HPV Population Profiling in Healthy Men by Next-Generation Deep Sequencing Coupled with HPV-QUEST
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants, Specimen Collection, and DNA Extraction
2.2. Generation of HPV L1 Amplicon Libraries and Deep Sequencing
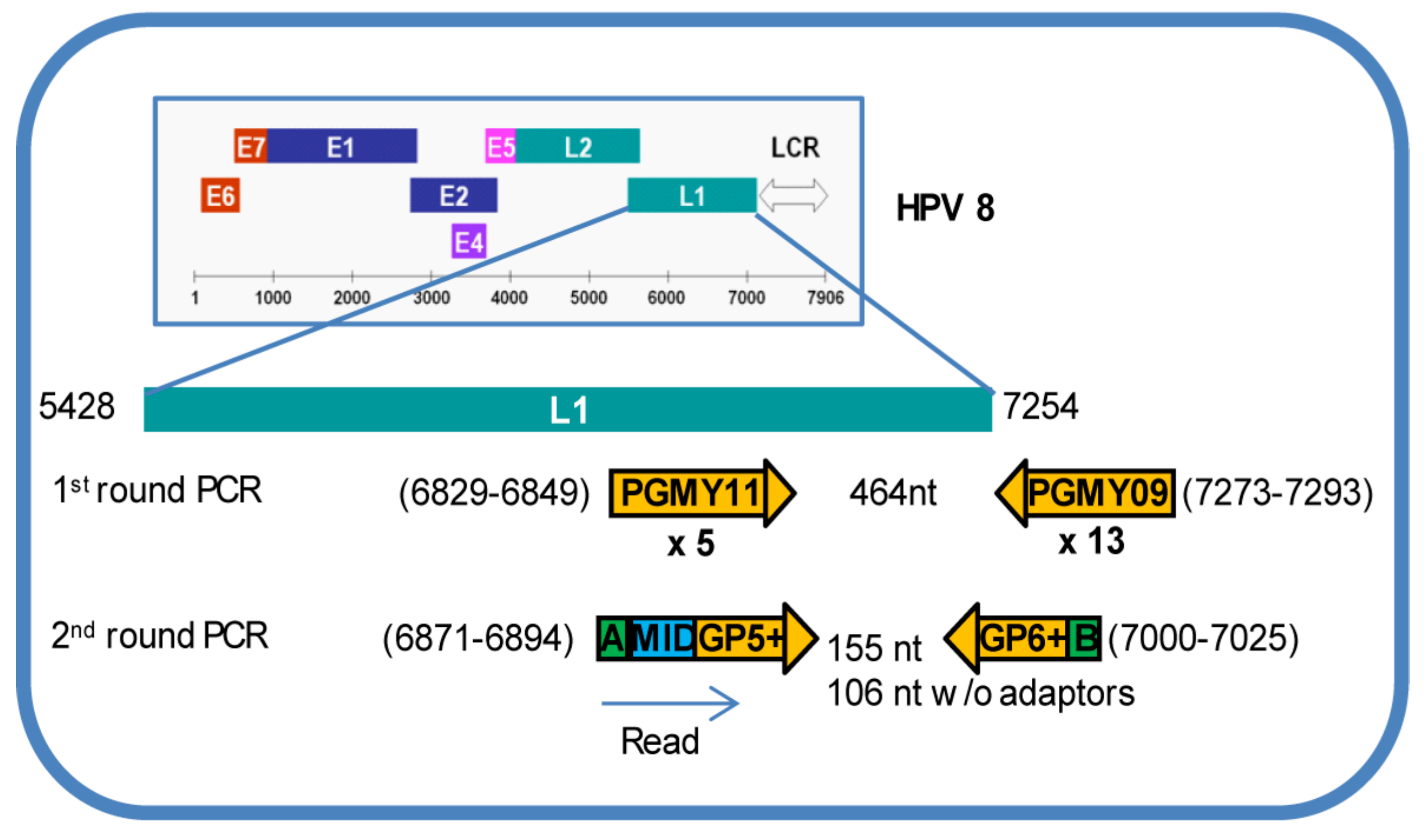
2.3. Sequence Analysis
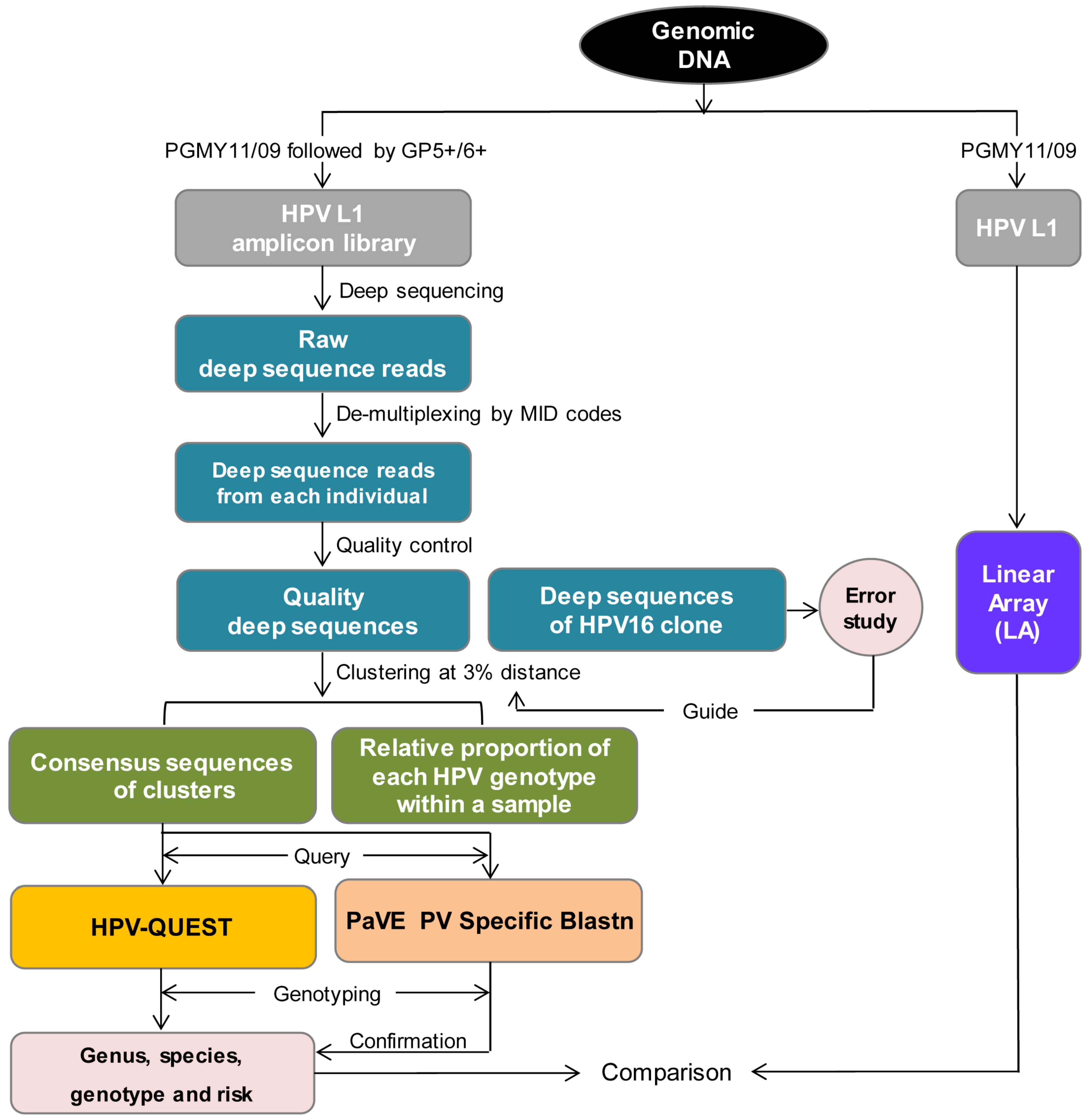
3. Results
3.1. HPV Genotype Infection and Diversity

3.2. HPV Genotype Variants

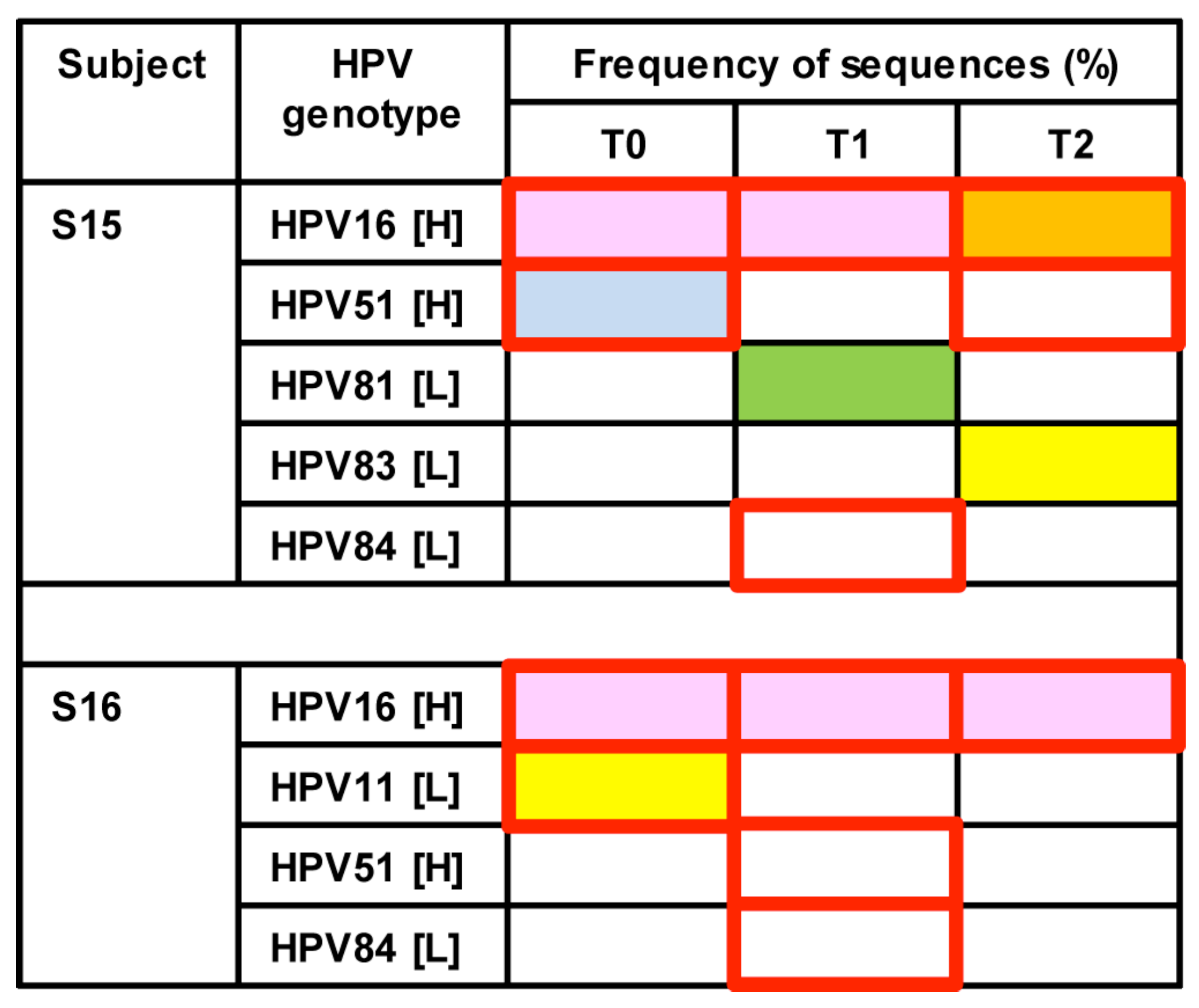
3.3. Longitudinal Changes of HPV Types within Two Healthy Individuals
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- D’Souza, G.; Kreimer, A.R.; Viscidi, R.; Pawlita, M.; Fakhry, C.; Koch, W.M.; Westra, W.H.; Gillison, M.L. Case-control study of human papillomavirus and oropharyngeal cancer. N. Engl. J. Med. 2007, 356, 1944–1956. [Google Scholar] [CrossRef] [PubMed]
- Klein, F.; Amin Kotb, W.F.; Petersen, I. Incidence of human papilloma virus in lung cancer. Lung Cancer 2009, 65, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, L.; Zhang, Y.; Zhao, P.; Zheng, T.; Dai, M. Human papillomavirus infection and bladder cancer risk: A meta-analysis. J. Infect. Dis. 2011, 204, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Marur, S.; D’Souza, G.; Westra, W.H.; Forastiere, A.A. HPV-associated head and neck cancer: A virus-related cancer epidemic. Lancet Oncol. 2010, 11, 781–789. [Google Scholar] [CrossRef]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Munoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Gallegos-Hernandez, J.F.; Paredes-Hernandez, E.; Flores-Diaz, R.; Minauro-Munoz, G.; Apresa-Garcia, T.; Hernandez-Hernandez, D.M. Human papillomavirus: Association with head and neck cancer. Cir. Cir. 2007, 75, 151–155. [Google Scholar] [PubMed]
- Giuliano, A.R.; Nyitray, A.G.; Kreimer, A.R.; Pierce Campbell, C.M.; Goodman, M.T.; Sudenga, S.L.; Monsonego, J.; Franceschi, S. EUROGIN 2014 roadmap: Differences in human papillomavirus infection natural history, transmission and human papillomavirus-related cancer incidence by gender and anatomic site of infection. Int. J. Cancer 2015, 136, 2752–2760. [Google Scholar] [CrossRef] [PubMed]
- Anwar, K.; Naiki, H.; Nakakuki, K.; Inuzuka, M. High frequency of human papillomavirus infection in carcinoma of the urinary bladder. Cancer 1992, 70, 1967–1973. [Google Scholar] [CrossRef]
- Fife, K.H.; Cramer, H.M.; Schroeder, J.M.; Brown, D.R. Detection of multiple human papillomavirus types in the lower genital tract correlates with cervical dysplasia. J. Med. Virol. 2001, 64, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Sasagawa, T.; Basha, W.; Yamazaki, H.; Inoue, M. High-risk and multiple human papillomavirus infections associated with cervical abnormalities in Japanese women. Cancer Epidemiol. Biomark. Prev. 2001, 10, 45–52. [Google Scholar]
