Measles Virus Hemagglutinin Protein Epitopes: The Basis of Antigenic Stability
Abstract
:1. Introduction
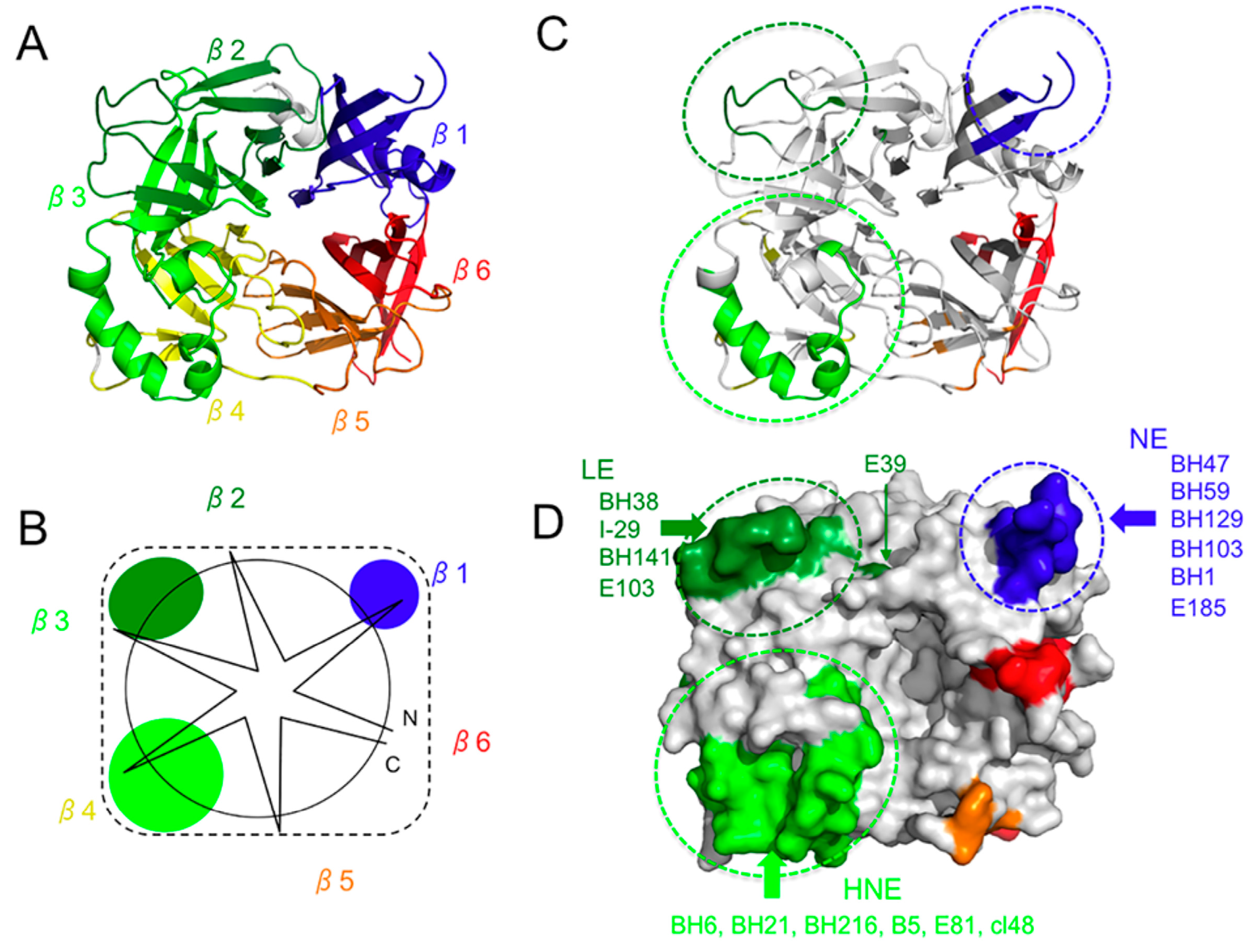
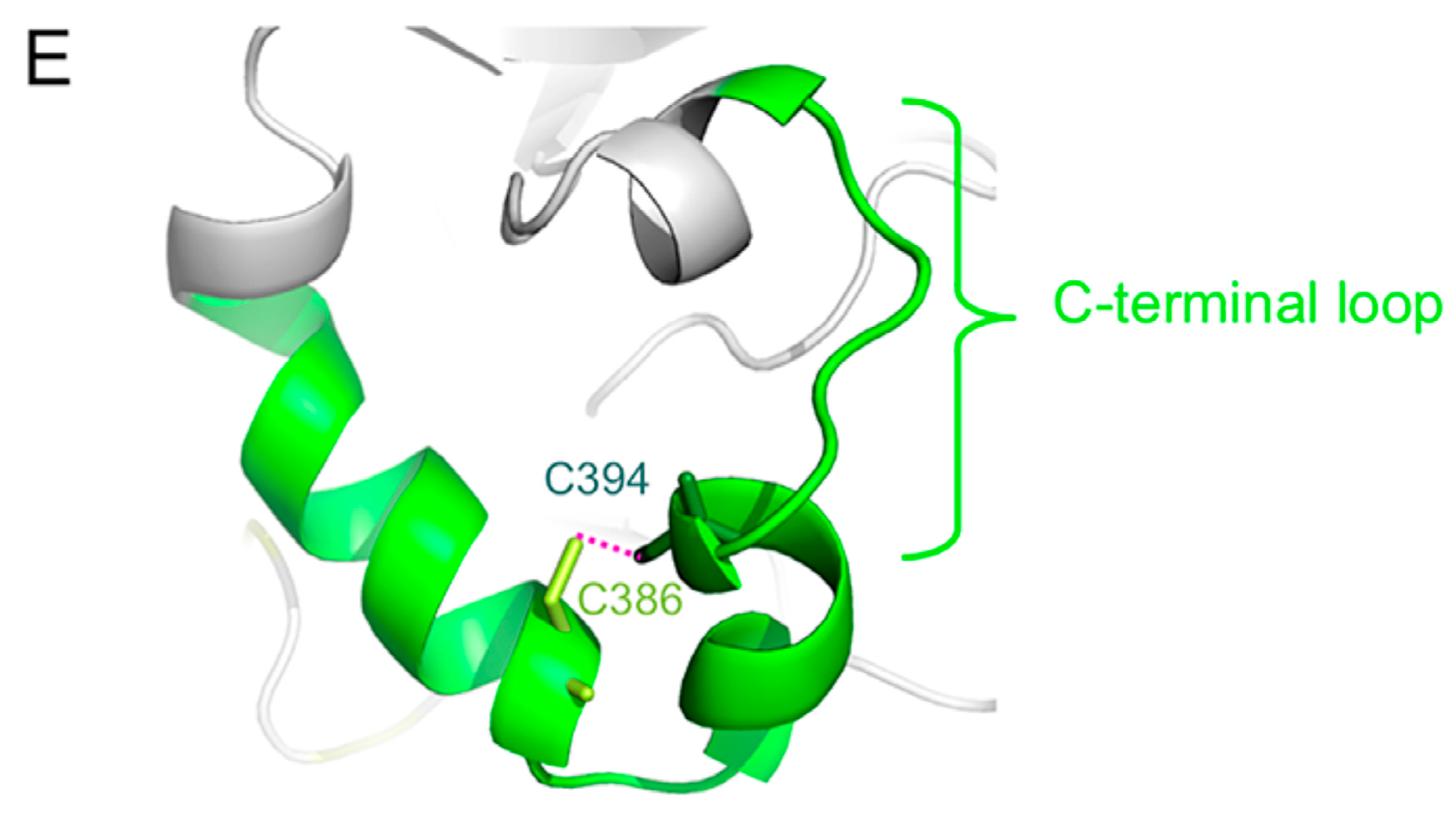
2. Overall Structure of the H Protein
3. Hemagglutinating and Noose Epitope (HNE)
4. Receptor-Binding Epitope (RBE)
5. Epitope Shielded by N416-Sugar (Sugar-Shielded Epitope, SSE)
6. Neutralizing Epitope (NE) and BH1-Binding Epitope
7. Epitope on the Loop Protruding from the β2 Sheet (Loop Epitope; LE)
8. Materials and Methods
8.1. Cells
8.2. Viruses
8.3. MAbs and Human Sera
8.4. Neutralizing Assay
8.5. HI Assay
8.6. Structures of H and F Proteins
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tahara, M.; Bürckert, J.-P.; Kanou, K.; Maenaka, K.; Muller, C.P.; Takeda, M. Measles Virus Hemagglutinin Protein Epitopes: The Basis of Antigenic Stability. Viruses 2016, 204, 8–216. [Google Scholar] [CrossRef] [PubMed]
- Bellini, W.J.; Rota, P.A. Biological feasibility of measles eradication. Virus Res. 2011, 162, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Strebel, P.M.; Cochi, S.L.; Hoekstra, E.; Rota, P.A.; Featherstone, D.; Bellini, W.J.; Katz, S.L. A world without measles. J. Infect. Dis. 2011, 204, 1–3. [Google Scholar] [CrossRef] [PubMed]
- De Swart, R.L.; Yuksel, S.; Osterhaus, A.D. Relative contributions of measles virus hemagglutinin- and fusion protein-specific serum antibodies to virus neutralization. J. Virol. 2005, 79, 11547–11551. [Google Scholar] [CrossRef] [PubMed]
- Bouche, F.B.; Ertl, O.T.; Muller, C.P. Neutralizing B cell response in measles. Viral Immunol. 2002, 15, 451–471. [Google Scholar] [CrossRef] [PubMed]
- De Swart, R.L.; Yuksel, S.; Langerijs, C.N.; Muller, C.P.; Osterhaus, A.D. Depletion of measles virus glycoprotein-specific antibodies from human sera reveals genotype-specific neutralizing antibodies. J. Gen. Virol. 2009, 90, 2982–2989. [Google Scholar] [CrossRef] [PubMed]
- Tatsuo, H.; Ono, N.; Tanaka, K.; Yanagi, Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature 2000, 406, 893–897. [Google Scholar] [PubMed]
- Noyce, R.S.; Bondre, D.G.; Ha, M.N.; Lin, L.T.; Sisson, G.; Tsao, M.S.; Richardson, C.D. Tumor cell marker PVRL4 (nectin 4) is an epithelial cell receptor for measles virus. PLoS Pathog. 2011, 7, e1002240. [Google Scholar] [CrossRef] [PubMed]
