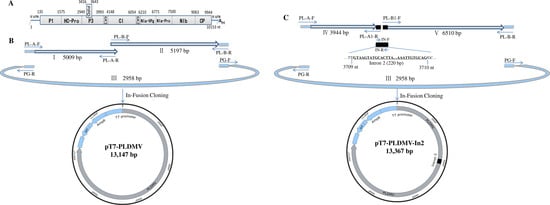
Rapid Construction of Stable Infectious Full-Length cDNA Clone of Papaya Leaf Distortion Mosaic Virus Using In-Fusion Cloning
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virus Source and RNA Extraction
2.2. In-Fusion Construction of A Full-Length cDNA Clone of PLDMV-DF
| Name | Primer Sequence (5′→3′) |
|---|---|
| PL-A-F a | CGACTCACTATAGGGAAAAATATAAAAACTCAACAAAACT |
| PL-A-R b | GGTGCGCCCATCGACTTTAGTCAC |
| PL-B-F | GTCGATGGGCGCACCATGAAAATTG |
| PL-B-R | GAATTCACTAGTGATGAGCTCTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTCCCTCCTTGCTTAGTCTGAAGTTC |
| PG-F | ATCACTAGTGAATTCGCGGCCGCCTGC |
| PG-R | CCCTATAGTGAGTCGTATTACAATTCAC |
| IN-F | GTAAGTATGCACTTAAAGAGTATGTGTG |
| IN-R | CTGCACAATTTCAAAGATTGAACCTAAGGA |
| PL-A1-R | TAAGTGCATACTTACAAGCACCACTTACACAAAGAGAATG |
| PL-B1-F | TTTGAAATTGTGCAGGCCTGATTGTTTGAAGTTTATAAAC |
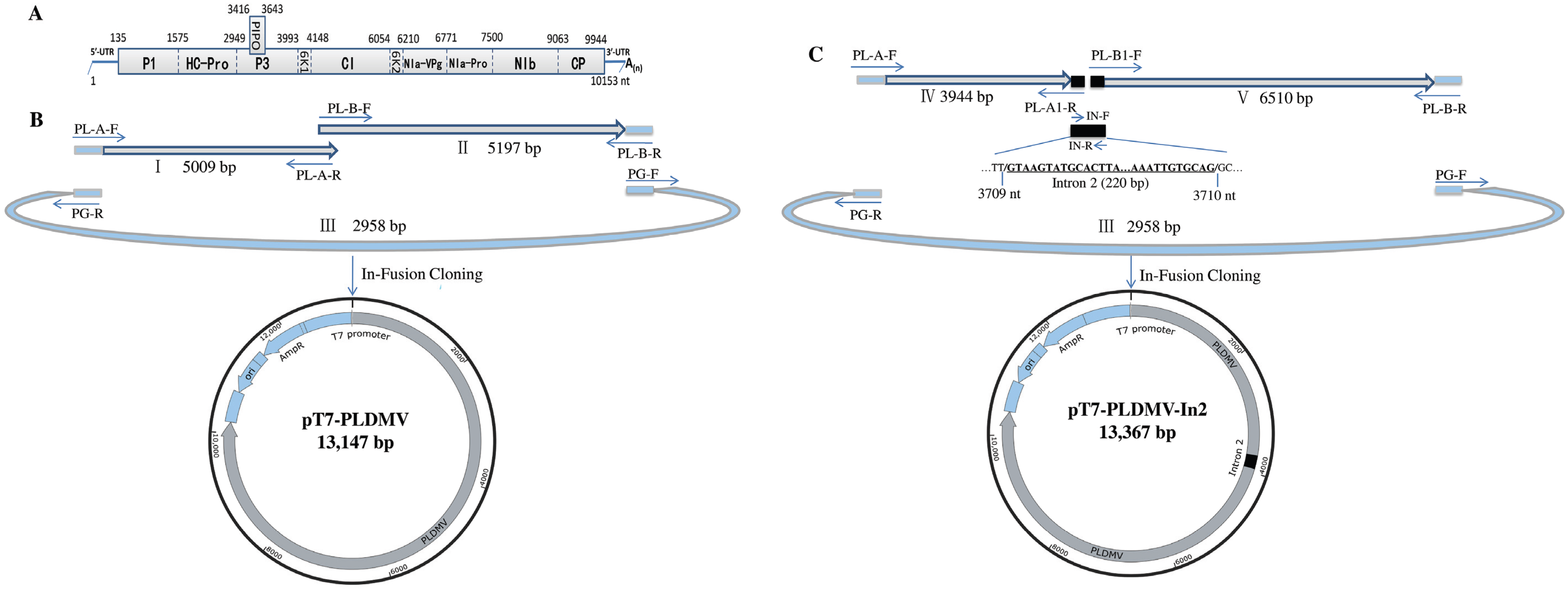
2.3. In-Fusion Construction of A Full-Length cDNA Clone of PLDMV-DF with An Inserted Plant Intron
2.4. In Vitro Transcription Reactions
2.5. Mechanical Inoculation of Plants
2.6. RT-PCR and Indirect Enzyme-Linked Immunosorbent Assay (ID-ELISA)
3. Results and Discussion

4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tripathi, S.; Suzuki, J.Y.; Ferreira, S.A.; Gonsalves, D. Papaya ringspot virus-P: Characteristics, pathogenicity, sequence variability and control. Mol. Plant Pathol. 