- Yang, Y.; Li, X.; Zhang, Z.; Qian, H.Z.; Ruan, Y.; Zhou, F.; Gao, C.; Li, M.; Jin, Q.; Gao, L. Association of human papillomavirus infection and abnormal anal cytology among HIV-infected MSM in Beijing, China. PLoS ONE 2012, 7, e35983. [Google Scholar] [CrossRef] [PubMed]
- Trottier, H.; Mahmud, S.; Costa, M.C.; Sobrinho, J.P.; Duarte-Franco, E.; Rohan, T.E.; Ferenczy, A.; Villa, L.L.; Franco, E.L. Human papillomavirus infections with multiple types and risk of cervical neoplasia. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Bachtiary, B.; Obermair, A.; Dreier, B.; Birner, P.; Breitenecker, G.; Knocke, T.H.; Selzer, E.; Potter, R. Impact of multiple HPV infection on response to treatment and survival in patients receiving radical radiotherapy for cervical cancer. Int. J. Cancer 2002, 102, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Munagala, R.; Dona, M.G.; Rai, S.N.; Jenson, A.B.; Bala, N.; Ghim, S.J.; Gupta, R.C. Significance of multiple HPV infection in cervical cancer patients and its impact on treatment response. Int. J. Oncol. 2009, 34, 263–271. [Google Scholar] [PubMed]
- Syrjanen, S. The role of human papillomavirus infection in head and neck cancers. Ann. Oncol. 2010, 21 (Suppl. 7), vii243–vii245. [Google Scholar] [CrossRef] [PubMed]
- Dayyani, F.; Etzel, C.J.; Liu, M.; Ho, C.H.; Lippman, S.M.; Tsao, A.S. Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC). Head Neck Oncol. 2010, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Ragin, C.C.; Taioli, E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: Review and meta-analysis. Int. J. Cancer 2007, 121, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Rautava, J.; Kuuskoski, J.; Syrjanen, K.; Grenman, R.; Syrjanen, S. HPV genotypes and their prognostic significance in head and neck squamous cell carcinomas. J. Clin. Virol. 2012, 53, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Nielson, C.M.; Harris, R.B.; Flores, R.; Abrahamsen, M.; Papenfuss, M.R.; Dunne, E.F.; Markowitz, L.E.; Giuliano, A.R. Multiple-type human papillomavirus infection in male anogenital sites: Prevalence and associated factors. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, S.K.; Munk, C.; Winther, J.F.; Jorgensen, H.O.; Meijer, C.J.; van den Brule, A.J. Acquisition and persistence of human papillomavirus infection in younger men: A prospective follow-up study among Danish soldiers. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Louvanto, K.; Syrjanen, K.J.; Rintala, M.A.; Grenman, S.E.; Syrjanen, S.M. Genotype-specific clearance of genital human papillomavirus (HPV) infections among mothers in the Finnish family HPV study. J. Clin. Microbiol. 2010, 48, 2665–2671. [Google Scholar] [CrossRef] [PubMed]