- Muhlebach, M.D.; Mateo, M.; Sinn, P.L.; Prufer, S.; Uhlig, K.M.; Leonard, V.H.; Navaratnarajah, C.K.; Frenzke, M.; Wong, X.X.; Sawatsky, B.; et al. Adherens junction protein nectin-4 is the epithelial receptor for measles virus. Nature 2011, 480, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Tahara, M.; Nagata, N.; Seki, F. Wild-type measles virus is intrinsically dual-tropic. Front. Microbiol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, Y.; Takeda, M.; Ohno, S.; Hashiguchi, T. Measles virus receptors. Curr. Top. Microbiol. Immunol. 2009, 329, 13–30. [Google Scholar] [PubMed]
- Jardetzky, T.S.; Lamb, R.A. Activation of paramyxovirus membrane fusion and virus entry. Curr. Opin. Virol. 2014, 5, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Finsterbusch, T.; Wolbert, A.; Deitemeier, I.; Meyer, K.; Mosquera, M.M.; Mankertz, A.; Santibanez, S. Measles viruses of genotype H1 evade recognition by vaccine-induced neutralizing antibodies targeting the linear haemagglutinin noose epitope. J. Gen. Virol. 2009, 90, 2739–2745. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zheng, J.; Huang, H.; Hu, Y.; Bian, J.; Xu, D.; Li, F. Measles incidence rate and a phylogenetic study of contemporary genotype H1 measles strains in China: Is an improved measles vaccine needed? Virus Genes 2011, 43, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Tamin, A.; Rota, P.A.; Wang, Z.D.; Heath, J.L.; Anderson, L.J.; Bellini, W.J. Antigenic analysis of current wild type and vaccine strains of measles virus. J. Infect. Dis. 1994, 170, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Kuhne, M.; Brown, D.W.; Jin, L. Genetic variability of measles virus in acute and persistent infections. Infect. Genet. Evol. 2006, 6, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Santibanez, S.; Niewiesk, S.; Heider, A.; Schneider-Schaulies, J.; Berbers, G.A.; Zimmermann, A.; Halenius, A.; Wolbert, A.; Deitemeier, I.; Tischer, A.; et al. Probing neutralizing-antibody responses against emerging measles viruses (MVs): Immune selection of MV by H protein-specific antibodies? J. Gen. Virol. 2005, 86, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, T.; Ose, T.; Kubota, M.; Maita, N.; Kamishikiryo, J.; Maenaka, K.; Yanagi, Y. Structure of the measles virus hemagglutinin bound to its cellular receptor SLAM. Nat. Struct. Mol. Biol. 2011, 18, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, G.; Qi, J.; Li, Y.; He, Y.; Xu, X.; Shi, J.; Zhang, C.W.; Yan, J.; Gao, G.F. Structure of measles virus