Recent Progress towards Novel EV71 Anti-Therapeutics and Vaccines
Abstract
:1. Introduction
2. Inactivated Vaccines
3. Baculovirus-Expressed Vaccines
3.1. Baculovirus-Expressed Virus-Like Particles (VLPs)
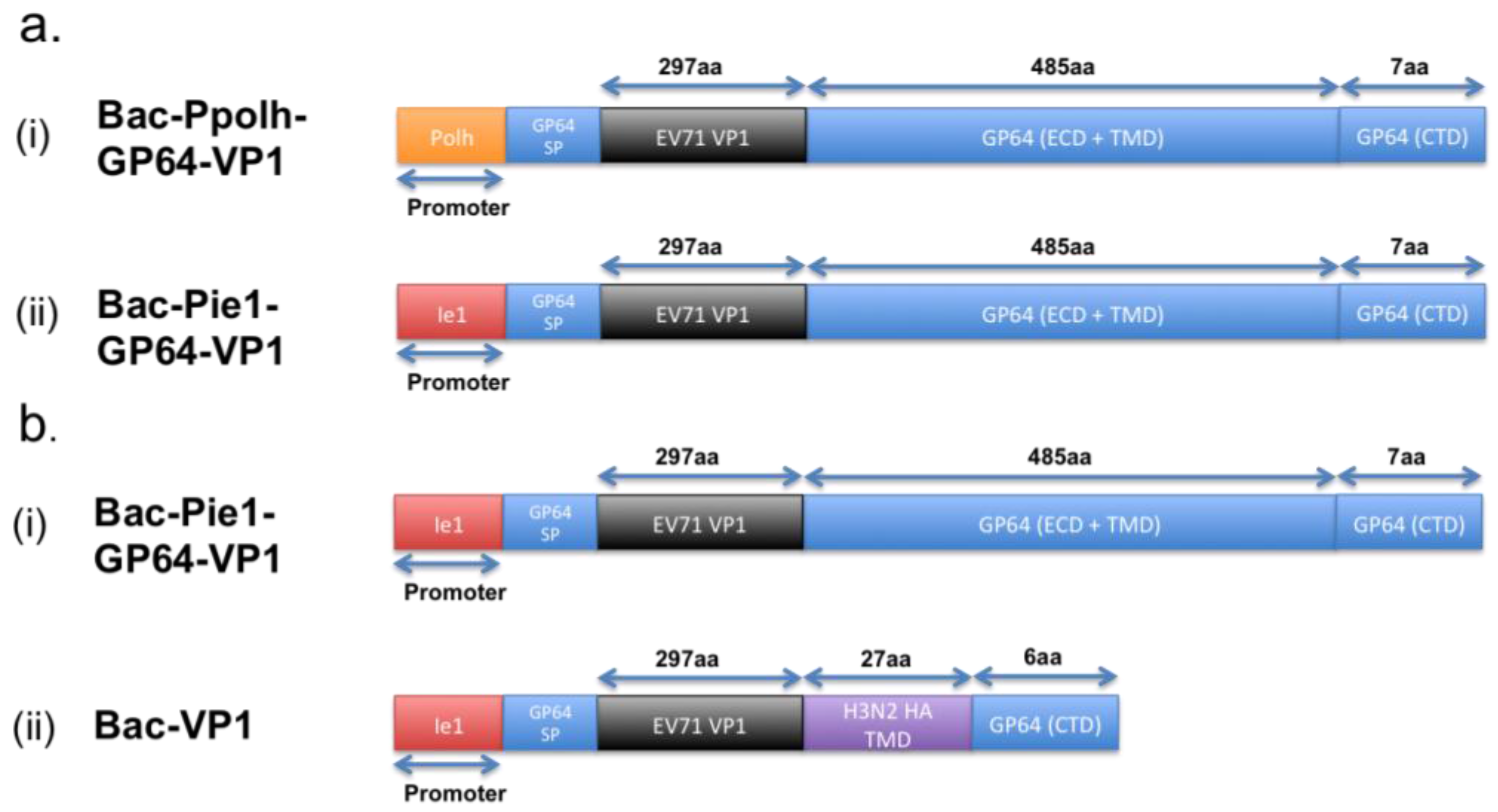
3.2. Baculovirus Surface Display
| Mab Name | Derived from EV71 Genotype | Target | Epitope Type | Epitope Sequence | Remarks |
|---|---|---|---|---|---|
| MAB51 [22] | NUH0083/subgenotype B5 | VP1 | Linear | KQEKD | Able to neutralize all 11 subgenotypes of EV71. Universal neutralizing antibody. |
| 10D3 [23] | 5865/SIN/000009/subgenotype B4 | VP3 | Conformational | Amino acid position 59, 62 and 67 formed the highly conserved knob | Able to neutralize all 11 subgenotypes of EV71. Universal neutralizing antibody. |
| 4E8 [24] | EV71 Hn2 strain/subgenotype C4 | VP1 | Linear | SSKSEYSLVI (aa 240–250); RIYMRMKHVR (aa 250–260) | Neutralization only tested on Hn2 Strain. RIYMRMKHVR epitope is highly conserved among 11 EV71 subgenotypes. SSKSEYSLVI epitope is not highly conserved. |
| 4C6 [24] | EV71 Hn2 strain/subgenotype C4 | VP1 | Linear | SSKSEYSLVI (aa 240–250); RIYMRMKHVR (aa 250–260) | Neutralization only tested on Hn2 Strain. RIYMRMKHVR epitope is highly conserved among 11 EV71 subgenotypes. SSKSEYSLVI epitope is not highly conserved. |
| MAb979 [56] | EV71/subgenotype C2 | VP2 | Linear | Amino acids 136–150, encompasses the exact epitope EDSHP | Neutralizes B4, B5, C2 with cross-reactivity with CVA16. |
| MA28-7 [57] | EV71 strain 1095/subgenotype C2 | VP1 | Conformational | VP1-145 and residues that map to the positively charged patches (VP1-98, VP1-242, and VP1-244) | Neutralizes EV71 subgenotype A, B1, B3, B4 and C2. Neutralizes only specific strains of EV71 that have a conserved glycine at amino acid VP1-145, a surface-exposed residue that maps to the five-fold vertex and that has been implicated in receptor binding. |
| BB1A5 [58] | EV71 strain 52-3/subgenotype C4 | VP2 | Linear | 136-155 (Thr (T141A), Glu (E142A), Ser (S144A) and His (H145A) being the most critical points) | Able to neutralize B3, B4, C2, C4 and C5 subgenogroups. |
| H3B10 [59] | Not known | VP1 | Linear | KQEK (aa 208–222) | Shown capable of neutralizing C2, C4, C5, B4 and B5 subgenogroups. Broad-neutralizing monoclonal antibodies. |
| K8G2 [59] | Not known | VP1 | Linear | KQEK (aa 208–222) | Shown capable of neutralizing C2, C4, C5, B4 and B5 subgenogroups. Broad-neutralizing monoclonal antibodies. |
| 2G8 [60] | EV71 strain AH/08/0 /subgenotype C4 | VP1 | Linear | YPTFGEHKQEKDLEYC | Shown capable of neutralizing C4 subgenogroup. Ala substitutions at Y208, T210, G212, K215, K218, L220, E221, and Y222 could completely abolish or significantly reduce the binding reactivity. |
| 22A12 [61] | EV71 strain 1095/Shiga /subgenotype C2 | VP1 | Linear/Conformational | YPTFGEHKQEKDLEYC (Amino acid residues 211 to 217 of the GH loop are disordered in the procapsid but form a structured loop in the infectious virus. 22A12 recognizes the disordered loop only) | Unable to neutralize infectious virus (native virus). Able to bind to procasid, which protects the live virus from neutralizing antibodies. When being binded to 22A12, the protective shield is lost, hence increasing the neutralizing ability of other antibodies. |
| D2 [62] | Yeast-produced virus-like particles | VP1 | Linear | Not known | Produced by immunizing with yeast-produced virus-like particles rather than whole virus. |
| G12 [62] | Yeast-produced virus-like particles | VP1 | Linear | Not known | Produced by immunizing with yeast-produced virus-like particles rather than whole virus. |