- Louvanto, K.; Rintala, M.A.; Syrjanen, K.J.; Grenman, S.E.; Syrjanen, S.M. Genotype-specific persistence of genital human papillomavirus (HPV) infections in women followed for 6 years in the Finnish Family HPV Study. J. Infect. Dis. 2010, 202, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, K.; Varnai, A.D.; Bollmann, M.; Bankfalvi, A.; Szendy, M.; Speich, N.; Schmitt, C.; Pajor, L.; Bollmann, R.; Hildenbrand, R. A 7.5-year prospective study of longer than 18 months type-specific human papillomavirus persistence in a routine cytology-based cervical screening population of about 31,000 women in West Germany. Eur. J. Cancer Prev. 2009, 18, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.L.; Hjerpe, A. Persistence of human papillomavirus (HPV) infections preceding cervical carcinoma. Cancer 1996, 77, 121–127. [Google Scholar] [CrossRef]
- Yang, Z.; Cuzick, J.; Hunt, W.C.; Wheeler, C.M. Concurrence of multiple human papillomavirus infections in a large US population-based cohort. Am. J. Epidemiol. 2014, 180, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, N.O.; Del Castillo, D.M.; Perone, C.; Januario, J.N.; Melo, V.H.; Brasileiro, F.G. Comparison of HPV genotyping by type-specific PCR and sequencing. Mem. Inst. Oswaldo Cruz 2010, 105, 73–78. [Google Scholar] [CrossRef]
- Castle, P.E.; Porras, C.; Quint, W.G.; Rodriguez, A.C.; Schiffman, M.; Gravitt, P.E.; Gonzalez, P.; Katki, H.A.; Silva, S.; Freer, E.; et al. Comparison of two PCR-based human papillomavirus genotyping methods. J. Clin. Microbiol. 2008, 46, 3437–3445. [Google Scholar] [CrossRef] [PubMed]
- Klug, S.J.; Molijn, A.; Schopp, B.; Holz, B.; Iftner, A.; Quint, W.; Snijders, J.F.; Petry, K.U.; Kruger, K.S.; Munk, C.; et al. Comparison of the performance of different HPV genotyping methods for detecting genital HPV types. J. Med. Virol. 2008, 80, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- Plummer, M.; Vaccarella, S.; Franceschi, S. Multiple human papillomavirus infections: The exception or the rule? J. Infect. Dis. 2011, 203, 891–893. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Lai, C.H.; Huang, H.J.; Chao, A.; Chang, C.J.; Chang, T.C.; Chou, H.H.; Hong, J.H. Clinical effect of human papillomavirus genotypes in patients with cervical cancer undergoing primary radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Gharizadeh, B.; Kalantari, M.; Garcia, C.A.; Johansson, B.; Nyren, P. Typing of human papillomavirus by pyrosequencing. Lab. Investig. 2001, 81, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Gharizadeh, B.; Oggionni, M.; Zheng, B.; Akom, E.; Pourmand, N.; Ahmadian, A.; Wallin, K.L.; Nyren, P. Type-specific multiple sequencing primers: A novel strategy for reliable and rapid genotyping of human papillomaviruses by pyrosequencing technology. J. Mol. Diagn. 2005, 7, 198–205. [Google Scholar] [CrossRef]