hemagglutinin bound to its epithelial receptor nectin-4. Nat. Struct. Mol. Biol. 2012, 20, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Santiago, C.; Celma, M.L.; Stehle, T.; Casasnovas, J.M. Structure of the measles virus hemagglutinin bound to the CD46 receptor. Nat. Struct. Mol. Biol. 2010, 17, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Jardetzky, T.S.; Lamb, R.A. Timing is everything: Fine-tuned molecular machines orchestrate paramyxovirus entry. Virology 2015, 479, 518–531. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, T.; Kajikawa, M.; Maita, N.; Takeda, M.; Kuroki, K.; Sasaki, K.; Kohda, D.; Yanagi, Y.; Maenaka, K. Crystal structure of measles virus hemagglutinin provides insight into effective vaccines. Proc. Natl. Acad. Sci. USA 2007, 104, 19535–19540. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Fournier, P.; Berbers, G.A.; Steuer, H.; Wiesmuller, K.H.; Fleckenstein, B.; Schneider, F.; Jung, G.; King, C.C.; Muller, C.P. Protection against measles virus encephalitis by monoclonal antibodies binding to a cystine loop domain of the H protein mimicked by peptides which are not recognized by maternal antibodies. J. Gen. Virol. 1996, 77, 2479–2489. [Google Scholar] [CrossRef] [PubMed]
- Putz, M.M.; Hoebeke, J.; Ammerlaan, W.; Schneider, S.; Muller, C.P. Functional fine-mapping and molecular modeling of a conserved loop epitope of the measles virus hemagglutinin protein. Eur. J. Biochem. FEBS 2003, 270, 1515–1527. [Google Scholar] [CrossRef]
- Lapthorn, A.J.; Janes, R.W.; Isaacs, N.W.; Wallace, B.A. Cystine nooses and protein specificity. Nat. Struct. Biol. 1995, 2, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Ertl, O.T.; Wenz, D.C.; Bouche, F.B.; Berbers, G.A.; Muller, C.P. Immunodominant domains of the Measles virus hemagglutinin protein eliciting a neutralizing human B cell response. Arch. Virol. 2003, 148, 2195–2206. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.A.; Fukuda, A.; Sugiura, A. Characterization of major structural proteins of measles virus with monoclonal antibodies. J. Gen. Virol. 1985, 66, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Ito, Y.; Brindley, M.A.; Ma, X.; He, J.; Xu, S.; Fukuhara, H.; Sakai, K.; Komase, K.; Rota, P.A.; Plemper, R.K.; Maenaka, K.; Takeda, M. Functional and structural characterization of neutralizing epitopes of measles virus hemagglutinin protein. J. Virol. 