| Mab 22 [63] | EV71 Tainan/4643/98/C2 | VP1 | Conformational | Not known | With neutralization capabilities but no data on the subgenogroups tested. |
| Mab 24 [63] | EV71 Tainan/4643/98/C2 | VP1 | Conformational | Not known | With neutralization capabilities but no data on the subgenogroups tested. |
4. Intravenous Immunoglobulin (IVIg) Treatment
5. Monoclonal Antibody Therapy against EV71
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ranganathan, S.; Singh, S.; Poh, C.L.; Chow, V.T. The hand, foot and mouth disease virus capsid: Sequence analysis and prediction of antigenic sites from homology modelling. Appl. Bioinform. 2002, 1, 43–52. [Google Scholar] [PubMed]
- Plevka, P.; Perera, R.; Cardosa, J.; Kuhn, R.J.; Rossmann, M.G.; Rossmann, M.G. Crystal structure of human enterovirus 71. Science 2012, 336. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Peng, W.; Ren, J.; Hu, Z.; Xu, J.; Lou, Z.; Li, X.; Yin, W.; Shen, X.; Porta, C.; et al. A sensor-adaptor mechanism for enterovirus uncoating from structures of EV71. Nat. Struct. Mol. Biol. 2012, 19, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-W.; Cheng, H.-L.; Hsieh, H.-Y.; Chang, C.-L.; Tsai, H.-P.; Kuo, P.-H.; Wang, S.-M.; Liu, C.-C.; Su, I.-J.; Wang, J.-R. Mutations in the non-structural protein region contribute to intra-genotypic evolution of Enterovirus 71. J. Biomed. Sci. 2014, 21. [Google Scholar] [CrossRef] [PubMed]
- Tee, K.K.; Lam, T.T.-Y.; Chan, Y.F.; Bible, J.M.; Kamarulzaman, A.; Tong, C.Y.W.; Takebe, Y.; Pybus, O.G. Evolutionary genetics of human enterovirus 71: Origin, population dynamics, natural selection, and seasonal periodicity of the VP1 gene. J. Virol. 2010, 84, 3339–3350. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.; Chen, E.R.; Hsu, K.H.; Twu, S.J.; Chen, K.T.; Tsai, S.F.; Wang, J.R.; Shih, S.R. An epidemic of Enterovirus 71 infection in taiwan. Taiwan enterovirus epidemic working group. N. Engl. J. Med. 1999, 341, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.J.; Lennette, E.H.; Ho, H.H. An apparently new enterovirus isolated from patients with disease of the central nervous system. J. Infect. Dis. 1974, 129, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, K.; Lye, M.S.; Norlijah, O.; Ong, F.; Looi, L.M.; Khuzaiah, R.; Marzuki, I.; Hussein, I.; Wong, S.L.; Mohan, J.; et al. Deaths in children during an outbreak of hand, foot and mouth disease in peninsular malaysia—Clinical and pathological characteristics. Med. J. Malays. 2005, 60, 297–304. [Google Scholar] [PubMed]
- Liu, C.C.; Tseng, H.W.; Wang, S.M.; Wang, J.R.; Su, I.J. An outbreak of Enterovirus 71 infection in taiwan, 1998: Epidemiologic and clinical manifestations. J. Clin. Virol. 2000, 17, 23–30. [Google Scholar] [CrossRef]
- Huang, S.W.; Hsu, Y.W.; Smith, D.J.; Kiang, D.; Tsai, H.-P.; Lin, K.-H.; Wang, S.-M.; Liu, C.-C.; Su, I.-J.; Wang, J.R. Reemergence of Enterovirus 71 in 2008 in Taiwan: Dynamics of genetic and antigenic evolution from 1998 to 2008. J. Clin. Microbiol. 2009, 47, 3653–3662. [Google Scholar] [CrossRef] [PubMed]
- Ang, L.W.; Koh, B.K.; Chan, K.P.; Chua, L.T.; James, L.; Goh, K.T. Epidemiology and control of hand, foot and mouth disease in Singapore, 2001–2007. Ann. Acad. Med. Singap. 2009, 38, 106–112. [Google Scholar] [PubMed]
- Emerging Disease Surveillance and Response. Available online: http://www.wpro.who.int/emerging_diseases/en/ (accessed on 1 September 2015).
- Fenner, F.; Henderson, D.A.; Arita, I.; Jezek, Z.; Ladnyi, I.D. Smallpox and Its Eradication; WHO: Geneva, Switzerland, 1988. [Google Scholar]
- Riedel, S. Edward jenner and the history of smallpox and vaccination. Proc. Baylor Univ. Med. Cent. 2005, 18, 21–25. [Google Scholar]
