Tight Junctions Go Viral!
Abstract
:1. Introduction
2. Tight Junction Structure and Function
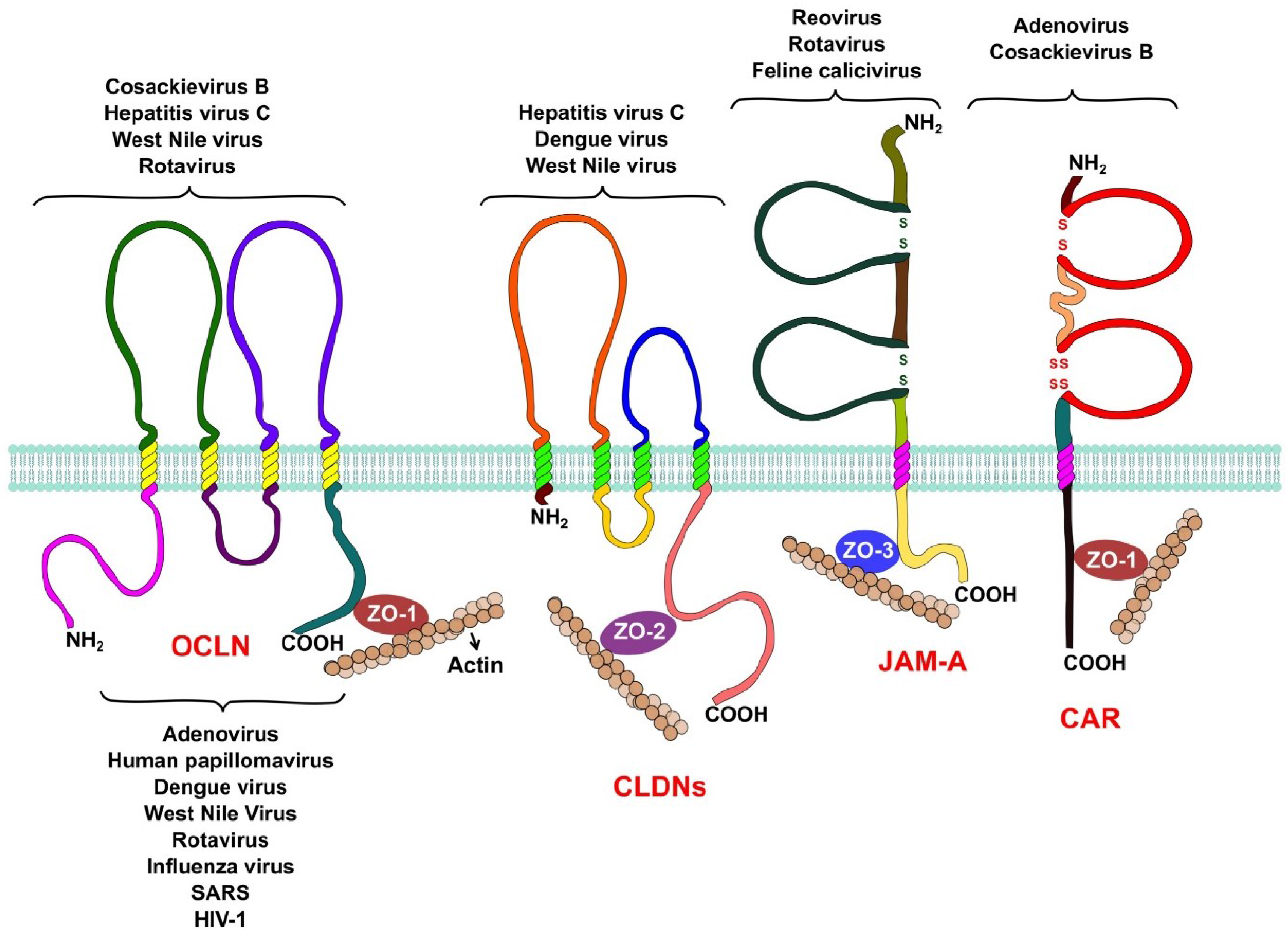
3. Viruses Open the Gate: Tight Junctions during Viral Entry
4. Tight Junctions: Opening the Gates into the Bloodstream
5. It Is Not All about the Entry
6. Modification of TJs for Viral Egress
| Virus | Target TJ Protein | Function | Reference | |
|---|---|---|---|---|
| DNA viruses | Adenovirus | CAR (CAREX7, CAREX8) | Viral receptor | [32,33,38,39] |
| MUPP-1, MAGI-1, PATJ, ZO-2 | Impair cell polarity for viral egress and dissemination | [65,66,67,72] | ||
| Human poliomavirus | PAT-1, MUPP1 | Alteration of cell polarity, carcinogenesis? | [68] | |
| RNA viruses | Coxsackievirus B | CAR | Viral receptor | [40,43] |
| Occludin | Viral internalization | [50] | ||
| Human hepatitis C virus | CLDN-1, CLDN-6, CLDN-9 | Viral entry | [48,49] | |
| Occludin | Viral entry | [50] | ||
| Dengue virus | CLDN-1 | Viral entry | [51] | |
| ZO-1 | Alteration of tight junction integrity | [61] | ||
| West Nile virus | JAM-A, ZO-1, Occludin, CLDN-1 | Impair tight junctions for hematogenous dissemination | [60] | |
| Rotavirus | JAM-A, Occludin, ZO-1 | Co-receptors | [52] | |
| RNA viruses | Reovirus | JAM-A | Receptor, hematogenous dissemination | [54,55,56,57,58,59] |
| Feline calicivirus | JAM-A | Receptor | [53] | |
| Influenza virus | MUPP1, MUPP2, MUPP3, ZO-1 | Alteration of cell polarity, spread outside the respiratory tract? | [69] | |
| Severe acute respiratory syndrome virus | MUPP1, MUPP2, MUPP3, ZO-1 | Alteration of cell polarity, spread outside the respiratory tract? | [70] | |
| Human immunodeficiency virus 1 | Several TJ proteins involved | Disruption of tight junctions, hematogenous dissemination, | [62,63,71] |
Acknowledgments
Conflicts of interest
References
- Steed, E.; Balda, M.S.; Matter, K. Dynamics and functions of tight junctions. Trends Cell Biol. 2010, 20, 142–149. [Google Scholar] [CrossRef]
- Shen, L. Tight junctions on the move: Molecular mechanisms for epithelial barrier regulation. Ann. N. Y. Acad. Sci. 2011, 1258, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Van Meer, G.; Simons, K. The function of tight junctions in maintaining differences in lipid composition between the apical and the basolateral cell surface domains of MDCK cells. EMBO J. 1986, 5, 1455–1464. [Google Scholar]
- Shin, K.; Fogg, V.C.; Margolis, B. Tight junctions and cell polarity. Annu. Rev. Cell Dev. Biol. 2006, 22, 207–235. [Google Scholar] [PubMed]
- Cereijido, M.; Contreras, R.G.; Gonzalez-Mariscal, L. Development and alteration of polarity. Annu. Rev. Physiol. 1989, 51, 785–795. [Google Scholar] [PubMed]
- Terry, S.; Nie, M.; Matter, K.; Balda, M.S. Rho signaling and tight junction functions. Physiology 2010, 25, 16–26. [Google Scholar] [PubMed]
- González-Mariscal, L.; Tapia, R.; Chamorro, D. Crosstalk of tight junction components with signaling pathways. Biochim. Biophys. Acta 2008, 1778, 729–756. [Google Scholar] [CrossRef] [PubMed]