2013, 87, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Lech, P.J.; Tobin, G.J.; Bushnell, R.; Gutschenritter, E.; Pham, L.D.; Nace, R.; Verhoeyen, E.; Cosset, F.L.; Muller, C.P.; Russell, S.J.; et al. Epitope dampening monotypic measles virus hemagglutinin glycoprotein results in resistance to cocktail of monoclonal antibodies. PLoS ONE 2013, 8, e52306. [Google Scholar] [CrossRef] [PubMed]
- Liebert, U.G.; Flanagan, S.G.; Loffler, S.; Baczko, K.; ter Meulen, V.; Rima, B.K. Antigenic determinants of measles virus hemagglutinin associated with neurovirulence. J. Virol. 1994, 68, 1486–1493. [Google Scholar] [PubMed]
- Xu, S.; Zhang, Y.; Zhu, Z.; Liu, C.; Mao, N.; Ji, Y.; Wang, H.; Jiang, X.; Li, C.; Tang, W.; et al. Genetic characterization of the hemagglutinin genes of wild-type measles virus circulating in China, 1993–2009. PLoS ONE 2013, 8, e73374. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Yasui, Y.; Minagawa, H.; Komase, K.; Takeda, M. Characterization of measles virus strains in Japan. Unpublished work.
- Makela, M.J.; Salmi, A.A.; Norrby, E.; Wild, T.F. Monoclonal antibodies against measles virus haemagglutinin react with synthetic peptides. Scand. J. Immunol. 1989, 30, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Lech, P.J.; Pappoe, R.; Nakamura, T.; Tobin, G.J.; Nara, P.L.; Russell, S.J. Antibody neutralization of retargeted measles viruses. Virology 2014, 454, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Sheshberadaran, H.; Norrby, E.; Kovamees, J. Molecular characterization of epitopes on the measles virus hemagglutinin protein. Virology 1993, 192, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Hummel, K.B.; Bellini, W.J. Localization of monoclonal antibody epitopes and functional domains in the hemagglutinin protein of measles virus. J. Virol. 1995, 69, 1913–1916. [Google Scholar] [PubMed]
- Kato, S.; Ohgimoto, S.; Sharma, L.B.; Kurazono, S.; Ayata, M.; Komase, K.; Takeda, M.; Takeuchi, K.; Ihara, T.; Ogura, H. Reduced ability of hemagglutinin of the CAM-70 measles virus vaccine strain to use receptors CD46 and SLAM. Vaccine 2009, 27, 3838–3848. [Google Scholar] [CrossRef] [PubMed]
- Masse, N.; Ainouze, M.; Neel, B.; Wild, T.F.; Buckland, R.; Langedijk, J.P. Measles virus (MV) hemagglutinin: Evidence that attachment sites for MV receptors SLAM and CD46 overlap on the globular head. J. Virol. 2004, 78, 9051–9063. [Google Scholar] [CrossRef] [PubMed]