2008, 9, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T.; Hataya, T. The complete nucleotide sequence and biotype variability of papaya leaf distortion mosaic virus. Phytopathology 2005, 95, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Bau, H.J.; Kung, Y.J.; Raja, J.A.; Chan, S.J.; Chen, K.C.; Chen, Y.K.; Wu, H.W.; Yeh, S.D. Potential threat of a new pathotype of Papaya leaf distortion mosaic virus infecting transgenic papaya resistant to Papaya ringspot virus. Phytopathology 2008, 98, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Tuo, D.; Shen, W.; Yan, P.; Li, C.; Gao, L.; Li, X.; Li, H.; Zhou, P. Complete genome sequence of an isolate of papaya leaf distortion mosaic virus from commercialized PRSV-resistant transgenic papaya in China. Acta Virol. 2013, 57, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Kung, Y.J.; Bau, H.J.; Wu, Y.L.; Huang, C.H.; Chen, T.M.; Yeh, S.D. Generation of transgenic papaya with double resistance to Papaya ringspot virus and papaya leaf-distortion mosaic virus. Phytopathology 2009, 99, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Aubry, F.; Nougairede, A.; Gould, E.A.; de Lamballerie, X. Flavivirus reverse genetic systems, construction techniques and applications: A historical perspective. Antiviral Res. 2015, 114, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Nagyová, A.; Subr, Z. Infectious full-length clones of plant viruses and their use for construction of viral vectors. Acta Virol. 2007, 51, 223–237. [Google Scholar] [PubMed]
- Virus Taxonomy: 2014 Release, EC 46, Montreal, Canada, July 2014, Email Ratification 2015. Available online: http://ictvonline.org/virusTaxonomy.asp?msl_id=29 (accessed on 11 September 2015).