- Zihni, C.; Balda, M.S.; Matter, K. Signalling at tight junctions during epithelial differentiation and microbial pathogenesis. J. Cell Sci. 2014, 15, 3401–3413. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, S.; Yamazaki, Y.; Katsuno, T.; Tamura, A. Tight junction-based epithelial microenvironment and cell proliferation. Oncogene 2008, 27, 6930–6938. [Google Scholar] [CrossRef]
- Furuse, M. Molecular basis of the core structure of tight junctions. Cold Spring Harb. Perspect. Biol. 2010, 2. [Google Scholar] [CrossRef]
- Furuse, M.; Izumi, Y.; Oda, Y.; Higashi, T.; Iwamoto, N. Molecular organization of tricellular tight junctions. Tissue Barriers 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Severson, E.A.; Parkos, C.A. Structural determinants of Junctional Adhesion Molecule A (JAM-A) function and mechanisms of intracellular signaling. Curr. Opin. Cell Biol. 2009, 21, 701–707. [Google Scholar] [PubMed]
- Garrido-Urbani, S.; Bradfield, P.F.; Imhof, B.A. Tight junction dynamics: The role of junctional adhesion molecules (JAMs). Cell Tissue Res. 2014, 355, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Markov, A.G.; Aschenbach, J.R.; Amasheh, S. Claudin clusters as determinants of epithelial barrier function. IUBMB Life 2015, 67, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Tani, K.; Tamura, A.; Tsukita, S.; Fujiyoshi, Y. Model for the architecture of claudin-based paracellular ion channels through tight junctions. J. Mol. Biol. 2015, 427, 291–297. [Google Scholar] [PubMed]
- Angelow, S.; Ahlstrom, R.; Yu, A.S. Biology of claudins. Am. J. Physiol. Ren. Physiol. 2008, 295, F867–F876. [Google Scholar] [CrossRef] [PubMed]
- Schulzke, J.D.; Gitter, A.H.; Mankertz, J.; Spiegel, S.; Seidler, U.; Amasheh, S.; Saitou, M.; Tsukita, S.; Fromm, M. Epithelial transport and barrier function in occludin-deficient mice. Biochim. Biophys. Acta 2005, 1669, 34–42. [Google Scholar] [PubMed]
- Yu, D.; Turner, J.R. Stimulus-induced reorganization of tight junction structure: the role of membrane traffic. Biochim. Biophys. Acta 2008, 1778, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pulido, L.; Martín-Belmonte, F.; Valencia, A.; Alonso, M.A. MARVEL: A conserved domain involved in membrane apposition events. Trends Biochem. Sci. 2002, 27, 599–601. [Google Scholar] [CrossRef]
- Yu, A.S.; McCarthy, K.M.; Francis, S.A.; McCormack, J.M.; Lai, J.; Rogers, R.A.; Lynch, R.D.; Schneeberger, E.E. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am. J. Physiol. Cell Physiol. 2005, 288, C1231–C1241. [Google Scholar] [PubMed]
- Raleigh, D.R.; Marchiando, A.M.; Zhang, Y.; Shen, L.; Sasaki, H.; Wang, Y.; Long, M.; Turner, J.R. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol. Biol. Cell 2010, 21, 1200–1213. [Google Scholar] [PubMed]
- Fanning, A.S.; Anderson, J.M. Zonula Occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann. N. Y. Acad. Sci. 2009, 1165, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Van Itallie, C.M.; Fanning, A.S.; Bridges, A.; Anderson, J.M. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol. Biol. Cell 2009, 20, 3930–3940. [Google Scholar] [CrossRef]
