.; et al. Transfusion-transmitted hepatitis E caused by apparently indigenous hepatitis E virus strain in Hokkaido, Japan. Transfusion 2004, 44, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Suzuki, H.; Toyota, T.; Takahashi, M.; Okamoto, H. Three male patients with sporadic acute hepatitis E in Sendai, Japan, who were domestically infected with hepatitis E virus of genotype iii or iv. J. Gastroenterol. 2004, 39, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, K.; Kang, J.H.; Sakata, H.; Takahashi, K.; Shindo, M.; Kato, M.; Sato, S.; Kato, T.; Nishimori, H.; Tsuji, K.; et al. A case of transfusion-transmitted hepatitis E caused by blood from a donor infected with hepatitis E virus via zoonotic food-borne route. Transfusion 2008, 48, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, P.E.; Ijaz, S.; Brailsford, S.R.; Brett, R.; Dicks, S.; Haywood, B.; Kennedy, I.T.; Kitchen, A.; Patel, P.; Poh, J.; et al. Hepatitis E virus in blood components: A prevalence and transmission study in Southeast England. Lancet 2014, 384, 1766–1773. [Google Scholar] [CrossRef]
- Aggarwal, R. Hepatitis E: Is it a blood-borne pathogen? J. Gastroenterol. Hepatol. 2004, 19, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Arankalle, V.A.; Chobe, L.P. Retrospective analysis of blood transfusion recipients: Evidence for post-transfusion hepatitis E. Vox Sang. 2000, 79, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Arankalle, V.A.; Chobe, L.P. Hepatitis E virus: Can it be transmitted parenterally? J. Viral Hepat. 1999, 6, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Sunaga, J.; Saito, N.; Fujimura, K.; Itoh, Y.; Sasaki, M.; Tsuda, F.; Takahashi, M.; Nishizawa, T.; Okamoto, H. Prevalence of antibodies to hepatitis E virus among Japanese blood donors: Identification of three blood donors infected with a genotype 3 hepatitis E virus. J. Med. Virol. 2004, 73, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, T.; Diekmann, J.; Johne, R.; Eberhardt, M.; Knabbe, C.; Dreier, J. Novel approach for detection of hepatitis E virus infection in German blood donors. J. Clin. Microbiol. 2012, 50, 2708–2713. [Google Scholar] [CrossRef] [PubMed]
- Holm, D.K.; Moessner, B.K.; Engle, R.E.; Zaaijer, H.L.; Georgsen, J.; Purcell, R.H.; Christensen, P.B. Declining prevalence of hepatitis E antibodies among Danish blood donors. Transfusion 2015, 55, 1662–1667. [Google Scholar] [CrossRef] [PubMed]
- Juhl, D.; Baylis, S.A.; Blumel, J.; Gorg, S.; Hennig, H. Seroprevalence and incidence of hepatitis E virus infection in German blood donors. Transfusion 2014, 54, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Gotanda, Y.; Iwata, A.; Ohnuma, H.; Yoshikawa, A.; Mizoguchi, H.; Endo, K.; Takahashi, M.; Okamoto, H. Ongoing subclinical infection of hepatitis E virus among blood donors with an elevated alanine aminotransferase level in Japan. J. Med. Virol. 2007, 79, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Li, J.; Yuan, Z.A.; Hu, J.Y.; Yu, Y.; Lu, Y.H. The development of a combined mathematical model to forecast the incidence of hepatitis E in Shanghai, China. BMC Infect. Dis. 2013, 13, 421. [Google Scholar] [CrossRef] [PubMed]
- Hogema, B.M.; Molier, M.; Slot, E.; Zaaijer, H.L. Past and present of hepatitis E in The Netherlands. Transfusion 2014, 54, 3092–3096. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Hofmann, M.; Danzer, M.; Hofer, K.; Kaar, J.; Gabriel, C. Seroprevalence and incidence of hepatitis E in blood donors in upper Austria. PLoS ONE 2015, 10, e0119576. [Google Scholar] [CrossRef] [PubMed]