- Seki, F.; Yamada, K.; Nakatsu, Y.; Okamura, K.; Yanagi, Y.; Nakayama, T.; Komase, K.; Takeda, M. The SI strain of measles virus derived from a patient with subacute sclerosing panencephalitis possesses typical genome alterations and unique amino acid changes that modulate receptor specificity and reduce membrane fusion activity. J. Virol. 2011, 85, 11871–11882. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Ohno, S.; Sakai, K.; Ito, Y.; Fukuhara, H.; Komase, K.; Brindley, M.A.; Rota, P.A.; Plemper, R.K.; Maenaka, K.; et al. The receptor-binding site of the measles virus hemagglutinin protein itself constitutes a conserved neutralizing epitope. J. Virol. 2013, 87, 3583–3586. [Google Scholar] [CrossRef] [PubMed]
- Seki, F.; Someya, K.; Komase, K.; Takeda, M. A chicken homologue of nectin-4 functions as a measles virus receptor. Vaccine 2016, 34, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Cattaneo, R.; Schwartz, S.; Norrby, E. Role of N-linked oligosaccharide chains in the processing and antigenicity of measles virus haemagglutinin protein. J. Gen. Virol. 1994, 75, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Santiago, C.; Bjorling, E.; Stehle, T.; Casasnovas, J.M. Distinct kinetics for binding of the CD46 and SLAM receptors to overlapping sites in the measles virus hemagglutinin protein. J. Biol. Chem. 2002, 277, 32294–32301. [Google Scholar] [CrossRef] [PubMed]
- Sheshberadaran, H.; Norrby, E. Characterization of epitopes on the measles virus hemagglutinin. Virology 1986, 152, 58–65. [Google Scholar] [CrossRef]
- Sheshberadaran, H.; Payne, L.G. Protein antigen-monoclonal antibody contact sites investigated by limited proteolysis of monoclonal antibody-bound antigen: Protein “footprinting”. Proc. Natl. Acad. Sci. USA 1988, 85, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sakata, H.; Kobune, F.; Sato, T.A.; Tanabayashi, K.; Yamada, A.; Sugiura, A. Variation in field isolates of measles virus during an 8-year period in Japan. Microbiol. Immunol. 1993, 37, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Rota, J.S.; Hummel, K.B.; Rota, P.A.; Bellini, W.J. Genetic variability of the glycoprotein genes of current wild-type measles isolates. Virology 1992, 188, 135–142. [Google Scholar] [CrossRef]