- Joberty, G.; Petersen, C.; Gao, L.; Macara, I.G. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat. Cell Biol. 2000, 2, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Ebnet, K.; Schulz, C.U.; Meyer Zu Brickwedde, M.K.; Pendl, G.G.; Vestweber, D. Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J. Biol. Chem. 2000, 275, 27979–27988. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Macara, I.G. Isoforms of the polarity protein par6 have distinct functions. J. Biol. Chem. 2004, 279, 41557–41562. [Google Scholar] [CrossRef] [PubMed]
- Dobrosotskaya, I.; Guy, R.K.; James, G.L. MAGI-1, a membrane-associated guanylate kinase with a unique arrangement of protein-protein interaction domains. J. Biol. Chem. 1997, 272, 31589–31597. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, L.S.; Beam, M.T.; Anderson, J.M.; Fanning, A.S. Epithelial barrier assembly requires coordinated activity of multiple domains of the tight junction protein ZO-1. J. Cell Sci. 2013, 126, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.C.; Chan, R.W.; Yu, W.C.; Ho, C.C.; Chui, W.H.; Lo, C.K.; Yuen, K.M.; Guan, Y.; Nicholls, J.M.; Peiris, J.S. Influenza H5N1 virus infection of polarized human alveolar epithelial cells and lung microvascular endothelial cells. Respir. Res. 2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Realpe, M.; Espinosa, R.; López, S.; Arias, C.F. Rotaviruses require basolateral molecules for efficient infection of polarized MDCKII cells. Virus Res. 2010, 147, 231–241. [Google Scholar] [CrossRef]
- Excoffon, K.J.; Guglielmi, K.M.; Wetzel, J.D.; Gansemer, N.D.; Campbell, J.A.; Dermody, T.S.; Zabner, J. Reovirus preferentially infects the basolateral surface and is released from the apical surface of polarized human respiratory epithelial cells. J. Infect. Dis. 2008, 197, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Lenman, A.; Liaci, A.M.; Liu, Y.; Årdahl, C.; Rajan, A.; Nilsson, E.; Bradford, W.; Kaeshammer, L.; Jones, M.S.; Frängsmyr, L.; et al. Human adenovirus 52 uses sialic acid-containing glycoproteins and the coxsackie and adenovirus receptor for binding to target cells. PLoS Pathog. 2015, 11, e1004657. [Google Scholar] [CrossRef] [PubMed]
- Freimuth, P.; Philipson, L.; Carson, S.D. The coxsackievirus and adenovirus receptor. Curr. Top. Microbiol. Immunol. 2008, 323, 67–87. [Google Scholar] [PubMed]
- Wickham, T.J.; Mathias, P.; Cheresh, D.A.; Nemerow, G.R. Integrins α v β 3 and α v β 5 promote adenovirus internalization but not virus attachment. Cell 1993, 73, 309–319. [Google Scholar] [CrossRef]
- Davison, E.; Diaz, R.M.; Hart, I.R.; Santis, G.; Marshall, J.F. Integrin alpha5beta1-mediated adenovirus infection is enhanced by the integrin-activating antibody TS2/16. J. Virol. 1997, 71, 6204–6207. [Google Scholar]
- Li, E.; Brown, S.L.; Stupack, D.G.; Puente, X.S.; Cheresh, D.A.; Nemerow, G.R. Integrin alpha(v)beta1 is an adenovirus coreceptor. J. Virol. 2001, 75, 5405–5409. [Google Scholar] [CrossRef] [PubMed]