- Khuroo, M.S.; Kamili, S.; Khuroo, M.S. Clinical course and duration of viremia in vertically transmitted hepatitis E virus (HEV) infection in babies born to HEV-infected mothers. J. Viral Hepat. 2009, 16, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mohanty, A.; Joshi, Y.K.; Deka, D.; Mohanty, S.; Panda, S.K. Mother-to-child transmission of hepatitis E virus infection. Indian J. Pediatr. 2003, 70, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Bose, P.D.; Das, B.C.; Hazam, R.K.; Kumar, A.; Medhi, S.; Kar, P. Evidence of extrahepatic replication of hepatitis E virus in human placenta. J. Gen. Virol. 2014, 95, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Khuroo, M.S. Association of severity of hepatitis E virus infection in the mother and vertically transmitted infection in the fetus. JK-Practitioner 2006, 13, 70–74. [Google Scholar]
- Kumar, A.; Beniwal, M.; Kar, P.; Sharma, J.B.; Murthy, N.S. Hepatitis E in pregnancy. Int. J. Gynaecol. Obstet. 2004, 85, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Chibber, R.M.; Usmani, M.A.; Al-Sibai, M.H. Should HEV infected mothers breast feed? Arch. Gynecol. Obstet. 2004, 270, 15–20. [Google Scholar] [CrossRef] [PubMed]
- El Sayed Zaki, M.; El Aal, A.A.; Badawy, A.; El-Deeb, D.R.; El-Kheir, N.Y. Clinicolaboratory study of mother-to-neonate transmission of hepatitis E virus in Egypt. Am. J. Clin. Pathol. 2013, 140, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Ayoola, E.A.; Want, M.A.; Gadour, M.O.; Al-Hazmi, M.H.; Hamza, M.K. Hepatitis E virus infection in haemodialysis patients: A case-control study in Saudi Arabia. J. Med. Virol. 2002, 66, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Hosseini-Moghaddam, S.M.; Zarei, A.; Alavian, S.M.; Mansouri, M. Hepatitis E virus infection: A general review with a focus on hemodialysis and kidney transplant patients. Am. J. Nephrol. 2010, 31, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Sylvan, S.P.; Jacobson, S.H.; Christenson, B. Prevalence of antibodies to hepatitis E virus among hemodialysis patients in Sweden. J. Med. Virol. 1998, 54, 38–43. [Google Scholar] [CrossRef]
- Ucar, E.; Cetin, M.; Kuvandik, C.; Helvaci, M.R.; Gullu, M.; Huzmeli, C. Hepatitis E virus seropositivity in hemodialysis patients in Hatay Province, Turkey. Mikrobiyoloji Bulteni 2009, 43, 299–302. [Google Scholar] [PubMed]
- Siddiqui, A.R.; Jooma, R.A.; Smego, R.A., Jr. Nosocomial outbreak of hepatitis E infection in Pakistan with possible parenteral transmission. Clin. Infect. Dis. 2005, 40, 908–909. [Google Scholar] [CrossRef] [PubMed]
- Ola, S.O.; Odaibo, G.N.; Olaleye, O.D.; Ayoola, E.A. Hepatitis B and E viral infections among Nigerian healthcare workers. Afr. J. Med. Med. Sci. 2012, 41, 387–391. [Google Scholar] [PubMed]
- Robson, S.C.; Adams, S.; Brink, N.; Woodruff, B.; Bradley, D. Hospital outbreak of hepatitis E. Lancet 1992, 339, 1424–1425. [Google Scholar] [CrossRef]
- Balayan, M.S.; Fedorova, O.E.; Mikhailov, M.I.; Rytick, P.G.; Eremin, V.F.; Danilova, T.I.; Shevelev, B.I.; Gorbacheva, E.C.; Pankova, G.Y. Antibody to hepatitis E virus in HIV-infected individuals and aids patients. J. Viral Hepat. 1997, 4, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.B.; Engle, R.E.; Jacobsen, S.E.; Krarup, H.B.; Georgsen, J.; Purcell, R.H. High prevalence of hepatitis E antibodies among Danish prisoners and drug users. J. Med. Virol. 