- Levy, C.; Amirache, F.; Costa, C.; Frecha, C.; Muller, C.P.; Kweder, H.; Buckland, R.; Cosset, F.L.; Verhoeyen, E. Lentiviral vectors displaying modified measles virus gp overcome pre-existing immunity in in vivo-like transduction of human T and B cells. Mol. Ther. 2012, 20, 1699–1712. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.B.; Scheiflinger, F.; Manchester, M.; Yilma, T.; Oldstone, M.B. Structural and functional studies of the measles virus hemagglutinin: Identification of a novel site required for CD46 interaction. Virology 1999, 256, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Saitoh, M.; Kobayashi, M.; Ishii, H.; Saraya, T.; Kurai, D.; Tsukagoshi, H.; Shirabe, K.; Nishina, A.; Kozawa, K.; et al. Molecular evolution of haemagglutinin (H) gene in measles virus. Sci. Rep. 2015. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Measles virus nomenclature update: 2012. Wkly. Epidemiol. Rec. 2012, 87, 73–81. [Google Scholar]
- Nagai, M.; Xin, J.Y.; Yoshida, N.; Miyata, A.; Fujino, M.; Ihara, T.; Yoshikawa, T.; Asano, Y.; Nakayama, T. Modified adult measles in outbreaks in Japan, 2007–2008. J. Med. Virol. 2009, 81, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.; Brons, N.H.; Berbers, G.A.; Wiesmuller, K.H.; Fleckenstein, B.T.; Schneider, F.; Jung, G.; Muller, C.P. Antibodies to a new linear site at the topographical or functional interface between the haemagglutinin and fusion proteins protect against measles encephalitis. J. Gen. Virol. 1997, 78, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Paal, T.; Brindley, M.A.; St Clair, C.; Prussia, A.; Gaus, D.; Krumm, S.A.; Snyder, J.P.; Plemper, R.K. Probing the spatial organization of measles virus fusion complexes. J. Virol. 2009, 83, 10480–10493. [Google Scholar] [CrossRef] [PubMed]
- Apte-Sengupta, S.; Negi, S.; Leonard, V.H.; Oezguen, N.; Navaratnarajah, C.K.; Braun, W.; Cattaneo, R. Base of the measles virus fusion trimer head receives the signal that triggers membrane fusion. J. Biol. Chem. 2012, 287, 33026–33035. [Google Scholar] [CrossRef] [PubMed]
- Brindley, M.A.; Plemper, R.K. Blue native PAGE and biomolecular complementation reveal a tetrameric or higher-order oligomer organization of the physiological measles virus attachment protein H. J. Virol. 2010, 84, 12174–12184. [Google Scholar] [CrossRef] [PubMed]