- Salone, B.; Martina, Y.; Piersanti, S.; Cundari, E.; Cherubini, G.; Franqueville, L.; Failla, C.M.; Boulanger, P.; Saggio, I. Integrin alpha3beta1 is an alternative cellular receptor for adenovirus serotype 5. J. Virol. 2003, 77, 13448–13454. [Google Scholar] [CrossRef] [PubMed]
- Kotha, P.L.; Sharma, P.; Kolawole, A.O.; Yan, R.; Alghamri, M.S.; Brockman, T.L.; Gomez-Cambronero, J.; Excoffon, K.J. Adenovirus entry from the apical surface of polarized epithelia is facilitated by the host innate immune response. PLoS Pathog. 2015, 11, e1004696. [Google Scholar] [CrossRef] [PubMed]
- Excoffon, K.J.; Bowers, J.R.; Sharma, P. 1. Alternative splicing of viral receptors: A review of the diverse morphologies and physiologies of adenoviral receptors. Recent Res. Dev. Virol. 2014, 9, 1–24. [Google Scholar] [PubMed]
- Coyne, C.B.; Bergelson, J.M. CAR: A virus receptor within the tight junction. Adv. Drug Deliv. Rev. 2005, 57, 869–882. [Google Scholar] [CrossRef]
- Milstone, A.M.; Petrella, J.; Sanchez, M.D.; Mahmud, M.; Whitbeck, J.C.; Bergelson, J.M. Interaction with coxsackievirus and adenovirus receptor, but not with decay-accelerating factor (DAF), induces A-particle formation in a DAF-binding coxsackievirus B3 isolate. J. Virol. 2005, 79, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Shieh, J.T.C.; Bergelson, J.M. Interaction with decay-accelerating factor facilitates coxsackievirus B infection of polarized epithelial cells. J. Virol. 2002, 76, 9474–9480. [Google Scholar] [CrossRef] [PubMed]
- Coyne, C.B.; Bergelson, J.M. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell 2006, 124, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Coyne, C.B.; Shen, L.; Turner, J.R.; Bergelson, J.M. Coxsackievirus entry from epithelial tight junctions requires occludin and the small GTPases Rab34 and Rab5. Cell Host Microbe 2007, 2, 181–192. [Google Scholar] [CrossRef]
- Sobo, K.; Rubbia-Brandt, L.; Brown, T.D.K.; Stuart, A.D.; McKee, T.A. Decay-accelerating factor binding determines the entry route of echovirus 11 in polarized epithelial cells. J. Virol. 2011, 85, 12376–12386. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Zhu, Y.; Xia, X.; Liu, Y.; Feng, Y.; Hua, X.; Qi, Z.T. Tupaia CD81, SR-BI, claudin-1, and occludin support hepatitis C virus infection. J. Virol. 2011, 85, 2793–2802. [Google Scholar] [CrossRef] [PubMed]
- Monazahian, M.; Böhme, I.; Bonk, S.; Koch, A.; Scholz, C.; Grethe, S.; Thomssen, R. Low density lipoprotein receptor as a candidate receptor for hepatitis C virus. J. Med. Virol. 1999, 57, 223–229. [Google Scholar] [CrossRef]
- Von Hahn, T.; Rice, C.M. Hepatitis C virus entry. J. Biol. Chem. 2008, 283, 3689–3693. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.; Yuan, F.; Li, Y.; Zhu, F.; Hou, P.; Li, J.; Song, X.; Ding, M.; Deng, H. Claudin-6 and claudin-9 function as additional coreceptors for hepatitis C virus. J. Virol. 2007, 81, 12465–12471. [Google Scholar] [CrossRef] [PubMed]