- Nakahara, K.S.; Nishino, K.; Uyeda, I. Construction of infectious cDNA clones derived from the potyviruses clover yellow vein virus and bean yellow mosaic virus. Methods Mol. Biol. 2015, 1236, 219–227. [Google Scholar] [PubMed]
- Kim, K.-S.; Oh, H.-Y.; Suranto, S.; Nurhayati, E.; Gough, K.; Shukla, D.; Pallaghy, C. Infectivity of in vitro transcripts of johnsongrass mosaic potyvirus full-length cDNA clones in maize and sorghum. Arch. Virol. 2003, 148, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Revers, F.; Souche, S.; Lot, H.; le Gall, O.; Candresse, T.; Dunez, J. Construction of full-length cDNA clones of lettuce mosaic virus (LMV) and the effects of intron-insertion on their viability in Escherichia coli and on their infectivity to plants. Arch. Virol. 1998, 143, 2443–2451. [Google Scholar] [CrossRef] [PubMed]
- Bordat, A.; Houvenaghel, M.C.; German-Retana, S. Gibson assembly: An easy way to clone potyviral full-length infectious cDNA clones expressing an ectopic VPg. Virol. J. 2015, 12. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.R.; Bouchard, R.; Redinbaugh, M.G.; Meulia, T. Complete sequence and development of a full-length infectious clone of an Ohio isolate of Maize dwarf mosaic virus (MDMV). Virus Res. 2012, 165, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-H.; Yeh, S.-D. Infectivity assays of in vitro and in vivo transcripts of papaya ringspot potyvirus. Bot. Bull. Acad. Sin. 1997, 38, 153–163. [Google Scholar]
- Chen, K.C.; Chiang, C.H.; Raja, J.A.; Liu, F.L.; Tai, C.H.; Yeh, S.D. A single amino acid of niapro of papaya ringspot virus determines host specificity for infection of papaya. Mol. Plant Microbe Interact. 2008, 21, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Desbiez, C.; Chandeysson, C.; Lecoq, H.; Moury, B. A simple, rapid and efficient way to obtain infectious clones of potyviruses. J. Virol. Methods 2012, 183, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Flasinski, S.; Gunasinghe, U.; Gonzales, R.A.; Cassidy, B.G. The cDNA sequence and infectious transcripts of peanut stripe virus. Gene 1996, 171, 299–300. [Google Scholar] [CrossRef]
- Naderpour, M.; Johansen, I.E. Visualization of resistance responses in Phaseolus vulgaris using reporter tagged clones of Bean common mosaic virus. Virus Res. 2011, 159, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Johansen, I.E. Intron insertion facilitates amplification of cloned virus cDNA in Escherichia coli while biological activity is reestablished after transcription in vivo. Proc. Natl. Acad. Sci. USA 1996, 93, 12400–12405. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Song, Y.S.; Ryu, K.H. Development of infectious transcripts from full-length and GFP-tagged cDNA clones of Pepper mottle virus and stable systemic expression of GFP in tobacco and pepper. Virus Res. 2011, 155, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Moya, J.J.; Garcia, J.A. Construction of a stable and highly infectious intron-containing cDNA clone of plum pox potyvirus and its use to infect plants by particle bombardment. Virus Res. 2000, 68, 99–107. [Google Scholar] [CrossRef]
- Puurand, Ü.; Valkonen, J.P.; Mäkinen, K.; Rabenstein, F.; Saarma, M. Infectious in vitro transcripts from cloned cDNA of the potato A potyvirus. Virus Res. 1996, 40, 135–140. [Google Scholar] [CrossRef]
