Identification and Application of Neutralizing Epitopes of Human Adenovirus Type 55 Hexon Protein
Abstract
:1. Introduction
2. Results
2.1. Identification of Neutralizing Epitopes Using Synthetic Peptide
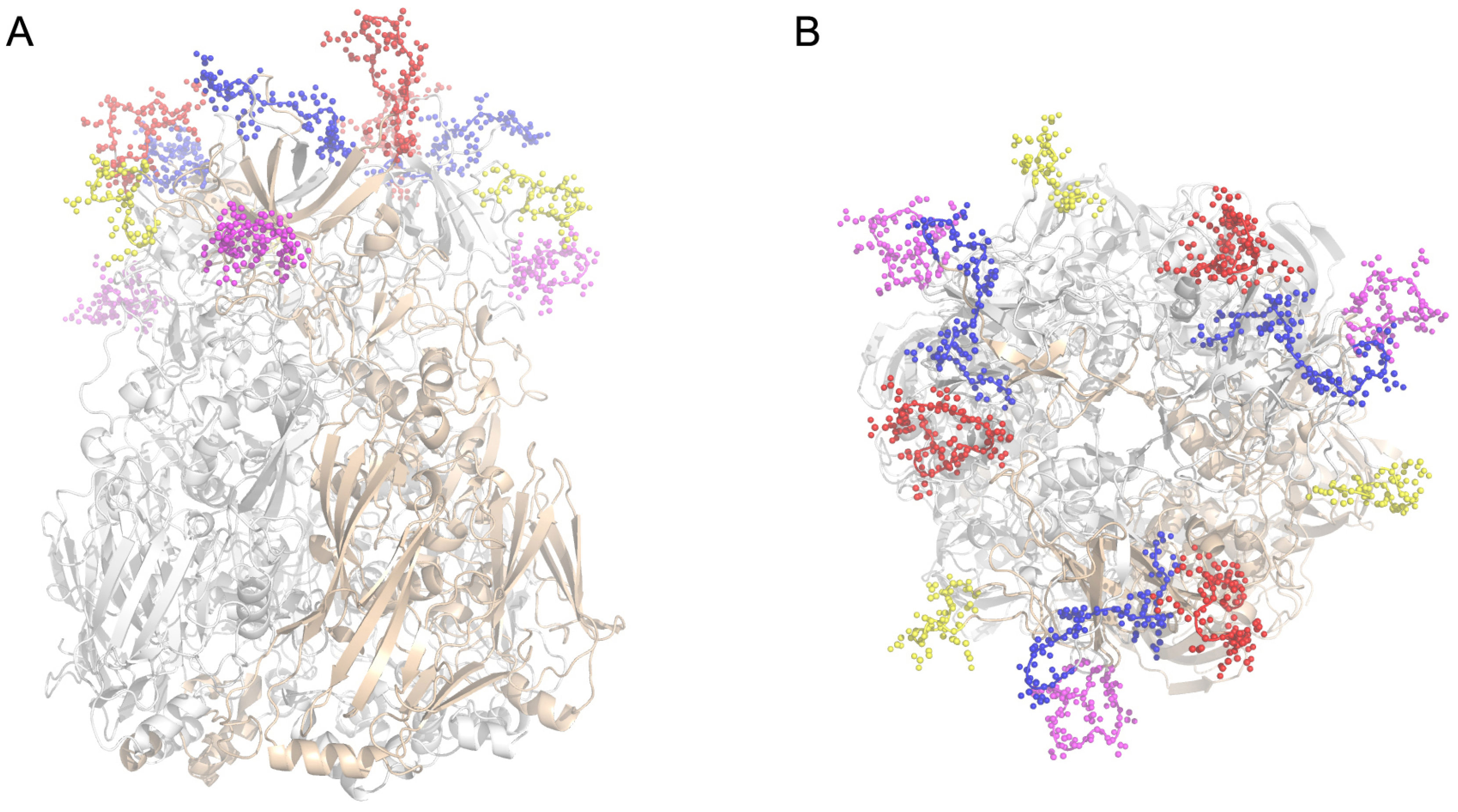
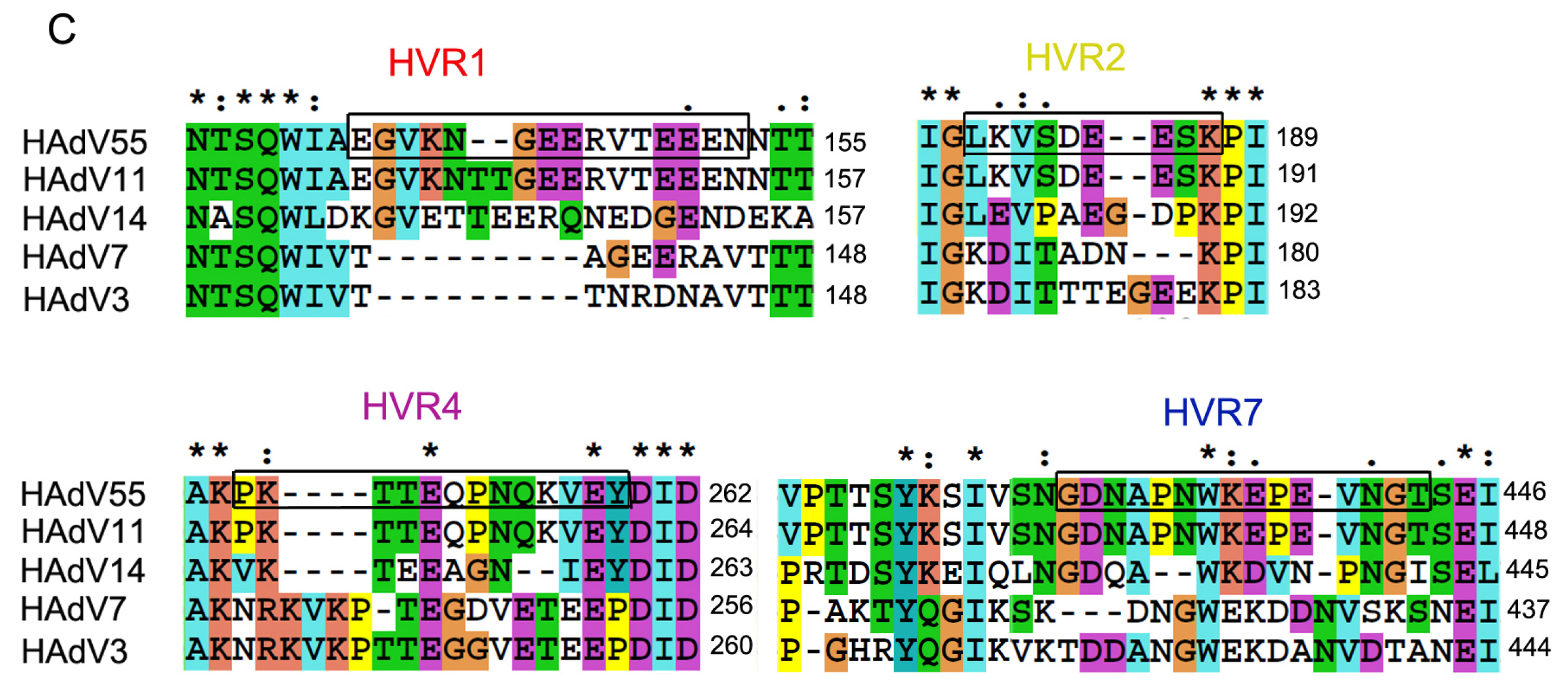
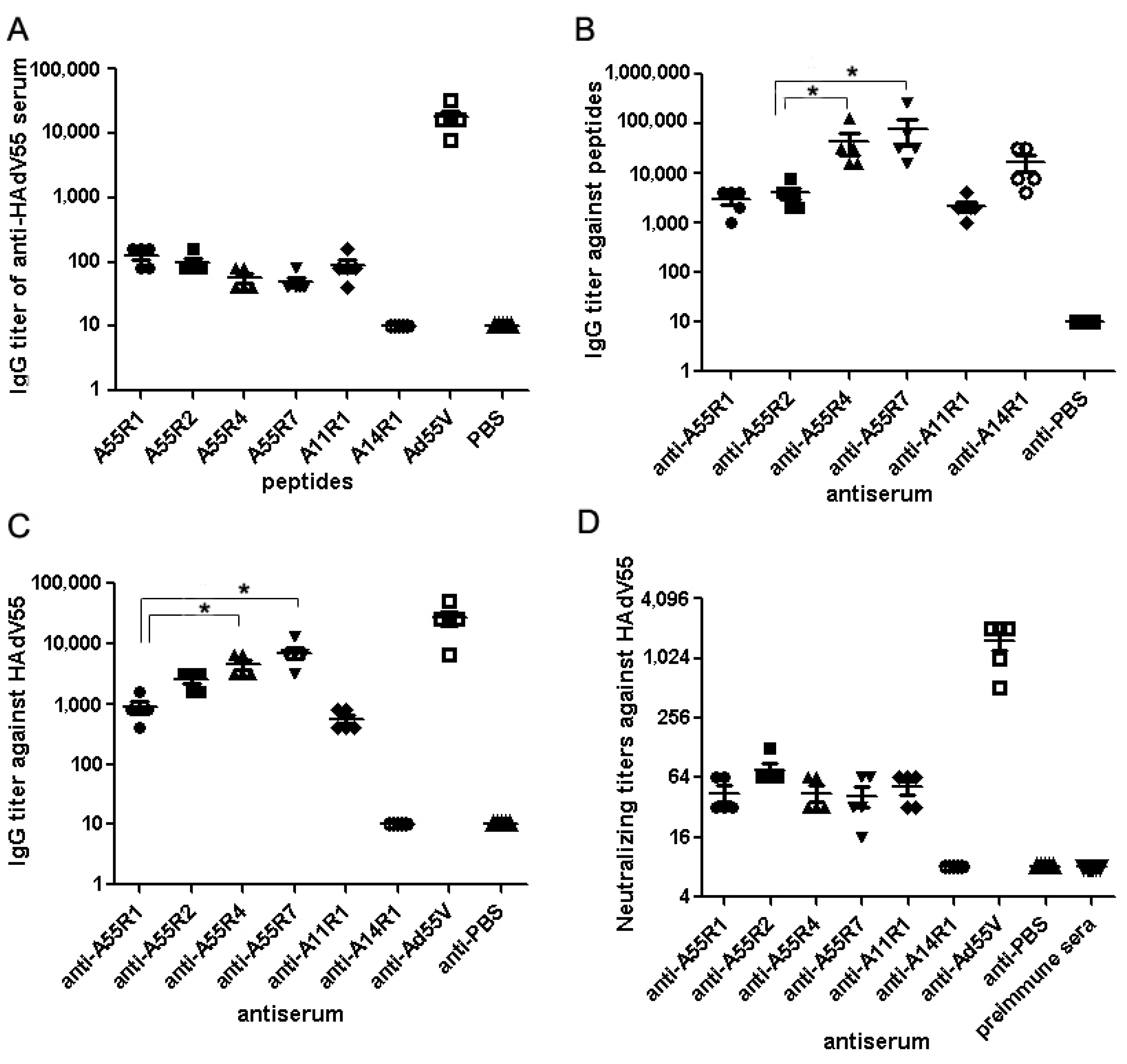