- Ploss, A.; Evans, M.J.; Gaysinskaya, V.A.; Panis, M.; You, H.; de Jong, Y.P.; Rice, C.M. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature 2009, 457, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Che, P.; Tang, H.; Li, Q. The interaction between claudin-1 and dengue viral prM/M protein for its entry. Virology 2013, 446, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Torres-Flores, J.M.; Silva-Ayala, D.; Espinoza, M.A.; López, S.; Arias, C.F. The tight junction protein JAM-A functions as coreceptor for rotavirus entry into MA104 cells. Virology 2015, 475, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Makino, A.; Shimojima, M.; Miyazawa, T.; Kato, K.; Tohya, Y.; Akashi, H. Junctional adhesion molecule 1 is a functional receptor for feline calicivirus. J. Virol. 2006, 80, 4482–4490. [Google Scholar] [CrossRef] [PubMed]
- Barton, E.S.; Forrest, J.C.; Conolly, J.L.; Chappell, J.D.; Liu, Y.; Schnell, F.J.; Nusrat, A.; Parkos, C.A.; Dermody, T.S. Junction adhesión molecule is a receptor for reovirus. Cell 2001, 104, 441–451. [Google Scholar] [CrossRef]
- Kirchner, E.; Guglielmi, K.M.; Strauss, H.M.; Dermody, T.S.; Stehle, T. Structure of reovirus sigma1 in complex with its receptor junctional adhesion molecule-A. PLoS Pathog. 2008, 4, e1000235. [Google Scholar] [CrossRef] [PubMed]
- Antar, A.A.; Konopka, J.L.; Campbell, J.A.; Henry, R.A.; Perdigoto, A.L.; Carter, B.D.; Pozzi, A.; Abel, T.W.; Dermody, T.S. Junctional adhesion molecule-A is required for hematogenous dissemination of reovirus. Cell Host Microbe 2009, 5, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Boehme, K.W.; Lai, C.M.; Dermody, T.S. Mechanisms of reovirus bloodstream dissemination. Adv. Virus Res. 2013, 87, 1–35. [Google Scholar] [PubMed]
- Konopka-Anstadt, J.L.; Mainou, B.A.; Sutherland, D.M.; Sekine, Y.; Strittmatter, S.M.; Dermody, T.S. The Nogo receptor NgR1 mediates infection by mammalian reovirus. Cell Host Microbe 2014, 15, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.M.; Boehme, K.W.; Pruijssers, A.J.; Parekh, V.V.; van Kaer, L.; Parkos, C.A.; Dermody, T.S. Endothelial JAM-A promotes reovirus viremia and bloodstream dissemination. J. Infect. Dis. 2015, 211, 383–393. [Google Scholar] [CrossRef]
- Roe, K.; Kumar, M.; Lum, S.; Orillo, B.; Nerurkar, V.R.; Verma, S. West Nile virus-induced disruption of the blood-brain barrier in mice is characterized by the degradation of the junctional complex proteins and increase in multiple matrix metalloproteinases. J. Gen. Virol. 2012, 93, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Kanlaya, R.; Pattanakitsakul, S.N.; Sinchaikul, S.; Chen, S.T.; Thongboonkerd, V. Alterations in actin cytoskeletal assembly and junctional protein complexes in human endothelial cells induced by dengue virus infection and mimicry of leukocyte transendothelial migration. J. Proteome Res. 2009, 8, 2551–2562. [Google Scholar] [CrossRef] [PubMed]
- Afonso, P.V.; Ozden, S.; Prevost, M.C.; Schmitt, C.; Seilhean, D.; Weksler, B.; Couraud, P.O.; Gessain, A.; Romero, I.A.; Ceccaldi, P.E. Human blood-brain barrier disruption by retroviral-infected lymphocytes: Role of myosin light chain kinase in endothelial tight-junction disorganization. J. Immunol. 2007, 179, 2576–2583. [Google Scholar] [CrossRef] [PubMed]
