Determinants of Disease Phenotype Differences Caused by Closely-Related Isolates of Begomovirus Betasatellites Inoculated with the Same Species of Helper Virus
Abstract
:1. Introduction
2. Results
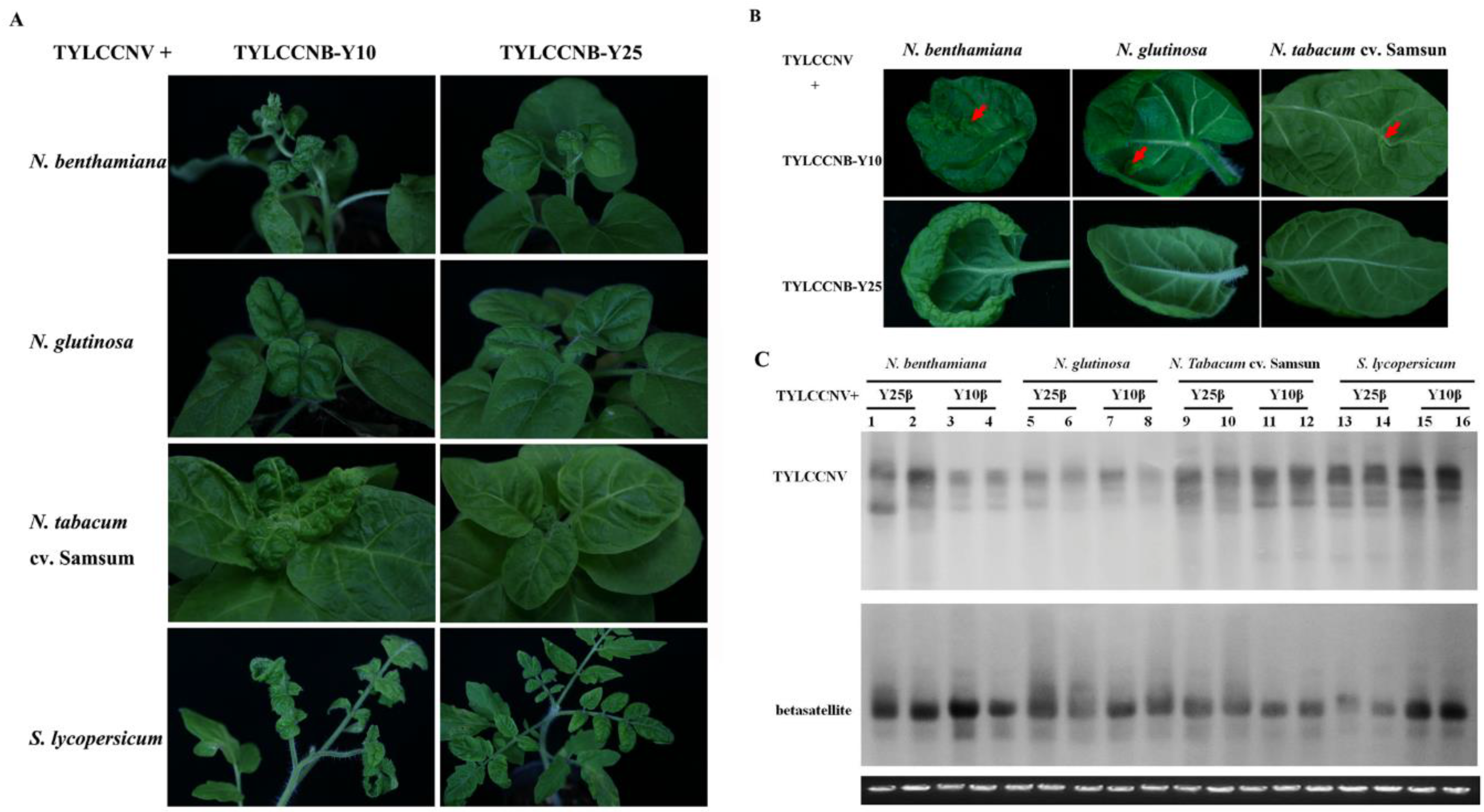
2.1. Infectivity of TYLCCNV and its Associated Betasatellite
2.2. Phenotypes of Transgenic Plants Expressing TYLCCNB-Y25 βC1

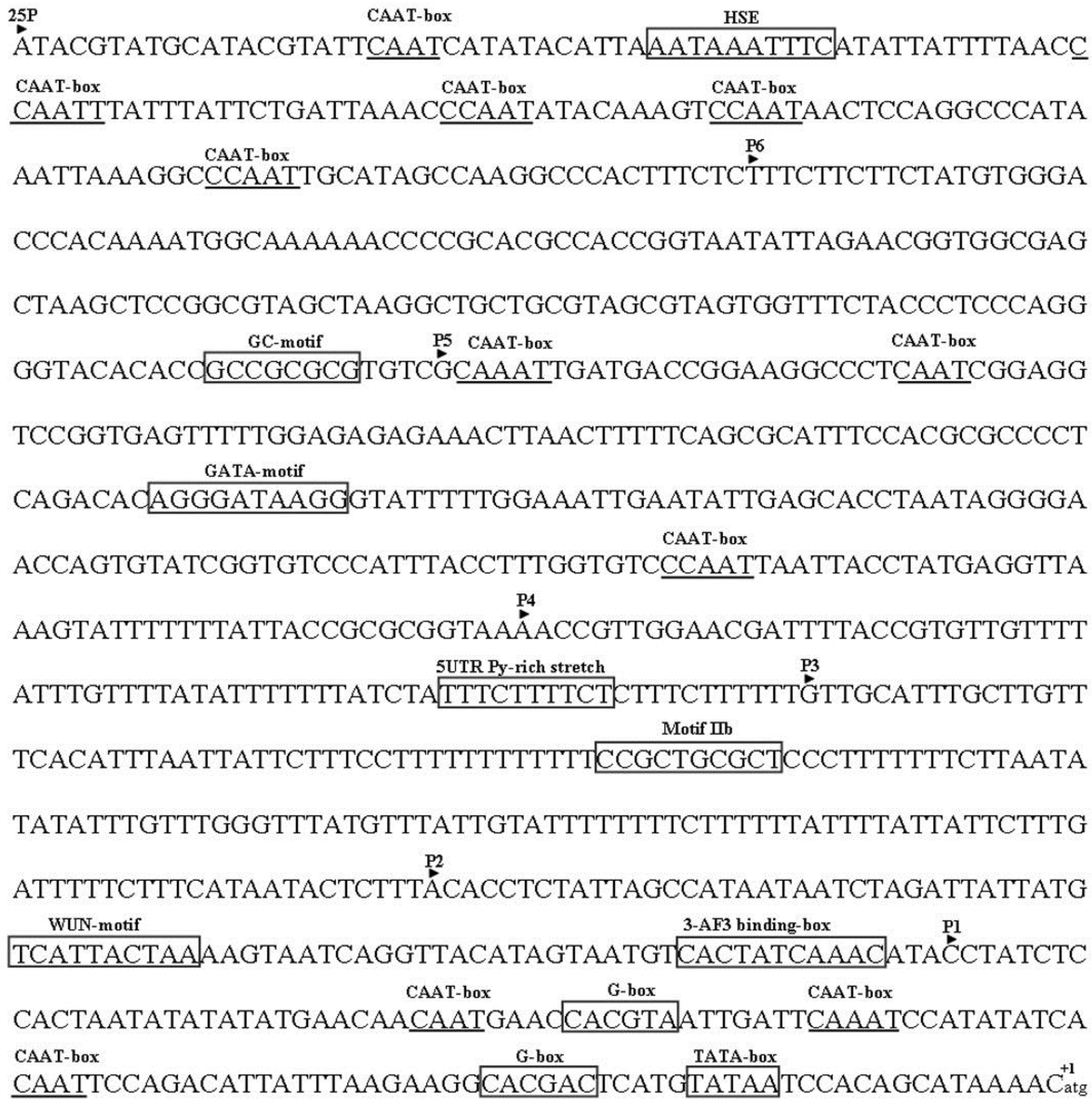
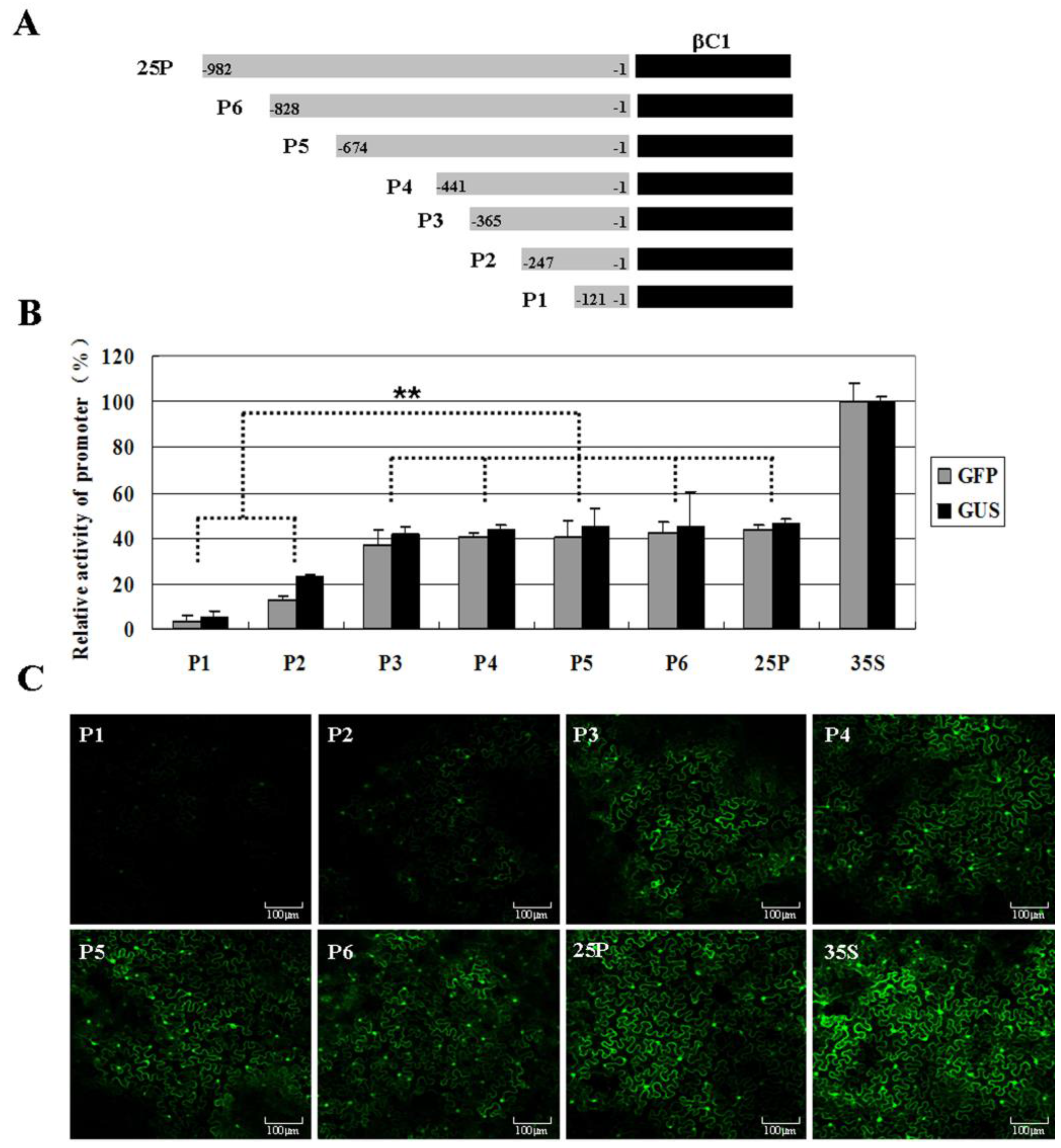
2.3. Analysis of the Putative Promoter of TYLCCNB-Y25
2.4. Infectivity and Symptoms of Hybrid Betasatellites
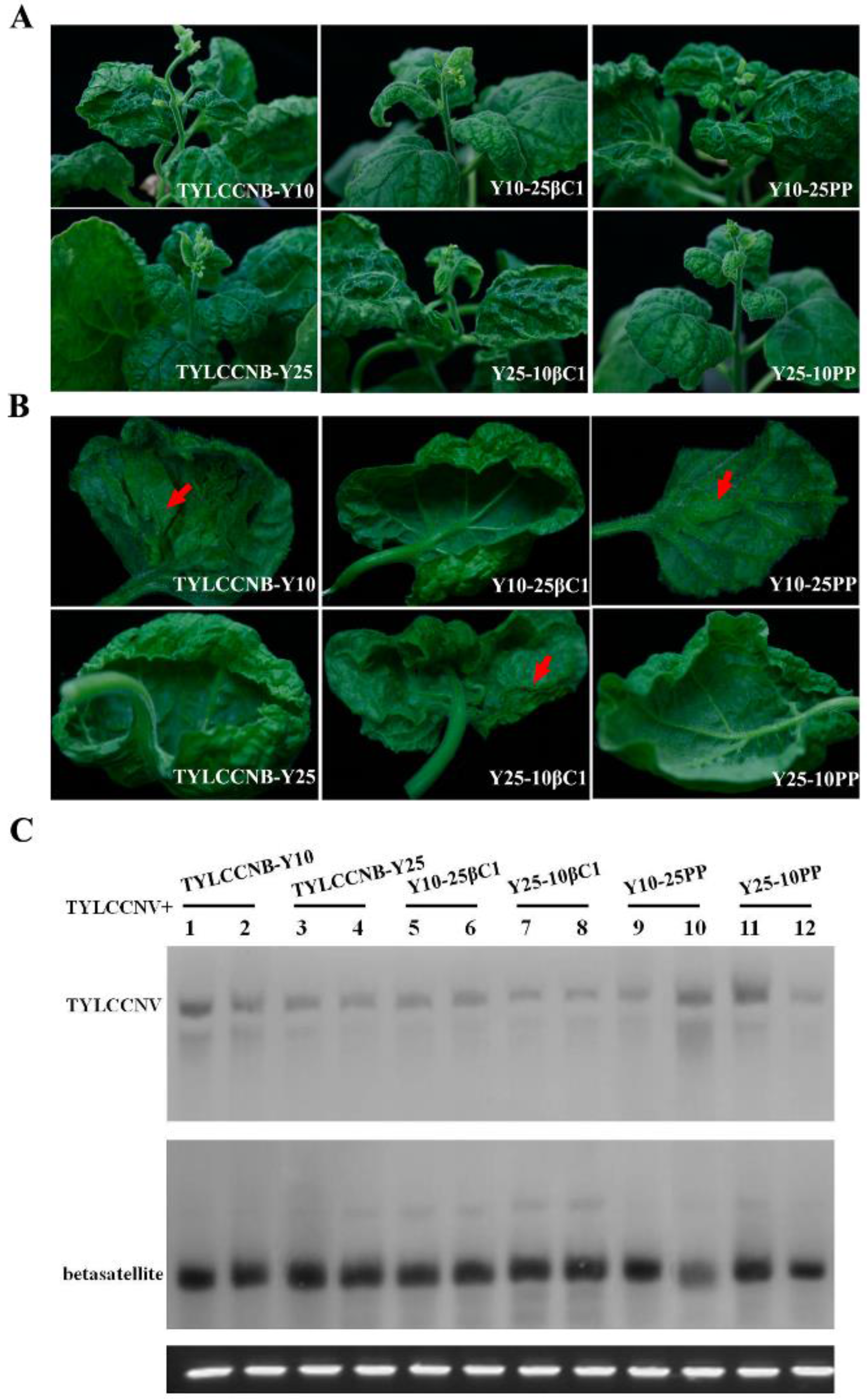
| Inoculum (TYLCCNV+) | Plant Species | Symptoms a | Infectivity b |
|---|---|---|---|
| TYLCCNB-Y10 | N. benthamiana | LC, YV, EN, SD | 35/35(34) |
| N. glutinosa | LC, YV, EN | 14/15(5) | |
| N. tabacum cv. Samsun | LC, YV, EN | 12/15(5) | |
| S. lycopersicum | LC, YV, EN | 8/14(3) | |
| TYLCCNB-Y25 | N. benthamiana | LC | 34/35 |
| N. glutinosa | LC | 15/15 | |
| N. tabacum cv. Samsun | LC | 9/15 | |
| S. lycopersicum | LC | 7/15 | |
| Y25-10βC1 | N. benthamiana | YV, LC, SD, EN | 30/30(30) |
| Y10-25βC1 | N. benthamiana | LC | 29/30 |
| Y25-10PP | N. benthamiana | LC | 20/25 |
| Y10-25PP | N. benthamiana | MYV, LC, SD, EN | 25/25(25) |
| Y10-25CβC1 | N. benthamiana | LC | 28/28 |
| Y10-25MβC1 | N. benthamiana | MYV, LC, SD, EN | 28/28(20) |
| Y10-25NβC1 | N. benthamiana | MYV, LC, SD, EN | 22/28(12) |
| Y25-10CβC1 | N. benthamiana | MYV, LC, SD, EN | 27/28(15) |
| Y25-10MβC1 | N. benthamiana | LC | 28/28 |
| Y25-10NβC1 | N. benthamiana | LC | 24/28 |
2.5. Minor Determinants of Symptoms within the TYLCCNB βC1 Gene


3. Discussion