- Yin, L.; Yao, J.; Gardner, B.P.; Chang, K.; Yu, F.; Goodenow, M.M. HPV-QUEST: A highly customized system for automated HPV sequence analysis capable of processing Next Generation sequencing data set. Bioinformation 2012, 8, 388–390. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.R.; Lazcano-Ponce, E.; Villa, L.L.; Flores, R.; Salmeron, J.; Lee, J.H.; Papenfuss, M.R.; Abrahamsen, M.; Jolles, E.; Nielson, C.M.; et al. The human papillomavirus infection in men study: Human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2036–2043. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.R.; Lee, J.H.; Fulp, W.; Villa, L.L.; Lazcano, E.; Papenfuss, M.R.; Abrahamsen, M.; Salmeron, J.; Anic, G.M.; Rollison, D.E.; et al. Incidence and clearance of genital human papillomavirus infection in men (HIM): A cohort study. Lancet 2011, 377, 932–940. [Google Scholar] [CrossRef]
- Lu, B.; Viscidi, R.P.; Lee, J.H.; Wu, Y.; Villa, L.L.; Lazcano-Ponce, E.; da Silva, R.J.; Baggio, M.L.; Quiterio, M.; Salmeron, J.; et al. Human papillomavirus (HPV) 6, 11, 16, and 18 seroprevalence is associated with sexual practice and age: Results from the multinational HPV Infection in Men Study (HIM Study). Cancer Epidemiol. Biomark. Prev. 2011, 20, 990–1002. [Google Scholar] [CrossRef] [PubMed]
- Vaccarella, S.; Plummer, M.; Franceschi, S.; Gravitt, P.; Papenfuss, M.; Smith, D.; Villa, L.; Ponce, E.L.; Giuliano, A.R. Clustering of human papillomavirus (HPV) types in the male genital tract: The HPV in men (HIM) study. J. Infect. Dis. 2011, 204, 1500–1504. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Abalos, A.T.; Nielson, C.M.; Abrahamsen, M.; Harris, R.B.; Giuliano, A.R. Reliability of sample collection and laboratory testing for HPV detection in men. J. Virol. Methods 2008, 149, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Benki, S.; McClelland, R.S.; Emery, S.; Baeten, J.M.; Richardson, B.A.; Lavreys, L.; Mandaliya, K.; Overbaugh, J. Quantification of genital human immunodeficiency virus type 1 (HIV-1) DNA in specimens from women with low plasma HIV-1 RNA levels typical of HIV-1 nontransmitters. J. Clin. Microbiol. 2006, 44, 4357–4362. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Vandegraaff, N.; Mundy, L.; Burrell, C.J.; Li, P. Evaluation of PCR-based methods for the quantitation of integrated HIV-1 DNA. J. Virol. Methods 2002, 105, 233–246. [Google Scholar] [CrossRef]