- Jakab, G.; Droz, E.; Brigneti, G.; Baulcombe, D.; Malnoe, P. Infectious in vivo and in vitro transcripts from a full-length cDNA clone of PVY-N605, a Swiss necrotic isolate of potato virus Y. J. Gen. Virol. 1997, 78, 3141–3145. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-K.; Lee, H.-G.; Kim, K.-H. Systemic gene delivery into soybean by simple rub-inoculation with plasmid DNA of a soybean mosaic virus-based vector. Arch. Virol. 2009, 154, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Bejerman, N.; Giolitti, F.; de Breuil, S.; Lenardon, S. Development of a full-length infectious clone of sunflower chlorotic mottle virus (SuCMoV). Arch. Virol. 2013, 158, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Bedoya, L.C.; Daros, J.A. Stability of tobacco etch virus infectious clones in plasmid vectors. Virus Res. 2010, 149, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Tian, Y.P.; Wang, J.; Yin, X.; Li, X.D.; Valkonen, J.P. Construction of an infectious cDNA clone and gene expression vector of tobacco vein banding mosaic virus (genus Potyvirus). Virus Res. 2012, 169, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, O.; Pirone, T.; Hellmann, G. Construction and analysis of infectious transcripts from a resistance-breaking strain of tobacco vein mottling potyvirus. Arch. Virol. 1996, 141, 1535–1552. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, F.; Martı́nez-Herrera, D.; Aguilar, I.; Ponz, F. Infectivity of turnip mosaic potyvirus cDNA clones and transcripts on the systemic host Arabidopsis thaliana and local lesion hosts. Virus Res. 1998, 55, 207–219. [Google Scholar] [CrossRef]
- Gal-On, A.; Antignus, Y.; Rosner, A.; Raccah, B. Infectious in vitro RNA transcripts derived from cloned cDNA of the cucurbit potyvirus, zucchini yellow mosaic virus. J. Gen. Virol 1991, 72, 2639–2643. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.C.; Haenni, A.L. Infectious transcripts and cDNA clones of RNA viruses. Virology 1994, 198, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Chikh Ali, M.; Said Omar, A.; Natsuaki, T. An infectious full-length cDNA clone of potato virus YNTN-NW, a recently reported strain of PVY that causes potato tuber necrotic ringspot disease. Arch. Virol. 2011, 156, 2039–2043. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Z.; Elledge, S.J. Harnessing homologous recombination in vitro to generate recombinant DNA via SLIC. Nat. Methods 2007, 4, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Z.; Elledge, S.J. SLIC: A method for sequence- and ligation-independent cloning. Methods Mol. Biol. 2012, 852, 51–59. [Google Scholar] [PubMed]
- Hill, R.E.; Eaton-Rye, J.J. Plasmid construction by SLIC or sequence and ligation-independent cloning. Methods Mol. Biol. 2014, 1116, 25–36. [Google Scholar] [PubMed]
- Berrow, N.S.; Alderton, D.; Sainsbury, S.; Nettleship, J.; Assenberg, R.; Rahman, N.; Stuart, D.I.; Owens, R.J. A versatile ligation-independent cloning method suitable for high-throughput expression screening applications. Nucleic Acids Res. 2007, 35. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.G.; Benders, G.A.; Andrews-Pfannkoch, C.; Denisova, E.A.; Baden-Tillson, H.; Zaveri, J.; Stockwell, T.B.; Brownley, A.; Thomas, D.W.; Algire, M.A.; et al. Complete chemical synthesis, assembly, and cloning of a Mycoplasma genitalium genome. Science 2008, 319, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Berrow, N.S.; Alderton, D.; Owens, R.J. The precise engineering of expression vectors using high-throughput In-Fusion PCR cloning. Methods Mol. Biol. 2009, 498, 75–90. [Google Scholar] [PubMed]
- Bird, L.E.; Rada, H.; Flanagan, J.; Diprose, J.M.; Gilbert, R.J.C.; Owens, R.J. Application of In-Fusion cloning for the parallel construction of E. coli expression vectors. Methods Mol. Biol. 2014, 1116, 209–234. [Google Scholar] [PubMed]
- Hildebrand, A.; Szewczyk, E.; Lin, H.; Kasuga, T.; Fan, Z. Engineering Neurospora crassa for improved cellobiose and cellobionate production. Appl. Environ. Microbiol. 2015, 81, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Kaas, Q.; Zhang, L.; Xu, K.; Li, N.; Zheng, W.; Lai, Q. Isolation and characterization of a cytosolic pyruvate kinase cDNA from loquat (Eriobotrya japonica Lindl.). Plant Mol. Biol. Rep. 2013, 31, 109–119. [Google Scholar] [CrossRef]