- Liang, Z.-L.; Mao, Q.-Y.; Wang, Y.-P.; Zhu, F.-C.; Li, J.-X.; Yao, X.; Gao, F.; Wu, X.; Xu, M.; Wang, J.-Z. Progress on the research and development of inactivated EV71 whole-virus vaccines. Hum. Vaccines Immunother. 2013, 9, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Wang, J. EV71 vaccine, an invaluable gift for children. Clin. Trans. Immunol. 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.-C.; Meng, F.-Y.; Li, J.-X.; Li, X.-L.; Mao, Q.-Y.; Tao, H.; Zhang, Y.-T.; Yao, X.; Chu, K.; Chen, Q.-H.; et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant Enterovirus 71 vaccine in children in China: A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2013, 381, 2024–2032. [Google Scholar] [CrossRef]
- Li, R.; Liu, L.; Mo, Z.; Wang, X.; Xia, J.; Liang, Z.; Zhang, Y.; Li, Y.; Mao, Q.; Wang, J.; et al. An inactivated Enterovirus 71 vaccine in healthy children. N. Engl. J. Med. 2014, 370, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Xu, W.; Xia, J.; Liang, Z.; Liu, Y.; Zhang, X.; Tan, X.; Wang, L.; Mao, Q.; Wu, J.; et al. Efficacy, safety, and immunogenicity of an Enterovirus 71 vaccine in China. N. Engl. J. Med. 2014, 370, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Kiener, T.K.; Premanand, B.; Kwang, J. Immune responses to baculovirus-displayed Enterovirus 71 VP1 antigen. Expert Rev. Vaccines 2013, 12, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Kolpe, A.B.; Kiener, T.K.; Chow, V.T.K.; Kwang, J. Display of VP1 on the surface of baculovirus and its immunogenicity against heterologous human Enterovirus 71 strains in mice. PLoS ONE 2011, 6, e21757. [Google Scholar] [CrossRef] [PubMed]
- Lim, X.F.; Jia, Q.; Khong, W.X.; Yan, B.; Premanand, B.; Alonso, S.; Chow, V.T.K.; Kwang, J. Characterization of an isotype-dependent monoclonal antibody against linear neutralizing epitope effective for prophylaxis of Enterovirus 71 infection. PLoS ONE 2012, 7, e29751. [Google Scholar] [CrossRef] [PubMed]
- Kiener, T.K.; Jia, Q.; Meng, T.; Chow, V.T.; Kwang, J. A novel universal neutralizing monoclonal antibody against Enterovirus 71 that targets the highly conserved “knob” region of VP3 protein. PLoS Negl. Trop. Dis. 2014, 8, e2895. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.-H.; Luo, Y.-J.; Wu, X.-Y.; Si, B.-Y.; Lin, L.; Zhu, Q.-Y. Monoclonal antibody induced with inactived EV71-Hn2 virus protects mice against lethal EV71-Hn2 virus infection. Virol. J. 2010, 7. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.H.; Liu, C.C.; Chang, J.Y.; Jiang, R.; Hsieh, Y.C.; Tsao, A.; Wu, C.L.; Huang, J.L.; Fung, C.P.; Hsieh, S.M.; et al. Formalin-inactivated EV71 vaccine candidate induced cross-neutralizing antibody against subgenotypes B1, B4, B5 and C4A in adult volunteers. PLoS ONE 2013, 8, e79783. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Cheng, T.; Zhu, F.; Li, J.; Wang, Y.; Li, Y.; Gao, F.; Yang, L.; Yao, X.; Shao, J.; et al. The cross-neutralizing activity of Enterovirus 71 subgenotype C4 vaccines in healthy chinese infants and children. PLoS ONE 2013, 8, e79599. [Google Scholar] [CrossRef] [PubMed]
- Wilton, T.; Dunn, G.; Eastwood, D.; Minor, P.D.; Martin, J. Effect of formaldehyde inactivation on poliovirus. J. Virol. 2014, 88, 11955–11964. [Google Scholar] [CrossRef] [PubMed]