- Montoya, J.G.; Wood, R.; Katzenstein, D.; Holodny, M.; Merigan, T.C. Peripheral blood mononuclear cell human immunodeficiency virus type 1 proviral DNA quantification by polymerase chain reaction: Relationship to immunodeficiency and drug effect. J. Clin. Microbiol. 1993, 31, 2692–2696. [Google Scholar] [PubMed]
- Vandegraaff, N.; Kumar, R.; Burrell, C.J.; Li, P. Kinetics of human immunodeficiency virus type 1 (HIV) DNA integration in acutely infected cells as determined using a novel assay for detection of integrated HIV DNA. J. Virol. 2001, 75, 11253–11260. [Google Scholar] [CrossRef] [PubMed]
- Yee, C.; Krishnan-Hewlett, I.; Baker, C.C.; Schlegel, R.; Howley, P.M. Presence and expression of human papillomavirus sequences in human cervical carcinoma cell lines. Am. J. Pathol. 1985, 119, 361–366. [Google Scholar] [PubMed]
- Gravitt, P.E.; Peyton, C.L.; Alessi, T.Q.; Wheeler, C.M.; Coutlee, F.; Hildesheim, A.; Schiffman, M.H.; Scott, D.R.; Apple, R.J. Improved amplification of genital human papillomaviruses. J. Clin. Microbiol. 2000, 38, 357–361. [Google Scholar] [PubMed]
- De Roda Husman, A.M.; Walboomers, J.M.; van den Brule, A.J.; Meijer, C.J.; Snijders, P.J. The use of general primers GP5 and GP6 elongated at their 3′ ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J. Gen. Virol. 1995, 76, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Fuessel Haws, A.L.; He, Q.; Rady, P.L.; Zhang, L.; Grady, J.; Hughes, T.K.; Stisser, K.; Konig, R.; Tyring, S.K. Nested PCR with the PGMY09/11 and GP5(+)/6(+) primer sets improves detection of HPV DNA in cervical samples. J. Virol. Methods 2004, 122, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Winder, D.M.; Ball, S.L.; Vaughan, K.; Hanna, N.; Woo, Y.L.; Franzer, J.T.; Sterling, J.C.; Stanley, M.A.; Sudhoff, H.; Goon, P.K. Sensitive HPV detection in oropharyngeal cancers. BMC Cancer 2009, 9, 440. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.V.; Snijders, P.J.; Voorhorst, F.J.; Dillner, J.; Forslund, O.; Johansson, B.; von Knebel, D.M.; Meijer, C.J.; Meyer, T.; Nindl, I.; et al. Reliable high risk HPV DNA testing by polymerase chain reaction: An intermethod and intramethod comparison. J. Clin. Pathol. 1999, 52, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Kornegay, J.R.; Roger, M.; Davies, P.O.; Shepard, A.P.; Guerrero, N.A.; Lloveras, B.; Evans, D.; Coutlee, F. International proficiency study of a consensus L1 PCR assay for the detection and typing of human papillomavirus DNA: Evaluation of accuracy and intralaboratory and interlaboratory agreement. J. Clin. Microbiol. 2003, 41, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Roche: Amplicon Fusion Primer Design Guidlines for GS FLX Titanium Seris Lib-A Chemistry; 454 Life Science Corp.: Branford, CT, USA, 2009; pp. 1–3.