- Szewczyk, E.; Kasuga, T.; Fan, Z. Efficient sequential repetitive gene deletions in Neurospora crassa employing a self-excising β-recombinase/six cassette. J. Microbiol. Methods 2013, 92, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.G.; Young, L.; Chuang, R.Y.; Venter, J.C.; Hutchison, C.A., 3rd; Smith, H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 2009, 6, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.G.; Smith, H.O.; Hutchison, C.A., 3rd; Venter, J.C.; Merryman, C. Chemical synthesis of the mouse mitochondrial genome. Nat. Methods 2010, 7, 901–903. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Donnelly, M.E.; Scholes, D.T.; St. George, K.; Hatta, M.; Kawaoka, Y.; Wentworth, D.E. Single-reaction genomic amplification accelerates sequencing and vaccine production for classical and swine origin human influenza A viruses. J. Virol. 2009, 83, 10309–10313. [Google Scholar] [CrossRef] [PubMed]
- Siridechadilok, B.; Gomutsukhavadee, M.; Sawaengpol, T.; Sangiambut, S.; Puttikhunt, C.; Chin-inmanu, K.; Suriyaphol, P.; Malasit, P.; Screaton, G.; Mongkolsapaya, J. A simplified positive-sense-RNA virus construction approach that enhances analysis throughput. J. Virol. 2013, 87, 12667–12674. [Google Scholar] [CrossRef] [PubMed]
- Vandergaast, R.; Hoover, L.I.; Zheng, K.; Fredericksen, B.L. Generation of west nile virus infectious clones containing amino acid insertions between capsid and capsid anchor. Viruses 2014, 6, 1637–1653. [Google Scholar] [CrossRef] [PubMed]
- Suhardiman, M.; Kramyu, J.; Narkpuk, J.; Jongkaewwattana, A.; Wanasen, N. Generation of porcine reproductive and respiratory syndrome virus by in vitro assembly of viral genomic cDNA fragments. Virus Res. 2015, 195, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Blawid, R.; Nagata, T. Construction of an infectious clone of a plant RNA virus in a binary vector using one-step Gibson Assembly. J. Virol. Methods 2015, 222, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Sander, L.; Jensen, P.E.; Back, L.F.; Stummann, B.M.; Henningsen, K.W. Structure and expression of a nitrite reductase gene from bean (Phaseolus vulgaris) and promoter analysis in transgenic tobacco. Plant Mol. Biol. 1995, 27, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Misteli, T.; Caceres, J.F.; Spector, D.L. The dynamics of a pre-mRNA splicing factor in living cells. Nature 1997, 387, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Côté, M.-J.; Turmel, M. In vitro self-splicing reactions of chloroplast and mitochondrial group-I introns in Chlamydomonas eugametos and Chlamydomonas moewusii. Curr. Genet. 1995, 27, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Stange, N.; Beier, H. A cell-free plant extract for accurate pre-tRNA processing, splicing and modification. EMBO J. 1987, 6, 2811–2818. [Google Scholar] [PubMed]
- Youssef, F.; Marais, A.; Faure, C.; Gentit, P.; Candresse, T. Strategies to facilitate the development of uncloned or cloned infectious full-length viral cDNAs: Apple chlorotic leaf spot virus as a case study. Virol. J. 2011, 8. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuo, D.; Shen, W.; Yan, P.; Li, X.; Zhou, P. Rapid Construction of Stable Infectious Full-Length cDNA Clone of Papaya Leaf Distortion Mosaic Virus Using In-Fusion Cloning. Viruses 2015, 7, 6241-6250. https://doi.org/10.3390/v7122935
Tuo D, Shen W, Yan P, Li X, Zhou P. Rapid Construction of Stable Infectious Full-Length cDNA Clone of Papaya Leaf Distortion Mosaic Virus Using In-Fusion Cloning. Viruses. 2015; 7(12):6241-6250. https://doi.org/10.3390/v7122935
Chicago/Turabian StyleTuo, Decai, Wentao Shen, Pu Yan, Xiaoying Li, and Peng Zhou. 2015. "Rapid Construction of Stable Infectious Full-Length cDNA Clone of Papaya Leaf Distortion Mosaic Virus Using In-Fusion Cloning" Viruses 7, no. 12: 6241-6250. https://doi.org/10.3390/v7122935