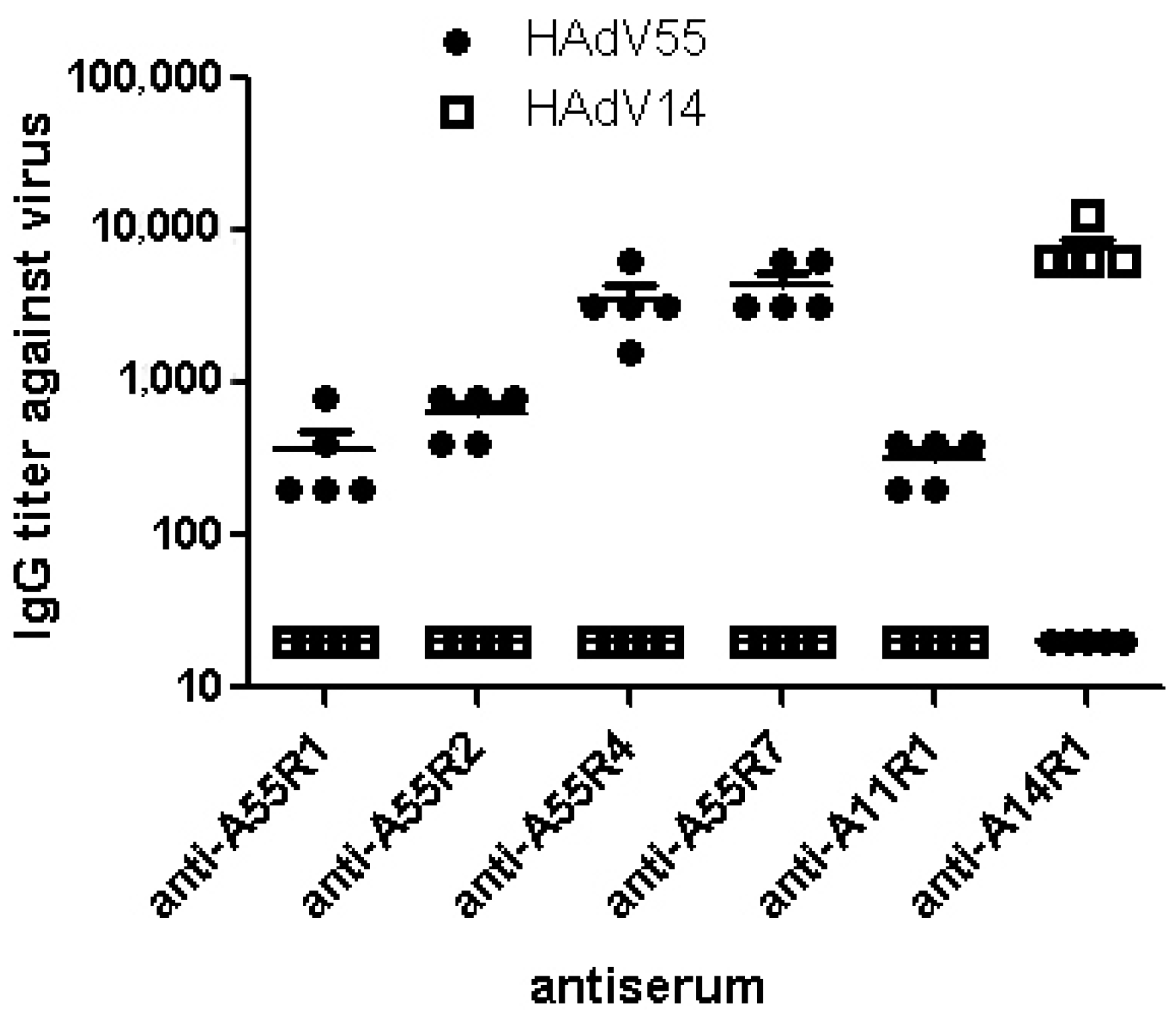
2.2. Anti-rAd3A55R2 Serum Could Neutralize Both HAdV3 and HAdV55 in Vitro
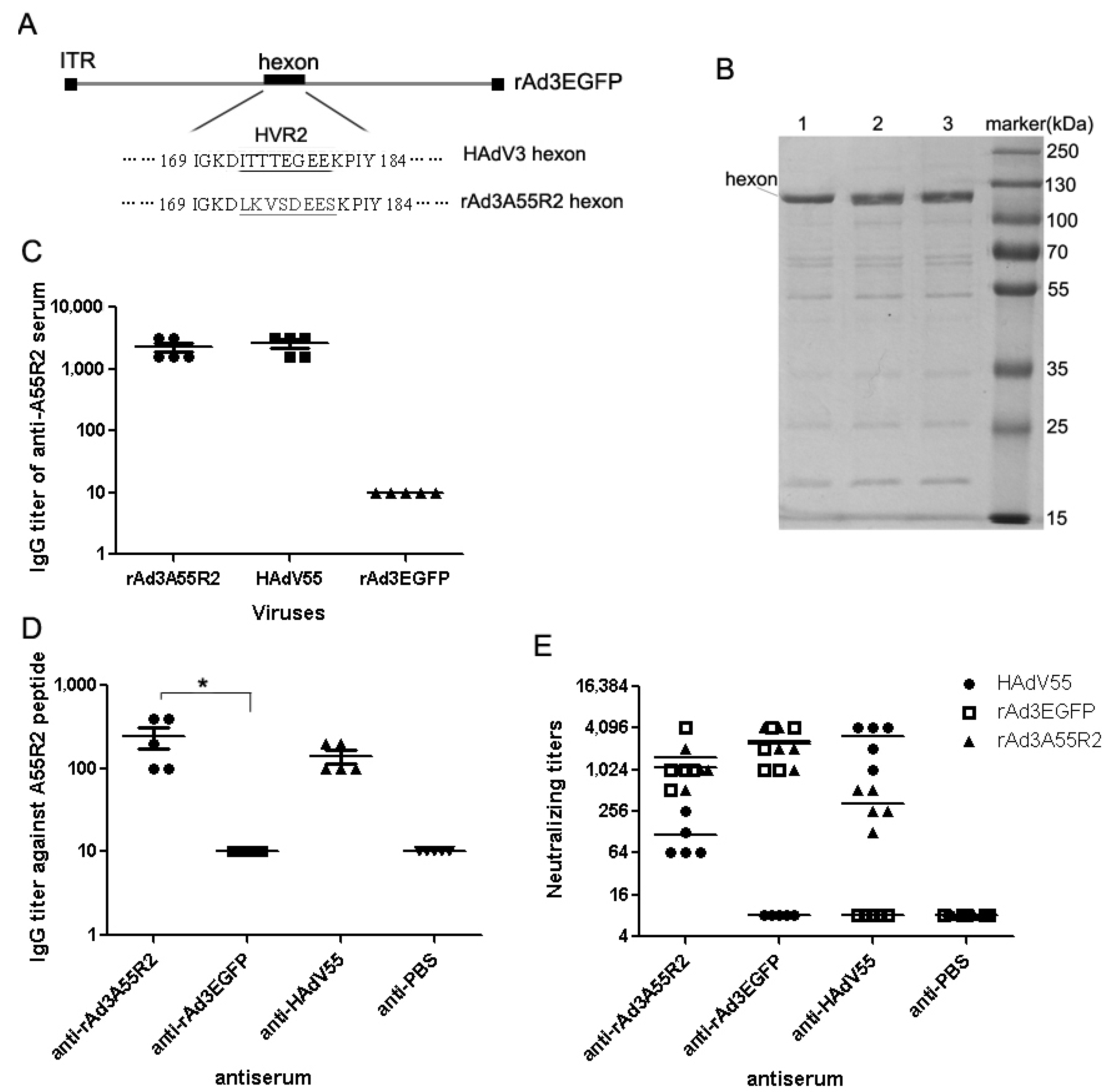
3. Discussion
4. Materials and Methods
4.1. Virus Strains and Cells
4.2. Defining the Potential NAb Epitopes of HAdV55 Hexon
4.3. Peptides Synthesis
4.4. Generating of Epitope Chimeric Adenoviruses
4.5. Mouse Immunization
4.6. ELISA
4.7. Neutralization Tests
4.8. Statistical Analyses
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Lenaerts, L.; de Clercq, E.; Naesens, L. Clinical features and treatment of adenovirus infections. Rev. Med. Virol. 2008, 18, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Sandkovsky, U.; Vargas, L.; Florescu, D.F. Adenovirus: Current epidemiology and emerging approaches to prevention and treatment. Curr. Infect. Dis. Rep. 2014, 16, 416. [Google Scholar] [CrossRef] [PubMed]
- Pavia, A.T. Viral infections of the lower respiratory tract: Old viruses, new viruses, and the role of diagnosis. Clin. Infect. Dis. 2011, 52, S284–S289. [Google Scholar] [CrossRef] [PubMed]
- Taxonomy browser (Mastadenovirus). Available online: http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?id=10509 (accessed on 1 September 2015).