- Sun, Y.; Cai, Y.; Liu, L.; Yu, F.; Farrell, M.L.; McKendree, W.; Farmerie, W. ESPRIT: Estimating species richness using large collections of 16S rRNA pyrosequences. Nucleic Acids Res. 2009, 37, e76. [Google Scholar] [CrossRef] [PubMed]
- Bernard, H.U.; Calleja-Macias, I.E.; Dunn, S.T. Genome variation of human papillomavirus types: Phylogenetic and medical implications. Int. J. Cancer 2006, 118, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- De Villiers, E.M.; Fauquet, C.; Broker, T.R.; Bernard, H.U.; Zur, H.H. Classification of papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Wheeler, C.M.; Halpern, A.L.; Stewart, A.C.; Hildesheim, A.; Jenison, S.A. Human papillomavirus type 16 variant lineages in United States populations characterized by nucleotide sequence analysis of the E6, L2, and L1 coding segments. J. Virol. 1995, 69, 7743–7753. [Google Scholar] [PubMed]
- NCBI Genebank. Available online: http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Tree&id=151340&lvl=3&lin=f&keep=1&srchmode=1&unlock (accessed on 10 December, 2015).
- Los Alamos HPV Sequence Database. Available online: http://hpv-web.lanl.gov/ (accessed on 10 December, 2015).
- Virus Sequence Database. Available online: http://kcdc.labkm.net/vsd/database/gene_search_7.jsp?orgId=7&reset=1 (accessed on 10 December, 2015).
- Chan, S.Y.; Delius, H.; Halpern, A.L.; Bernard, H.U. Analysis of genomic sequences of 95 papillomavirus types: Uniting typing, phylogeny, and taxonomy. J. Virol. 1995, 69, 3074–3083. [Google Scholar] [PubMed]
- Papillomavirus Episteme Blastn. Available online: http://pave.niaid.nih.gov/#search/pv_specific_blast (accessed on 10 December, 2015).
- NCBI Blastn. Available online: http://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome (accessed on 10 December, 2015).
- Bernard, H.U.; Chan, S.Y.; Manos, M.M.; Ong, C.K.; Villa, L.L.; Delius, H.; Peyton, C.L.; Bauer, H.M.; Wheeler, C.M. Identification and assessment of known and novel human papillomaviruses by polymerase chain reaction amplification, restriction fragment length polymorphisms, nucleotide sequence, and phylogenetic algorithms. J. Infect. Dis. 1994, 170, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Delius, H.; Hofmann, B. Primer-directed sequencing of human papillomavirus types. Curr. Top. Microbiol. Immunol. 1994, 186, 13–31. [Google Scholar] [PubMed]
- Schwarz, E.; Durst, M.; Demankowski, C.; Lattermann, O.; Zech, R.; Wolfsperger, E.; Suhai, S.; zur Hausen, H. DNA sequence and genome organization of genital human papillomavirus type 6b. EMBO J. 1983, 2, 2341–2348. [Google Scholar] [PubMed]
- Chaturvedi, A.K.; Katki, H.A.; Hildesheim, A.; Rodriguez, A.C.; Quint, W.; Schiffman, M.; van Doorn, L.J.; Porras, C.; Wacholder, S.; Gonzalez, P.; et al. Human papillomavirus infection with multiple types: Pattern of coinfection and risk of cervical disease. J. Infect. Dis. 2011, 203, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Westra, W.H. Detection of human papillomavirus (HPV) in clinical samples: Evolving methods and strategies for the accurate determination of HPV status of head and neck carcinomas. Oral Oncol. 2014, 50, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Mirghani, H.; Amen, F.; Moreau, F.; Guigay, J.; Ferchiou, M.; Melkane, A.E.; Hartl, D.M.; Lacau St, G.J. Human papilloma virus testing in oropharyngeal squamous cell carcinoma: What the clinician should know. Oral Oncol. 2014, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.; Sloan, P.; Shaw, R. Refining the diagnosis of oropharyngeal squamous cell carcinoma using human papillomavirus testing. Oral Oncol. 2010, 46, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Kleter, B.; van Doorn, L.J.; ter Schegget, J.; Schrauwen, L.; van, K.K.; Burger, M.; ter Harmsel, B.; Quint, W. Novel short-fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. Am. J. Pathol. 1998, 153, 1731–1739. [Google Scholar] [CrossRef]
- Kleter, B.; van Doorn, L.J.; Schrauwen, L.; Molijn, A.; Sastrowijoto, S.; ter Schegget, J.; Lindeman, J.; ter Harmsel, B.; Burger, M.; Quint, W. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J. Clin. Microbiol. 1999, 37, 2508–2517. [Google Scholar] [PubMed]
- Hwang, T.S.; Jeong, J.K.; Park, M.; Han, H.S.; Choi, H.K.; Park, T.S. Detection and typing of HPV genotypes in various cervical lesions by HPV oligonucleotide microarray. Gynecol. Oncol. 2003, 90, 51–56. [Google Scholar] [CrossRef]