- Ader, N.; Brindley, M.A.; Avila, M.; Origgi, F.C.; Langedijk, J.P.; Orvell, C.; Vandevelde, M.; Zurbriggen, A.; Plemper, R.K.; Plattet, P. Structural rearrangements of the central region of the morbillivirus attachment protein stalk domain trigger F protein refolding for membrane fusion. J. Biol. Chem. 2012, 287, 16324–16334. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Shirogane, Y.; Hashiguchi, T.; Yanagi, Y. Mutations in the putative dimer-dimer interfaces of the measles virus hemagglutinin head domain affect membrane fusion triggering. J. Biol. Chem. 2013, 288, 8085–8091. [Google Scholar] [CrossRef] [PubMed]
- Ayata, M.; Tanaka, M.; Kameoka, K.; Kuwamura, M.; Takeuchi, K.; Takeda, M.; Kanou, K.; Ogura, H. Amino acid substitutions in the heptad repeat A and C regions of the F protein responsible for neurovirulence of measles virus Osaka-1 strain from a patient with subacute sclerosing panencephalitis. Virology 2016, 487, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, N.; Sekizuka, T.; Seki, F.; Sakai, K.; Kubota, T.; Nakatsu, Y.; Chen, S.; Fukuhara, H.; Maenaka, K.; Yamaguchi, R.; et al. Canine distemper virus with the intact C protein has the potential to replicate in human epithelial cells by using human nectin4 as a receptor. Virology 2013, 435, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Ohno, S.; Tahara, M.; Takeuchi, H.; Shirogane, Y.; Ohmura, H.; Nakamura, T.; Yanagi, Y. Measles viruses possessing the polymerase protein genes of the Edmonston vaccine strain exhibit attenuated gene expression and growth in cultured cells and SLAM knock-in mice. J. Virol. 2008, 82, 11979–11984. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Takeuchi, K.; Miyajima, N.; Kobune, F.; Ami, Y.; Nagata, N.; Suzaki, Y.; Nagai, Y.; Tashiro, M. Recovery of pathogenic measles virus from cloned cDNA. J. Virol. 2000, 74, 6643–6647. [Google Scholar] [CrossRef] [PubMed]
| Amino Acids 1 | MAb | Epitope Name | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| β1 | 235 | E185 | iv | ||||||||
| 233–240 | BH1 | ||||||||||
| 235, 244–250 | BH47, BH59, BH103, BH129 | NE | |||||||||
| β2 | 302 | E39 | v | ||||||||
| 310 | BH38 | LE | E4 | ||||||||
| 310 | BH141 | LE | E4 | ||||||||
| 309–318 | I-29 | LE | I | E4 | IA | 1 | |||||
| n.d. 2 | I-12 | LE | I | IA | 1 | ||||||
| 311 | E103 | LE | vi | ||||||||
| β3 | 377–378 | L77 | F | ||||||||
| 391 | B5 | I | |||||||||
| 391 | E81 | I | |||||||||
| 379–400 | BH6, BH21, BH216 | HNE | |||||||||
| 395, 398 | cl48 | E3 | |||||||||
| n.d. | K71 | D | |||||||||
| n.d. | NC32 | D/E | |||||||||
| β4 | 473–477 | E128 | SSE | II | |||||||
| 491 | 16-CD11 | SSE | II | II | 2 | ||||||
| n.d. | BH99 | SSE | |||||||||