4. Materials and Methods
4.1. Construction of Infectious Clones of Wild-Type and Hybrid Betasatellites

4.2. Construction of Promoter Expression Vectors
4.3. Construction of the TYLCCNB-Y25 βC1 Transgenic Expression Vector
4.4. Agroinoculation of Plants
4.5. Plant Transformation
4.6. Analysis of DNAs in Inoculated Plants or RNAs in Transformed Plants
4.7. Fluorometric GUS Assay
4.8. Fluorometric GFP Assay
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Adams, M.J.; King, A.M.Q.; Carstens, E.B. Ratification vote on taxonomic proposals to the international committee on taxonomy of viruses. Arch. Virol. 2013, 18, 2023–2030. [Google Scholar] [CrossRef] [PubMed]
- Fauquet, C.M.; Bisaro, D.M.; Briddon, R.W.; Brown, J.K.; Harrison, B.D.; Rybicki, E.P.; Stenger, D.C.; Stanley, J. Revision of taxonomic criteria for species demarcation in the family Geminiviridae, and an updated list of begomovirus species. Arch. Virol. 2003, 148, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Briddon, R.W.; Bull, S.E.; Amin, I.; Idris, A.M.; Mansoor, S.; Bedford, I.D.; Dhawan, P.; Rishi, N.; Siwatch, S.S.; Abdel-Salam, A.M.; et al. Diversity of DNA β, a satellite molecule associated with some monopartite begomoviruses. Virology 2003, 312, 106–121. [Google Scholar] [CrossRef]
- Zhou, X.; Xie, Y.; Tao, X.; Zhang, Z.; Li, Z.; Fauquet, C.M. Characterization of DNA β associated with begomoviruses in China and evidence for co-evolution with their cognate viral DNA-A. J. Gen. Virol. 2003, 84, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Saunders, K.; Bedford, I.D.; Briddon, R.W.; Markham, P.G.; Wong, S.M.; Stanley, J. A unique virus complex causes Ageratum yellow vein disease. Proc. Natl. Acad. Sci. USA 2000, 97, 6890–6895. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Tao, X.; Xie, Y.; Fauquet, C.M.; Zhou, X. A DNA β associated with Tomato yellow leaf curl China virus is required for symptom induction. J. Virol. 2004, 78, 13966–13974. [Google Scholar] [CrossRef] [PubMed]
- Kon, T.; Sharma, P.; Ikegami, M. Suppressor of RNA silencing encoded by the monopartite tomato leaf curl Java begomovirus. Arch. Virol. 2007, 152, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Saunders, K.; Norman, A.; Gucciardo, S.; Stanley, J. The DNA β satellite component associated with ageratum yellow vein disease encodes an essential pathogenicity protein (βC1). Virology 2004, 324, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Behjatnia, S.A.; Mansoor, S.; Zafar, Y.; Hasnain, S.; Rezaian, M.A. A single complementary-sense transcript of a geminiviral DNA β satellite is determinant of pathogenicity. Mol. Plant Microbe Interact. 2005, 18, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Xie, Y.; Raja, P.; Li, S.Z.; Wolf, J.N.; Shen, Q.T.; Bisaro, D.M.; Zhou, X.P. Suppression of methylation-mediated transcriptional gene silencing by βC1-SAHH protein interaction during geminivirus-betasatellite infection. PLoS Pathog. 2011, 7, e1002329. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.H.; Luo, Y.Q.; Ding, M.; Zhang, Z.K.; Yang, C.K. First report of Tomato yellow leaf curl China virus infecting kidney bean in China. Plant Pathol. 2007, 56, 342. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhou, X.P.; Zhang, X.; Xie, Y. Molecular characterization of tomato-infecting begomoviruses in Yunnan, China. Arch. Virol. 