- Tirado, S.M.; Yoon, K.J. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 2003, 16, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-M.; Chen, I.C.; Su, L.-Y.; Huang, K.-J.; Lei, H.-Y.; Liu, C.-C. Enterovirus 71 infection of monocytes with antibody-dependent enhancement. Clin. Vaccine Immunol. CVI 2010, 17, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-F.; Cao, R.-Y.; Deng, Y.-Q.; Tian, X.; Jiang, T.; Qin, E.D.; Qin, C.-F. Antibody dependent enhancement infection of Enterovirus 71 in vitro and in vivo. Virol. J. 2011, 8. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.Y.; Dong, D.Y.; Liu, R.J.; Han, J.F.; Wang, G.C.; Zhao, H.; Li, X.F.; Deng, Y.Q.; Zhu, S.Y.; Wang, X.Y.; et al. Human IgG subclasses against enterovirus type 71: Neutralization versus antibody dependent enhancement of infection. PLoS ONE 2013, 8, e64024. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Lee, Y.P.; Wang, Y.-F.; Yu, C.K. Formaldehyde-inactivated human Enterovirus 71 vaccine is compatible for co-immunization with a commercial pentavalent vaccine. Vaccine 2011, 29, 2772–2776. [Google Scholar] [CrossRef] [PubMed]
- Ogra, P.L.; Faden, H.; Welliver, R.C. Vaccination strategies for mucosal immune responses. Clin. Microbiol. Rev. 2001, 14, 430–445. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, D.L. Baculovirus-insect cell expression systems. Methods Enzymol. 2009, 463, 191–222. [Google Scholar] [PubMed]
- Pennock, G.D.; Shoemaker, C.; Miller, L.K. Strong and regulated expression of Escherichia coli β-galactosidase in insect cells with a baculovirus vector. Mol. Cell. Biol. 1984, 4, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.E.; Summers, M.D.; Fraser, M.J. Production of human β interferon in insect cells infected with a baculovirus expression vector. Mol. Cell. Biol. 1983, 3, 2156–2165. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.K. Baculoviruses as gene expression vectors. Annu. Rev. Microbiol. 1988, 42, 177–199. [Google Scholar] [CrossRef] [PubMed]
- Luckow, V.A.; Summers, M.D. Trends in the development of baculovirus expression vectors. Nat. Biotechnol. 1988, 6, 47–55. [Google Scholar] [CrossRef]
- O’Reilly, D.R.; Miller, L.K.; Luckow, V.A. Baculovirus Expression Vectors: A Laboratory Manual; Oxford University Press on Demand: Oxford, UK, 1994. [Google Scholar]
- DuBois, R.M.; Aguilar-Yañez, J.M.; Mendoza-Ochoa, G.I.; Oropeza-Almazán, Y.; Schultz-Cherry, S.; Alvarez, M.M.; White, S.W.; Russell, C.J. The receptor-binding domain of influenza virus hemagglutinin produced in Escherichia coli folds into its native, immunogenic structure. J. Virol. 2011, 85, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Jifeng, D.; Juan, P.; Yingchun, Z.; Jinhua, L. Expression of H5N1 avian influenza virus haemagglutinin in a baculovirus expression system. Chin. J. Agric. Biotechnol. 2007, 4, 233–237. [Google Scholar] [CrossRef]
- Ruiz-Gonzalvo, F.; Rodriguez, F.; Escribano, J.M. Functional and immunological properties of the baculovirus-expressed hemagglutinin of African swine fever virus. Virology 1996, 218, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Rajesh Kumar, S.; Syed Khader, S.M.; Kiener, T.K.; Szyporta, M.; Kwang, J. Intranasal immunization of baculovirus displayed hemagglutinin confers complete protection against mouse adapted highly pathogenic H7N7 reassortant influenza virus. PLoS ONE 2013, 8, e63856. [Google Scholar] [CrossRef] [PubMed]
- Berger, I.; Fitzgerald, D.J.; Richmond, T.J. Baculovirus expression system for heterologous multiprotein complexes. Nat. Biotechnol. 2004, 22, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Kost, T.A.; Condreay, J.P.; Jarvis, D.L. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat. Biotechnol. 2005, 23, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Lv, Z.; Chen, Q.; Quan, Y.; Zhang, H.; Li, S.; Chen, G.; Zheng, Q.; Jin, L.; Wu, X.; et al. Safety and immunogenicity of H5N1 influenza vaccine based on baculovirus surface display system of Bombyx mori. PLoS ONE 2008, 3, e3933. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.G.; Wang, Z.S.; Zhang, Q.; Li, Z.C.; Zhao, H.N.; Li, W.; Tong, D.W.; Liu, H.J. Baculovirus surface display of e envelope glycoprotein of Japanese encephalitis virus and its immunogenicity of the displayed proteins in mouse and swine models. Vaccine 2011, 29, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Premanand, B.; Kiener, T.K.; Meng, T.; Tan, Y.R.; Jia, Q.; Chow, V.T.; Kwang, J. Induction of protective immune responses against EV71 in mice by baculovirus encoding a novel expression cassette for capsid protein VP1. Antivir. Res. 2012, 95, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Roldão, A.; Mellado, M.C.M.; Castilho, L.R.; Carrondo, M.J.T.; Alves, P.M. Virus-like particles in vaccine development. Expert Rev. Vaccines 2010, 9, 1149–1176. [Google Scholar] [CrossRef] [PubMed]
- Mariani, L.; Venuti, A. Hpv vaccine: An overview of immune response, clinical protection, and new approaches for the future. J. Transl. Med. 2010, 8. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.-Y.; Chen, C.-Y.; Lin, S.-Y.; Chung, Y.-C.; Chiu, H.-Y.; Chi, W.-K.; Lin, Y.-L.; Chiang, B.-L.; Chen, W.-J.; Hu, Y.-C. Enterovirus 71 virus-like particle vaccine: Improved production conditions for enhanced yield. Vaccine 2010, 28, 6951–6957. [Google Scholar] [CrossRef] [PubMed]
- Ku, Z.; Ye, X.; Huang, X.; Cai, Y.; Liu, Q.; Li, Y.; Su, Z.; Huang, Z. Neutralizing antibodies induced by recombinant virus-like particles of Enterovirus 71 genotype C4 inhibit infection at pre- and post-attachment steps. PLoS ONE 2013, 8, e57601. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Yeh, C.T.; Li, W.H.; Yu, C.P.; Lin, W.C.; Yang, J.Y.; Wu, H.L.; Hu, Y.C. Enhanced Enterovirus 71 virus-like particle yield from a new baculovirus design. Biotechnol. Bioeng. 2015, 112, 2005–2015. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Yi, Y.; Song, J.-D.; Tian, M.-M.; Tian, R.-G.; Meng, Q.-L.; Qiu, F.; Jia, Z.-Y.; Bi, S.L. The assemblage, purification and characterization of EV71 VLPs expressed in baculovirus. Chin. J. Virol. 2012, 28, 201–206. (In Chinese) [Google Scholar]
- Whitford, M.; Stewart, S.; Kuzio, J.; Faulkner, P. Identification and sequence analysis of a gene encoding GP67, an abundant envelope glycoprotein of the baculovirus autographa californica nuclear polyhedrosis virus. J. Virol. 1989, 63, 1393–1399. [Google Scholar] [PubMed]
- Liu, C.-C.; Chou, A.-H.; Lien, S.-P.; Lin, H.-Y.; Liu, S.-J.; Chang, J.-Y.; Guo, M.-S.; Chow, Y.-H.; Yang, W.-S.; Chang, K.H.-W. Identification and characterization of a cross-neutralization epitope of Enterovirus 71. Vaccine 2011, 29, 4362–4372. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Cifuente, J.O.; Ashley, R.E.; Conway, J.F.; Makhov, A.M.; Tano, Y.; Shimizu, H.; Nishimura, Y.; Hafenstein, S. A strain-specific epitope of Enterovirus 71 identified by cryo-electron microscopy of the complex with fab from neutralizing antibody. J. Virol. 2013, 87, 11363–11370. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; He, D.; Li, Z.; Zheng, J.; Yang, L.; Yu, M.; Yu, H.; Chen, Y.; Que, Y.; Shih, J.W.K.; et al. Protection against lethal enterovirus 71 challenge in mice by a recombinant vaccine candidate containing a broadly cross-neutralizing epitope within the VP2 EF loop. Theranostics 2014, 4, 498–513. [Google Scholar] [CrossRef] [PubMed]
- He, D.L.; Xia, N.S.; Xu, F.H.; Cheng, T.; Weng, Z.X.; Ge, S.X.; Chen, Y.X.; Chen, Z.M. Enterovirus 71 Neutralized Epitope Polypeptide and Application Thereof. CN102690327 A, 26 September 2012. [Google Scholar]
- Deng, Y.-Q.; Ma, J.; Xu, L.-J.; Li, Y.-X.; Zhao, H.; Han, J.-F.; Tao, J.; Li, X.-F.; Zhu, S.-Y.; Qin, E.D.; et al. Generation and characterization of a protective mouse monoclonal antibody against human enterovirus 71. Appl. Microbiol. Biotechnol. 2015, 99, 7663–7671. [Google Scholar] [PubMed]