- van den Brule, A.J.; Pol, R.; Fransen-Daalmeijer, N.; Schouls, L.M.; Meijer, C.J.; Snijders, P.J. GP5+/6+ PCR followed by reverse line blot analysis enables rapid and high-throughput identification of human papillomavirus genotypes. J. Clin. Microbiol. 2002, 40, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, L.S.; Smelov, V.; Bzhalava, D.; Eklund, C.; Hultin, E.; Dillner, J. Next generation sequencing for human papillomavirus genotyping. J. Clin. Virol. 2013, 58, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Vernon, S.D.; Unger, E.R.; Williams, D. Comparison of human papillomavirus detection and typing by cycle sequencing, line blotting, and hybrid capture. J. Clin. Microbiol. 2000, 38, 651–655. [Google Scholar] [PubMed]
- Barzon, L.; Lavezzo, E.; Militello, V.; Toppo, S.; Palu, G. Applications of next-generation sequencing technologies to diagnostic virology. Int. J. Mol. Sci. 2011, 12, 7861–7884. [Google Scholar] [CrossRef] [PubMed]
- Meiring, T.L.; Salimo, A.T.; Coetzee, B.; Maree, H.J.; Moodley, J.; Hitzeroth, I.I.; Freeborough, M.J.; Rybicki, E.P.; Williamson, A.L. Next-generation sequencing of cervical DNA detects human papillomavirus types not detected by commercial kits. Virol. J. 2012, 9, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Militello, V.; Lavezzo, E.; Costanzi, G.; Franchin, E.; Di, C.B.; Toppo, S.; Palu, G.; Barzon, L. Accurate human papillomavirus genotyping by 454 pyrosequencing. Clin. Microbiol. Infect. 2013, 19, E428–E434. [Google Scholar] [CrossRef] [PubMed]
- Ekstrom, J.; Bzhalava, D.; Svenback, D.; Forslund, O.; Dillner, J. High throughput sequencing reveals diversity of Human Papillomaviruses in cutaneous lesions. Int. J. Cancer 2011, 129, 2643–2650. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.; Bzhalava, D.; Ekstrom, J.; Hultin, E.; Dillner, J.; Forslund, O. Metagenomic sequencing of “HPV-negative” condylomas detects novel putative HPV types. Virology 2013, 440, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Chan, S.Y.; Burk, R.D.; Das, B.C.; Fujinaga, K.; Icenogle, J.P.; Kahn, T.; Kiviat, N.; Lancaster, W.; Mavromara-Nazos, P.; et al. The genetic drift of human papillomavirus type 16 is a means of reconstructing prehistoric viral spread and the movement of ancient human populations. J. Virol. 1993, 67, 6413–6423. [Google Scholar] [PubMed]
- Lichtig, H.; Algrisi, M.; Botzer, L.E.; Abadi, T.; Verbitzky, Y.; Jackman, A.; Tommasino, M.; Zehbe, I.; Sherman, L. HPV16 E6 natural variants exhibit different activities in functional assays relevant to the carcinogenic potential of E6. Virology 2006, 350, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.K.; Chan, S.Y.; Campo, M.S.; Fujinaga, K.; Mavromara-Nazos, P.; Labropoulou, V.; Pfister, H.; Tay, S.K.; ter Meulen, J.; Villa, L.L.; et al. Evolution of human papillomavirus type 18: An ancient phylogenetic root in Africa and intratype diversity reflect coevolution with human ethnic groups. J. Virol. 1993, 67, 6424–6431. [Google Scholar] [PubMed]
- Pillai, M.R.; Hariharan, R.; Babu, J.M.; Lakshmi, S.; Chiplunkar, S.V.; Patkar, M.; Tongaonkar, H.; Dinshaw, K.; Jayshree, R.S.; Reddy, B.K.; et al. Molecular variants of HPV-16 associated with cervical cancer in Indian population. Int. J. Cancer 2009, 125, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Travasso, C.M.; Anand, M.; Samarth, M.; Deshpande, A.; Kumar-Sinha, C. Human papillomavirus genotyping by multiplex pyrosequencing in cervical cancer patients from India. J. Biosci. 2008, 33, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.K.; Cheung, T.H.; Tam, A.O.; Lo, K.W.; Yim, S.F.; Yu, M.M.; To, K.F.; Wong, Y.F.; Cheung, J.L.; Chan, D.P.; et al. Biases in human papillomavirus genotype prevalence assessment associated with commonly used consensus primers. Int. J. Cancer 2006, 118, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Eklund, C.; Forslund, O.; Wallin, K.L.; Zhou, T.; Dillner, J. The 2010 global proficiency study of human papillomavirus genotyping in vaccinology. J. Clin. Microbiol. 2012, 50, 2289–2298. [Google Scholar] [CrossRef] [PubMed]