2004, 149, 1721–1732. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Qing, L.; Li, Z.; Liu, Y.; Qian, Y.; Zhou, X. Genetic determinants of symptoms on viral DNA satellites. Appl. Environ. Microb. 2009, 75, 5380–5389. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Zhou, X. Phloem specific promoter from a satellite associated with a DNA virus. Virus Res. 2006, 115, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Eini, O.; Behjatnia, S.A.; Dogra, S.; Dry, I.B.; Randles, J.W.; Rezaian, M.A. Identification of sequence elements regulating promoter activity and replication of a monopartite begomovirus-associated DNA β satellite. J. Gen. Virol. 2009, 90, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.Y.; Wang, Y.Q.; Hou, H.W.; Qian, Y.J. Characterization of sequence elements from Malvastrum yellow vein betasatellite regulating promoter activity and DNA replication. Virol. J. 2012, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.; Briddon, R.W.; Zafar, Y.; Stanley, J. Geminivirus disease complexes: an emerging threat. Trends Plant Sci. 2003, 8, 128–134. [Google Scholar] [CrossRef]
- Mansoor, S.; Zafar, Y.; Briddon, R.W. Geminivirus disease complexes: The threat is spreading. Trends Plant Sci. 2006, 11, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Briddon, R.W.; Mansoor, S.; Bedford, I.D.; Pinner, M.S.; Saunders, K.; Stanley, J.; Zafar, Y.; Malik, K.A.; Markham, P.G. Identification of DNA components required for induction of cotton leaf curl disease. Virology 2001, 285, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.; Briddon, R.W.; Bull, S.E.; Bedford, I.D.; Bashir, A.; Hussain, M.; Saeed, M.; Zafar, Y.; Malik, K.A.; Fauquet, C.; Markham, P.G. Cotton leaf curl disease is associated with multiple monopartite begomoviruses supported by single DNAβ. Arch. Virol. 