- Shingler, K.L.; Cifuente, J.O.; Ashley, R.E.; Makhov, A.M.; Conway, J.F.; Hafenstein, S. The enterovirus 71 procapsid binds neutralizing antibodies and rescues virus infection in vitro. J. Virol. 2015, 89, 1900–1908. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Xianyu, L.; Lyu, S. Monoclonal neutralizing antibodies against ev71 screened from mice immunized with yeast-produced virus-like particles. Virol. Sin. 2015, 30, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Huang, K.J.; Ho, T.S.; Liu, C.C.; Lee, Y.R.; Lin, C.Y.; Shiuan, D.; Jiang, X.H. Monoclonal antibodies for diagnosis of Enterovirus 71. Monoclon. Antib. Immunodiagn. Immunother. 2013, 32, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, M.; Velumani, S.; He, F.; Karuppannan, A.K.; Geng, G.Y.; Yin, L.K.; Kwang, J. Protective immunity against influenza h5n1 virus challenge in mice by intranasal co-administration of baculovirus surface-displayed HA and recombinant CTB as an adjuvant. Virology 2008, 380, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Chang, M.O.; Kitajima, M.; Takaku, H. Baculovirus activates murine dendritic cells and induces non-specific NK cell and T cell immune responses. Cell. Immunol. 2010, 262, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, M.; Meng, T.; He, F.; Yunrui, T.; Qiang, J.; Lin, R.T.; Kwang, J. Subcutaneous immunization with baculovirus surface-displayed hemagglutinin of pandemic H1N1 influenza A virus induces protective immunity in mice. Clin. Vaccine Immunol. 2011, 18, 1582–1585. [Google Scholar] [CrossRef] [PubMed]
- Gronowski, A.M.; Hilbert, D.M.; Sheehan, K.C.; Garotta, G.; Schreiber, R.D. Baculovirus stimulates antiviral effects in mammalian cells. J. Virol. 1999, 73, 9944–9951. [Google Scholar] [PubMed]
- Syed Musthaq, S.; Madhan, S.; Sahul Hameed, A.S.; Kwang, J. Localization of VP28 on the baculovirus envelope and its immunogenicity against white spot syndrome virus in penaeus monodon. Virology 2009, 391, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Murges, D.; Kremer, A. Knebel-Morsdorf, D. Baculovirus transactivator ie1 is functional in mammalian cells. J. Gen. Virol. 1997, 78, 1507–1510. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.S.K. Endogenous antigen presentation of MHC class II epitopes through non-autophagic pathways. Front. Immunol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Diebold, S.S.; Cotten, M.; Koch, N.; Zenke, M. Mhc class II presentation of endogenously expressed antigens by transfected dendritic cells. Gene Ther. 2001, 8, 487–493. [Google Scholar] [CrossRef] [PubMed]
- McHeyzer-Williams, M.; Okitsu, S.; Wang, N.; McHeyzer-Williams, L. Molecular programming of B cell memory. Nat. Rev. Immunol. 2012, 12, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.P.; Travers, P.; Walport, M.; Janeway, C. Janeway’s Immunobiology; Garland Science: New York, NY, USA, 2008. [Google Scholar]
- Cruz, I.; Meijer, C.J.L.M.; Walboomers, J.M.M.; Snijders, P.J.F.; Waal, I.V.D. Lack of MHC class I surface expression on neoplastic cells and poor activation of the secretory pathway of cytotoxic cells in oral squamous cell carcinomas. Br. J. Cancer 1999, 81, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Ura, T.; Okuda, K.; Shimada, M. Developments in viral vector-based vaccines. Vaccines 2014, 2, 624–641. [Google Scholar] [CrossRef] [PubMed]