| 488 | BH97 | ||||||||||
| 483 | 2F4 | RBE | vii | ||||||||
| β5 | 505, 541, 543, 533 | 2F4 | RBE | vii | |||||||
| 505, 506 | 80-II-B2 | RBE | |||||||||
| 533 | cl55 | RBE | |||||||||
| 532, 533 | 16-DE6 | RBE | III | E2 | 3 | ||||||
| 547, 546 | 20H6 | RBE | |||||||||
| 552 | I-41 | RBE | III | E2 | IIIB | 3 | |||||
| β6 | 187 | I-44 | RBE | IV | IV | 4 | |||||
| 190 | 2F4 | RBE | vii | ||||||||
| 190–200, 571–579 | BH26 | ||||||||||
| References 3 | This | [23] | [27] | [28] | [44] | [29] | [30] | [36] | [45] | ||
| review | [53] | [40] | |||||||||
| Neutralizing Titer | HI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell | B95a | II-18 | Vero | RBC 3 | ||||||||
| Receptor | SLAM | Nectin-4 | CD46 | |||||||||
| Virus 1 | A | D3 | D3/Q391R | A | D3 | D3/Q391R | A | |||||
| MAb 2 | E81 | 2560 | 5120 | <40 | 81,920 | 81,920 | <1280 | 163,840 | 40,960 | |||
| B5 | 81,920 | 20,480 | <1280 | 81,920 | 10,240 | <1280 | 163,840 | 20,480 | ||||
| E128 | 5120 | 40 | n.d. 4 | 163,840 | <2560 | n.d. | 655,360 | 40,960 | ||||
| E103 | 2560 | 10,240 | 20,480 | 10,240 | 20,480 | 20,480 | 20,480 | 2560 | ||||
| E185 | 640 | 40 | n.d. | n.d. | n.d. | n.d. | 2560 | 2560 | ||||
| Virus 2 | MAb 3 | Vaccinees | Measles Patients | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E128 | #v9 | #v12 | #v14 | #v27 | #v29 | #p1 | #p2 | #p3 | #p4 | |||
| A | 163,840 | 80 | 640 | 160 | 80 | 80 | 40 | 40 | 320 | 5120 | ||
| D3 | <2560 | 160 | 640 | 160 | 80 | 160 | 320 | 160 | 640 | 20,480 | ||
| Virus 2 | A | A/E235G | D3 | |
|---|---|---|---|---|
| MAb 3 | BH47 | 81,920 | 10,240 | <640 |
| BH59 | 20,480 | 1280 | <160 | |
| BH129 | 20,480 | 640 | <160 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tahara, M.; Bürckert, J.-P.; Kanou, K.; Maenaka, K.; Muller, C.P.; Takeda, M. Measles Virus Hemagglutinin Protein Epitopes: The Basis of Antigenic Stability. Viruses 2016, 8, 216. https://doi.org/10.3390/v8080216
Tahara M, Bürckert J-P, Kanou K, Maenaka K, Muller CP, Takeda M. Measles Virus Hemagglutinin Protein Epitopes: The Basis of Antigenic Stability. Viruses. 2016; 8(8):216. https://doi.org/10.3390/v8080216
Chicago/Turabian StyleTahara, Maino, Jean-Philippe Bürckert, Kazuhiko Kanou, Katsumi Maenaka, Claude P. Muller, and Makoto Takeda. 2016. "Measles Virus Hemagglutinin Protein Epitopes: The Basis of Antigenic Stability" Viruses 8, no. 8: 216. https://doi.org/10.3390/v8080216