2003, 148, 1969–1986. [Google Scholar] [CrossRef] [PubMed]
- Qing, L.; Zhou, X. Trans-replication of, and competition between, DNA β satellites in plants inoculated with Tomato yellow leaf curl China virus and Tobacco curly shoot virus. Phytopathology 2009, 99, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.M.; Paplomatas, E.J.; Gilbertson, R.L. Host adaptation and replicationproperties of two bipartite geminiviruses and their pseudorecombinants. Mol. Plant Microbe Interact. 1998, 11, 208–217. [Google Scholar] [CrossRef]
- Morra, M.R.; Petty, I.T.D. Tissue specificity of geminivirus infection is genetically determined. Plant Cell 2000, 12, 2259–2270. [Google Scholar] [CrossRef] [PubMed]
- Petty, I.T.; Carter, S.C.; Morra, M.R.; Jeffrey, J.L.; Olivey, H.E. Bipartite geminivirus host adaptation determined cooperatively by coding and noncoding sequences of the genome. Virology 2000, 277, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Gopal, P.; Pravin Kumar, P.; Sinilal, B.; Jose, J.; Kasin Yadunandam, A.; Usha, R. Differential roles of C4 and betaC1 in mediating suppression of post-transcriptional gene silencing: evidence for transactivation by the C2 of Bhendi yellow vein mosaic virus, a monopartite begomovirus. Virus Res. 2007, 123, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Qazi, J.; Amin, I.; Mansoor, S.; Iqbal, M.J.; Briddon, R.W. Contribution of the satellite encoded gene