- Al-Shabanah, O.A.; Hafez, M.M.; Hassan, Z.K.; Sayed-Ahmed, M.M.; Abozeed, W.N.; Al-Rejaie, S.S.; Alsheikh, A.A. Human papillomavirus genotyping and integration in ovarian cancer Saudi patients. Virol. J. 2013, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- Briolat, J.; Dalstein, V.; Saunier, M.; Joseph, K.; Caudroy, S.; Pretet, J.L.; Birembaut, P.; Clavel, C. HPV prevalence, viral load and physical state of HPV-16 in cervical smears of patients with different grades of CIN. Int. J. Cancer 2007, 121, 2198–2204. [Google Scholar] [CrossRef] [PubMed]
- Cricca, M.; Morselli-Labate, A.M.; Venturoli, S.; Ambretti, S.; Gentilomi, G.A.; Gallinella, G.; Costa, S.; Musiani, M.; Zerbini, M. Viral DNA load, physical status and E2/E6 ratio as markers to grade HPV16 positive women for high-grade cervical lesions. Gynecol. Oncol. 2007, 106, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.M.; Lee, B.H.; Chang, S.F.; Chien, T.Y.; Huang, S.H.; Yan, C.C.; Cheng, W.F. Integration of human papillomavirus correlates with high levels of viral oncogene transcripts in cervical carcinogenesis. Virus Res. 2011, 161, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Melsheimer, P.; Vinokurova, S.; Wentzensen, N.; Bastert, G.; von Knebel, D.M. DNA aneuploidy and integration of human papillomavirus type 16 e6/e7 oncogenes in intraepithelial neoplasia and invasive squamous cell carcinoma of the cervix uteri. Clin. Cancer Res. 2004, 10, 3059–3063. [Google Scholar] [CrossRef] [PubMed]
- Pett, M.; Coleman, N. Integration of high-risk human papillomavirus: A key event in cervical carcinogenesis? J. Pathol. 2007, 212, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Joo, J.; Yoon, J.H.; Yoo, C.W.; Kim, J.Y. Physical status of human papillomavirus integration in cervical cancer is associated with treatment outcome of the patients treated with radiotherapy. PLoS ONE 2014, 9, e78995. [Google Scholar] [CrossRef] [PubMed]
- Wentzensen, N.; Vinokurova, S.; von Knebel, D.M. Systematic review of genomic integration sites of human papillomavirus genomes in epithelial dysplasia and invasive cancer of the female lower genital tract. Cancer Res. 2004, 64, 3878–3884. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chotewutmontri, S.; Wolf, S.; Klos, U.; Schmitz, M.; Durst, M.; Schwarz, E. Multiplex identification of human papillomavirus 16 DNA integration sites in cervical carcinomas. PLoS ONE 2013, 8, e66693. [Google Scholar] [CrossRef] [PubMed]
- Ziegert, C.; Wentzensen, N.; Vinokurova, S.; Kisseljov, F.; Einenkel, J.; Hoeckel, M.; von Knebel, D.M. A comprehensive analysis of HPV integration loci in anogenital lesions combining transcript and genome-based amplification techniques. Oncogene 2003, 22, 3977–3984. [Google Scholar] [CrossRef] [PubMed]
- Speich, N.; Schmitt, C.; Bollmann, R.; Bollmann, M. Human papillomavirus (HPV) study of 2916 cytological samples by PCR and DNA sequencing: Genotype spectrum of patients from the west German area. J. Med. Microbiol. 2004, 53, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Polz, M.F.; Cavanaugh, C.M. Bias in template-to-product ratios in multitemplate PCR. Appl. Environ. Microbiol. 1998, 64, 3724–3730. [Google Scholar] [PubMed]
- Qu, W.; Jiang, G.; Cruz, Y.; Chang, C.J.; Ho, G.Y.; Klein, R.S.; Burk, R.D. PCR detection of human papillomavirus: Comparison between MY09/MY11 and GP5+/GP6+ primer systems. J. Clin. Microbiol. 1997, 35, 1304–1310. [Google Scholar] [PubMed]
- Suzuki, M.T.; Giovannoni, S.J. Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl. Environ. Microbiol. 1996, 62, 625–630. [Google Scholar] [PubMed]
- Lei, Y.J.; Makhaola, K.; Pittayakhajonwut, D.; Wood, C.; Angeletti, P.C. Human papillomavirus 16 variants from Zambian women with normal pap smears. J. Med. Virol. 2011, 83, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, L.; Yao, J.; Chang, K.; Gardner, B.P.; Yu, F.; Giuliano, A.R.; Goodenow, M.M. HPV Population Profiling in Healthy Men by Next-Generation Deep Sequencing Coupled with HPV-QUEST. Viruses 2016, 8, 28. https://doi.org/10.3390/v8020028
Yin L, Yao J, Chang K, Gardner BP, Yu F, Giuliano AR, Goodenow MM. HPV Population Profiling in Healthy Men by Next-Generation Deep Sequencing Coupled with HPV-QUEST. Viruses. 2016; 8(2):28. https://doi.org/10.3390/v8020028
Chicago/Turabian StyleYin, Li, Jin Yao, Kaifen Chang, Brent P. Gardner, Fahong Yu, Anna R. Giuliano, and Maureen M. Goodenow. 2016. "HPV Population Profiling in Healthy Men by Next-Generation Deep Sequencing Coupled with HPV-QUEST" Viruses 8, no. 2: 28. https://doi.org/10.3390/v8020028