- Singh, G.; Robinson, C.M.; Dehghan, S.; Jones, M.S.; Dyer, D.W.; Seto, D.; Chodosh, J. Homologous recombination in E3 genes of human adenovirus species D. J. Virol. 2013, 87, 12481–12488. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Liu, Z.; Li, X.; Qu, J.; Guan, W.; Liu, Y.; Song, S.; Yu, X.; Cao, B. Severe community-acquired pneumonia caused by adenovirus type 11 in immunocompetent adults in Beijing. J. Clin. Virol. 2012, 54, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.B.; Tong, Y.G.; Wo, Y.; Wang, H.Y.; Liu, E.M.; Gray, G.C.; Liu, W.; Cao, W.C. Epidemiology of human adenovirus and molecular characterization of human adenovirus 55 in China, 2009–2012. Influenza Other Respir. Viruses 2014, 8, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Huang, G.H.; Pu, Z.H.; Qu, J.X.; Yu, X.M.; Zhu, Z.; Dong, J.P.; Gao, Y.; Zhang, Y.X.; Li, X.H.; et al. Emergence of community-acquired adenovirus type 55 as a cause of community-onset pneumonia. Chest 2014, 145, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Qian, Y.; Zhao, L.Q.; Zhu, R.N.; Sun, Y.; Tian, R. Identification and typing of adenovirus from acute respiratory infections in pediatric patients in Beijing from 2003 to 2012. Bing Du Xue Bao 2013, 29, 615–620. (In Chinese) [Google Scholar] [PubMed]
- Sun, B.; He, H.; Wang, Z.; Qu, J.; Li, X.; Ban, C.; Wan, J.; Cao, B.; Tong, Z.; Wang, C. Emergent severe acute respiratory distress syndrome caused by adenovirus type 55 in immunocompetent adults in 2013: A prospective observational study. Crit. Care 2014, 18, 456. [Google Scholar] [CrossRef] [PubMed]
- Chmielewicz, B.; Benzler, J.; Pauli, G.; Krause, G.; Bergmann, F.; Schweiger, B. Respiratory disease caused by a species B2 adenovirus in a military camp in Turkey. J. Med. Virol. 2005, 77, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Kajon, A.E.; Dickson, L.M.; Metzgar, D.; Houng, H.S.; Lee, V.; Tan, B.H. Outbreak of febrile respiratory illness associated with adenovirus 11a infection in a Singapore military training cAMP. J. Clin. Microbiol. 2010, 48, 1438–1441. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kong, M.; Su, X.; Zou, M.; Guo, L.; Dong, X.; Li, L.; Gu, Q. An outbreak of acute respiratory disease in China caused by human adenovirus type B55 in a physical training facility. Int. J. Infect. Dis. 2014, 28, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Hierholzer, J.C.; Pumarola, A.; Rodriguez-Torres, A.; Beltran, M. Occurrence of respiratory illness due to an atypical strain of adenovirus type 11 during a large outbreak in Spanish military recruits. Am. J. Epidemiol. 1974, 99, 434–442. [Google Scholar] [PubMed]
- Hierholzer, J.C.; Pumarola, A. Antigenic characterization of intermediate adenovirus 14–11 strains associated with upper respiratory illness in a military camp. Infect. Immun. 1976, 13, 354–359. [Google Scholar] [PubMed]
- Li, Q.G.; Hambraeus, J.; Wadell, G. Genetic relationship between thirteen genome types of adenovirus 11, 34, and 35 with different tropisms. Intervirology 1991, 32, 338–350. [Google Scholar] [PubMed]
- Kajon, A.E.; de Jong, J.C.; Dickson, L.M.; Arron, G.; Murtagh, P.; Viale, D.; Carballal, G.; Echavarria, M. Molecular and serological characterization of species B2 adenovirus strains isolated from children hospitalized with acute respiratory disease in Buenos Aires, Argentina. J. Clin. Virol. 2013, 58, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, Y.; Xu, S.; Yu, P.; Tian, X.; Wang, L.; Liu, Z.; Tang, L.; Mao, N.; Ji, Y.; et al. Outbreak of acute respiratory disease in China caused by B2 species of adenovirus type 11. J. Clin. Microbiol. 2009, 47, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhu, Z.; Tang, L.; Wang, L.; Tan, X.; Yu, P.; Zhang, Y.; Tian, X.; Wang, J.; Li, D.; et al. Genomic analyses of recombinant adenovirus type 11a in China. J. Clin. Microbiol. 2009, 47, 3082–3090. [Google Scholar] [CrossRef] [PubMed]