βC1 to cotton leaf curl disease symptoms. Virus Res. 2007, 128, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Iwasaki, M.; Machida, C.; Machida, Y.; Zhou, X.; Chua, N.H. βC1, the pathogenicity factor of TYLCCNV, interacts with AS1 to alter leaf development and suppress selective jasmonic acid responses. Gene. Dev. 2008, 22, 2564–2577. [Google Scholar] [CrossRef] [PubMed]
- Boulton, M.I.; King, D.I.; Donson, J.; Davies, J.W. Point substitutions in a promoter-like region and the V1 gene affect the host range and symptoms of maize streak virus. Virology 1991, 183, 114–121. [Google Scholar] [CrossRef]
- Roth, B.M.; Pruss, G.J.; Vance, V.B. Plant viral suppressors of RNA silencing. Virus Res. 2004, 102, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.B.; Metzlaff, M. RNA silencing and antiviral defense in plants. Curr. Opin. Plant Biol. 2005, 8, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Dogra, S.C.; Eini, O.; Rezaian, M.A.; Randles, J.W. A novel shaggy-like kinase interacts with the Tomato leaf curl virus pathogenicity determinant C4 protein. Plant Mol. Biol. 2009, 71, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Liu, Z.; Song, F.; Xie, Q.; Hanley-Bowdoin, L.; Zhou, X. Tomato SlSnRK1 protein interacts with and phosphorylates βC1, a pathogenesis protein encoded by a geminivirus β-satellite. Plant Physiol. 2011, 157, 1394–1406. [Google Scholar] [CrossRef] [PubMed]