- Jolles, S.; Sewell, W.A.C.; Misbah, S.A. Clinical uses of intravenous immunoglobulin. Clin. Exp. Immunol. 2005, 142, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.A.; Wijdicks, E.F.; Barohn, R.; Benson, E.; Cornblath, D.R.; Hahn, A.F.; Meythaler, J.M.; Miller, R.G.; Sladky, J.T.; Stevens, J.C. Practice parameter: Immunotherapy for guillain-barre syndrome: Report of the quality standards subcommittee of the american academy of neurology. Neurology 2003, 61, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Lei, H.Y.; Huang, M.-C.; Su, L.-Y.; Lin, H.-C.; Yu, C.-K.; Wang, J.-L.; Liu, C.C. Modulation of cytokine production by intravenous immunoglobulin in patients with Enterovirus 71-associated brainstem encephalitis. J. Clin. Virol. 2006, 37, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Ho, T.S.; Shen, C.-F.; Liu, C.C. Enterovirus 71, one virus and many stories. Pediatr. Neonatol. 2008, 49, 113–115. [Google Scholar] [CrossRef]
- Cao, R.; Han, J.; Deng, Y.; Yu, M.; Qin, E.; Qin, C. Presence of high-titer neutralizing antibodies against Enterovirus 71 in intravenous immunoglobulin manufactured from Chinese donors. Clin. Infect. Dis. 2010, 50, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Bayry, J.; Lacroix-Desmazes, S.; Kazatchkine, M.D.; Kaveri, S.V. Intravenous immunoglobulin for infectious diseases: Back to the pre-antibiotic and passive prophylaxis era? Trends Pharmacol. Sci. 2004, 25, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Teeling, J.L.; Jansen-Hendriks, T.; Kuijpers, T.W.; de Haas, M.; van de Winkel, J.G.; Hack, C.E.; Bleeker, W.K. Therapeutic efficacy of intravenous immunoglobulin preparations depends on the immunoglobulin G dimers: Studies in experimental immune thrombocytopenia. Blood 2001, 98, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F.; Ravetch, J.V. The antiinflammatory activity of IgG: The intravenous IgG paradox. J. Exp. Med. 2007, 204, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Ameratunga, R.; Sinclair, J.; Kolbe, J. Increased risk of adverse events when changing intravenous immunoglobulin preparations. Clin. Exp. Immunol. 2004, 136, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Feldmeyer, L.; Benden, C.; Haile, S.R.; Boehler, A.; Speich, R.; French, L.E.; Hofbauer, G.F. Not all intravenous immunoglobulin preparations are equally well tolerated. Acta Derm. Venereol. 2010, 90, 494–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiff, R.I. Transmission of viral infections through intravenous immune globulin. N. Engl. J. Med. 1994, 331, 1649–1650. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, E.Q.; Balthasar, J.P. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin. Pharmacol. Ther. 2008, 84, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, W.M.; Christensen, M.; Dos Santos, G.; Miller, D.; Ho, J.; Wu, T.; Dziegelewski, M.; Neethling, F.A. Production of monoclonal antibodies. Curr. Prot. Immunol. 2006. [Google Scholar] [CrossRef]
- Sgro, C. Side-effects of a monoclonal antibody, muromonab CD3/orthoclone OKT3: Bibliographic review. Toxicology 1995, 105, 23–29. [Google Scholar] [CrossRef]
- Lubeck, M.D.; Steplewski, Z.; Baglia, F.; Klein, M.H.; Dorrington, K.J.; Koprowski, H. The interaction of murine IgG subclass proteins with human monocyte fc receptors. J. Immunol. 1985, 135, 1299–1304. [Google Scholar] [PubMed]
- McCool, D.; Birshtein, B.K.; Painter, R.H. Structural requirements of immunoglobulin G for binding to the Fc γ receptors of the human tumor cell lines U937, HL-60, ML-1, AND K562. J. Immunol. 1985, 135, 1975–1980. [Google Scholar] [PubMed]