- Kanmogne, G.D.; Schall, K.; Leibhart, J.; Knipe, B.; Gendelman, H.E.; Persidsky, Y. HIV-1 gp120 compromises blood-brain barrier integrity and enhances monocyte migration across blood-brain barrier: Implication for viral neuropathogenesis. J. Cereb. Blood Flow Metab. 2007, 27, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Zheng, J.J. PDZ domains and their binding partners: Structure, specificity, and modification. Cell Commun. Signal. 2010, 8. [Google Scholar] [CrossRef] [PubMed]
- Glaunsinger, B.A.; Lee, S.S.; Thomas, M.; Banks, L.; Javier, R. Interactions of the PDZ-protein MAGI-1 with adenovirus E4-ORF1 and high-risk papillomavirus E6 oncoproteins. Oncogene 2000, 19, 5270–5280. [Google Scholar] [CrossRef] [PubMed]
- Glaunsinger, B.A.; Weiss, R.S.; Lee, S.S.; Javier, R. Link of the unique oncogenic properties of adenovirus type 9 E4-ORF1 to a select interaction with the candidate tumor suppressor protein ZO-2. EMBO J. 2001, 20, 5578–5586. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Glaunsinger, B.; Mantovani, F.; Banks, L.; Javier, R.T. Multi-PDZ domain protein MUPP1 is a cellular target for both adenovirus E4-ORF1 and high-risk papillomavirus type 18 E6 oncoproteins. J. Virol. 2000, 74, 9680–9693. [Google Scholar] [CrossRef] [PubMed]
- Latorre, I.J.; Roh, M.H.; Frese, K.K.; Weiss, R.S.; Margolis, B.; Javier, R.T. Viral oncoprotein-induced mislocalization of select PDZ proteins disrupts tight junctions and causes polarity defects in epithelial cells. J. Cell Sci. 2005, 118, 4283–4293. [Google Scholar] [CrossRef] [PubMed]
- Golebiewski, L.; Liu, H.; Javier, R.T.; Rice, A.P. The avian influenza virus NS1 ESEV PDZ binding motif associates with Dlg1 and Scribble to disrupt cellular tight junctions. J. Virol. 2011, 85, 10639–10648. [Google Scholar] [CrossRef] [PubMed]
- Teoh, K.T.; Siu, Y.L.; Chan, W.L.; Schlüter, M.A.; Liu, C.J.; Peiris, J.S.; Bruzzone, R.; Margolis, B.; Nal, B. The SARS coronavirus E protein interacts with PALS1 and alters tight junction formation and epithelial morphogenesis. Mol. Biol. Cell 2010, 21, 3838–3852. [Google Scholar] [CrossRef] [PubMed]
- Sufiawati, I.; Tugizov, S.M. HIV-associated disruption of tight and adherens junctions of oral epithelial cells facilitates HSV-1 infection and spread. PLoS ONE 2014, 9, e88803. [Google Scholar] [CrossRef] [PubMed]
- Walters, R.W.; Freimuth, P.; Moninger, T.O.; Ganske, I.; Zabner, J.; Welsh, M.J. Adenovirus fiber disrupts CAR-mediated intercellular adhesion allowing virus escape. Cell 2002, 110, 789–799. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Flores, J.M.; Arias, C.F. Tight Junctions Go Viral! Viruses 2015, 7, 5145-5154. https://doi.org/10.3390/v7092865
Torres-Flores JM, Arias CF. Tight Junctions Go Viral! Viruses. 2015; 7(9):5145-5154. https://doi.org/10.3390/v7092865
Chicago/Turabian StyleTorres-Flores, Jesús M., and Carlos F. Arias. 2015. "Tight Junctions Go Viral!" Viruses 7, no. 9: 5145-5154. https://doi.org/10.3390/v7092865