2002, 66, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Keane, F.; Gompels, M.; Bendall, R.; Drayton, R.; Jennings, L.; Black, J.; Baragwanath, G.; Lin, N.; Henley, W.; Ngui, S.L.; et al. Hepatitis E virus coinfection in patients with HIV infection. HIV Med. 2012, 13, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Pawlotsky, J.M.; Belec, L.; Gresenguet, G.; Deforges, L.; Bouvier, M.; Duval, J.; Dhumeaux, D. High prevalence of hepatitis B, C, and E markers in young sexually active adults from the Central African Republic. J. Med. Virol. 1995, 46, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Montella, F.; Rezza, G.; Di Sora, F.; Pezzotti, P.; Recchia, O. Association between hepatitis E virus and HIV infection in homosexual men. Lancet 1994, 344, 1433. [Google Scholar] [CrossRef]
- Rivero-Juarez, A.; Frias, M.; Rodriguez-Cano, D.; Cuenca-Lopez, F.; Rivero, A.I. Isolation of hepatitis E virus from breast milk during acute infection. Clin. Infect. Dis. 2016, 62, 1464. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Li, Y.; Yu, W.; Jing, S.; Wang, J.; Long, F.; He, Z.; Yang, C.; Bi, Y.; Cao, W.; et al. Excretion of infectious hepatitis E virus into milk in cows imposes high risks of zoonosis. Hepatology 2016, 64, 350–359. [Google Scholar] [CrossRef] [PubMed]


| Place | Year | Population Exposed | Icteric Cases | Deaths |
|---|---|---|---|---|
| Gulmarg | 1978–1979 | 600,000 | 20,083 | 600 |
| Sopore | 1979–1980 | 200,000 | 6000 | 200 |
| Handwara | 1980–1981 | 400,000 | 11,500 | 400 |
| Jammu Army Camp | 1981–1982 | 845 | 206 | 0 |
| Kupwara | 1981–1982 | 500,000 | 15,000 | 550 |
| Jammu | 1983–1984 | 176,833 | 518 | 2 |
| Pinglina | 1993–1994 | 10,000 | 156 | 2 |
| Shopian | 1994–1995 | 60,000 | 1500 | 17 |
| Maharajpora, Sopore | 2007–2008 | 720 | 21 | 2 |
| Pattan, Gulmarg | 2012–2013 | 20,000 | 600 | 2 |
| Total | 1978–2013 | 1,968,398 | 55,563 | 1775 |
| Reference | Region | HEV RNA (%) | HEV Infected Transfusion vs. Total Donations |
|---|---|---|---|
| Arankalle et al., 2000 [120] | Pune India | 3/200 (1.5%) | 1:67 |
| Khuroo et al., 2004 [114] | Kashmir India | 4/107 (3.7%) | 1:27 |
| Gotanda et al., 2007 [126] | Japan | 9/6700 (0.13%) | 1:745 |
| Ren et al., 2013 [127] | China | 6/10,741 (0.06%) | 1:1790 |
| Juhl et al., 2014 [125] | Germany | 35/23,500 (0.14%) | 1:671 |
| Hewitt et al., 2014 [118] | England | 79/225,000 (0.04%) | 1:2848 |
| Hogema et al., 2014 [128] | Netherlands | 20/35,220 (0.06%) | 1:1761 |
| Fischer et al., 2015 [129] | Austria | 7/58,915 (0.01%) | 1:8416 |
| Reference | Region | Mothers | Vertical Transmission (%) | Deaths |
|---|---|---|---|---|
| Khuroo, et al., 1995 [14] | Kashmir, India | 8 | 6 (75%) | 3 (50%) |
| Kumar, et al., 2001 [50] | Al-Khobar, KSA | 26 | 26 (100%) | 2 (7.7%) |
| Kumar, et al., 2004 [134] | New Delhi, India | 18 | 6 (33.3%) | - |
| Singh, et al., 2003 [131] | New Delhi, India | 6 | 3 (50%) | - |
| Chibber, et al., 2004 * [135] | Al-Khobar, KSA | 6 | 4 (66.6%) | - |
| Khuroo, et al., 2009 [130] | Kashmir, India | 19 | 15 (78.9%) | - |
| Zaki, et al., 2013 [136] | Mansoura, Egypt | 9 | 6 (66.6%) | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khuroo, M.S.; Khuroo, M.S.; Khuroo, N.S. Transmission of Hepatitis E Virus in Developing Countries. Viruses 2016, 8, 253. https://doi.org/10.3390/v8090253
Khuroo MS, Khuroo MS, Khuroo NS. Transmission of Hepatitis E Virus in Developing Countries. Viruses. 2016; 8(9):253. https://doi.org/10.3390/v8090253
Chicago/Turabian StyleKhuroo, Mohammad S., Mehnaaz S. Khuroo, and Naira S. Khuroo. 2016. "Transmission of Hepatitis E Virus in Developing Countries" Viruses 8, no. 9: 253. https://doi.org/10.3390/v8090253