- Wang, H.; Ma, C.; Lu, Y.; Ji, X.; Pang, Y.; Hua, F.; Cui, L.; Ba, D.; He, W. Generation of human neutralizing monoclonal antibodies against the 2009 pandemic H1N1 virus from peripheral blood memory b lymphocytes. Cell. Mol. Immunol. 2013, 10, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Traggiai, E.; Becker, S.; Subbarao, K.; Kolesnikova, L.; Uematsu, Y.; Gismondo, M.R.; Murphy, B.R.; Rappuoli, R.; Lanzavecchia, A. An efficient method to make human monoclonal antibodies from memory B cells: Potent neutralization of SARS coronavirus. Nat. Med. 2004, 10, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Harding, F.A.; Stickler, M.M.; Razo, J.; DuBridge, R.B. The immunogenicity of humanized and fully human antibodies: Residual immunogenicity resides in the CDR regions. mAbs 2010, 2, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Jakobovits, A. Production of fully human antibodies by transgenic mice. Curr. Opin. Biotechnol. 1995, 6, 561–566. [Google Scholar] [CrossRef]
- Lonberg, N. Human antibodies from transgenic animals. Nat. Biotechnol. 2005, 23, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Lonberg, N.; Taylor, L.D.; Harding, F.A.; Trounstine, M.; Higgins, K.M.; Schramm, S.R.; Kuo, C.C.; Mashayekh, R.; Wymore, K.; McCabe, J.G.; et al. Antigen-specific human antibodies from mice comprising four distinct genetic modifications. Nature 1994, 368, 856–859. [Google Scholar] [CrossRef] [PubMed]
- Green, L.L.; Hardy, M.C.; Maynard-Currie, C.E.; Tsuda, H.; Louie, D.M.; Mendez, M.J.; Abderrahim, H.; Noguchi, M.; Smith, D.H.; Zeng, Y.; David, N.E.; et al. Antigen-specific human monoclonal antibodies from mice engineered with human Ig heavy and light chain YACs. Nat. Genet. 1994, 7, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.J. Potent antibody therapeutics by design. Nat. Rev. Immunol. 2006, 6, 343–357. [Google Scholar] [PubMed]
- Brüggemann, M.; Caskey, H.M.; Teale, C.; Waldmann, H.; Williams, G.T.; Surani, M.A.; Neuberger, M.S. A repertoire of monoclonal antibodies with human heavy chains from transgenic mice. Proc. Natl. Acad. Sci. USA 1989, 86, 6709–6713. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, M.S.; Williams, G.T.; Mitchell, E.B.; Jouhal, S.S.; Flanagan, J.G.; Rabbitts, T.H. A hapten-specific chimaeric IgE antibody with human physiological effector function. Nature 1985, 314, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.L.; Johnson, M.J.; Herzenberg, L.A.; Oi, V.T. Chimeric human antibody molecules: Mouse antigen-binding domains with human constant region domains. Proc. Natl. Acad. Sci. USA 1984, 81, 6851–6855. [Google Scholar] [CrossRef] [PubMed]
- Gorman, S.D.; Clark, M.R. Humanisation of monoclonal antibodies for therapy. Semin. Immunol. 1990, 2, 457–466. [Google Scholar] [PubMed]
- Hwang, W.Y.; Foote, J. Immunogenicity of engineered antibodies. Methods 2005, 36, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Ng, Q.; Jia, Q.; Kwang, J.; He, F. A novel humanized antibody neutralizes H5N1 influenza virus via two different mechanisms. J. Virol. 2015, 89, 3712–3722. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A.; Travers, P.; Walport, M.; Shlomchik, M.J. Structural variation in immunoglobulin constant regions. Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Stewart, R.; Hammond, S.A.; Oberst, M.; Wilkinson, R.W. The role of Fc γ receptors in the activity of immunomodulatory antibodies for cancer. J. ImmunoTher. Cancer 2014, 2, 29. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, Q.; He, F.; Kwang, J. Recent Progress towards Novel EV71 Anti-Therapeutics and Vaccines. Viruses 2015, 7, 6441-6457. https://doi.org/10.3390/v7122949
Ng Q, He F, Kwang J. Recent Progress towards Novel EV71 Anti-Therapeutics and Vaccines. Viruses. 2015; 7(12):6441-6457. https://doi.org/10.3390/v7122949
Chicago/Turabian StyleNg, Qingyong, Fang He, and Jimmy Kwang. 2015. "Recent Progress towards Novel EV71 Anti-Therapeutics and Vaccines" Viruses 7, no. 12: 6441-6457. https://doi.org/10.3390/v7122949