- Briddon, R.W.; Bull, S.E.; Mansoor, S.; Amin, I.; Markham, P.G. Universal primers for the PCR-mediated amplification of DNA β: A molecule associated with some monopartite begomoviruses. Mol. Biotechnol. 2002, 20, 315–318. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Wang, J.L.; Huang, X.; Xu, W.H.; Liu, Z.M.; Fang, R.X. The promoter of a rice glycine-rich protein gene, Osgrp-2, confers vascular-specific expression in transgenic plants. Planta 2003, 216, 824–833. [Google Scholar] [PubMed]
- Horsch, R.B.; Fry, J.E.; Hoffmann, N.L.; Eichholtz, D.; Rogers, S.G.; Fraley, R.T. A simple and general-method for transferring genes into plants. Science 1985, 227, 1229–1231. [Google Scholar]
- Xie, Y.; Zhou, X.P.; Zhang, Z.K.; Qi, Y.J. Tobacco curly shoot virus isolated in Yunnan is a distinct species of Begomovirus. Chin. Sci. Bull. 2002, 47, 197–200. [Google Scholar] [CrossRef]
- Jefferson, R.A.; Kavanagh, T.A.; Bevan, M.W. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987, 6, 3901–3907. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Dang, M.; Huang, Q.; Qian, Y. Determinants of Disease Phenotype Differences Caused by Closely-Related Isolates of Begomovirus Betasatellites Inoculated with the Same Species of Helper Virus. Viruses 2015, 7, 4945-4959. https://doi.org/10.3390/v7092853
Zhang J, Dang M, Huang Q, Qian Y. Determinants of Disease Phenotype Differences Caused by Closely-Related Isolates of Begomovirus Betasatellites Inoculated with the Same Species of Helper Virus. Viruses. 2015; 7(9):4945-4959. https://doi.org/10.3390/v7092853
Chicago/Turabian StyleZhang, Jie, Mingqing Dang, Qingqing Huang, and Yajuan Qian. 2015. "Determinants of Disease Phenotype Differences Caused by Closely-Related Isolates of Begomovirus Betasatellites Inoculated with the Same Species of Helper Virus" Viruses 7, no. 9: 4945-4959. https://doi.org/10.3390/v7092853