- Seto, D.; Jones, M.S.; Dyer, D.W.; Chodosh, J. Characterizing, typing, and naming human adenovirus type 55 in the era of whole genome data. J. Clin. Virol. 2013, 58, 741–742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Seto, D.; Cao, B.; Zhao, S.; Wan, C. Genome sequence of human adenovirus type 55, a re-emergent acute respiratory disease pathogen in China. J. Virol. 2012, 86, 12441–12442. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.P.; Seto, J.; Jones, M.S.; Chodosh, J.; Xu, W.; Seto, D. Computational analysis identifies human adenovirus type 55 as a re-emergent acute respiratory disease pathogen. J. Clin. Microbiol. 2010, 48, 991–993. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Dmitriev, I.; Kashentseva, E.; Seki, T.; Wang, M.; Curie, D.T. Construction and characterization of adenovirus serotype 5 packaged by serotype 3 hexon. J. Virol. 2002, 76, 12775–12782. [Google Scholar] [CrossRef] [PubMed]
- Sumida, S.M.; Truitt, D.M.; Lemckert, A.A.; Vogels, R.; Custers, J.H.; Addo, M.M.; Lockman, S.; Peter, T.; Peyerl, F.W.; Kishko, M.G.; et al. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J. Immunol. 2005, 174, 7179–7185. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Su, X.; Li, H.; Li, X.; Zhou, Z.; Liu, W.; Zhou, R. Construction and characterization of human adenovirus serotype 3 packaged by serotype 7 hexon. Virus Res. 2011, 160, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Dong, J.; Wang, C.; Zhan, Y.; Zhang, H.; Wu, J.; Kong, W.; Yu, X. Characteristics of neutralizing antibodies to adenovirus capsid proteins in human and animal sera. Virology 2013, 437, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Crawford-Miksza, L.; Schnurr, D.P. Analysis of 15 adenovirus hexon proteins reveals the location and structure of seven hypervariable regions containing serotype-specific residues. J. Virol. 1996, 70, 1836–1844. [Google Scholar] [PubMed]
- Rux, J.J.; Kuser, P.R.; Burnett, R.M. Structural and phylogenetic analysis of adenovirus hexons by use of high-resolution x-ray crystallographic, molecular modeling, and sequence-based methods. J. Virol. 2003, 77, 9553–9566. [Google Scholar] [CrossRef] [PubMed]
- Ebner, K.; Pinsker, W.; Lion, T. Comparative sequence analysis of the hexon gene in the entire spectrum of human adenovirus serotypes: Phylogenetic, taxonomic, and clinical implications. J. Virol. 2005, 79, 12635–12642. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.H.; Wang, Y.C.; Jin, W.J.; Zhao, B.B.; Chen, C.F.; Yang, J.; Wang, J.F.; Guo, Y.Y.; Liu, J.J.; Zhang, D.; et al. Structure-based high-throughput epitope analysis of hexon proteins in B and C species human adenoviruses (HAdVs). PLoS ONE 2012, 7, e32938. [Google Scholar] [CrossRef] [PubMed]
- Bradley, R.R.; Maxfield, L.F.; Lynch, D.M.; Iampietro, M.J.; Borducchi, E.N.; Barouch, D.H. Adenovirus serotype 5-specific neutralizing antibodies target multiple hexon hypervariable regions. J. Virol. 2012, 86, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Pichla-Gollon, S.L.; Drinker, M.; Zhou, X.; Xue, F.; Rux, J.J.; Gao, G.P.; Wilson, J.M.; Ertl, H.C.; Burnett, R.M.; Bergelson, J.M. Structure-based identification of a major neutralizing site in an adenovirus hexon. J. Virol. 2007, 81, 1680–1689. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Qu, Z.; Wu, X.; Wang, Y.; Liu, L.; Wei, F.; Gao, H.; Shang, L.; Zhang, H.; Cui, H.; et al. Molecular modeling and epitopes mapping of human adenovirus type 3 hexon protein. Vaccine 2009, 27, 5103–5110. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Li, X.; Tian, X.; Zhou, Z.; Xing, K.; Li, H.; Tang, N.; Liu, W.; Bai, P.; Zhou, R. Serotype-specific neutralizing antibody epitopes of human adenovirus type 3 (HAdV-3) and HAdV-7 reside in multiple hexon hypervariable regions. J. Virol. 2012, 86, 7964–7975. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Liu, M.; Su, X.; Jiang, Z.; Ma, Q.; Liao, X.; Li, X.; Zhou, Z.; Li, C.; Zhou, R. Mapping the epitope of neutralizing monoclonal antibodies against human adenovirus type 3. Virus Res. 2015, 208, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Tian, X.; Li, X.; Zhou, Z.; Li, C.; Zhou, R. Generation of neutralizing monoclonal antibodies against a conformational epitope of human adenovirus type 7. PLoS ONE 2014, 9, e103058. [Google Scholar] [CrossRef] [PubMed]
- Myers, N.D.; Skorohodova, K.V.; Gounder, A.P.; Smith, J.G. Directed evolution of mutator adenoviruses resistant to antibody neutralization. J. Virol. 2013, 87, 6047–6050. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.; Sali, A. Protein structure modeling with MODELLER. Methods Mol. Biol. 2014, 1137, 1–15. [Google Scholar] [PubMed]
- Zhang, Q.; Su, X.; Seto, D.; Zheng, B.J.; Tian, X.; Sheng, H.; Li, H.; Wang, Y.; Zhou, R. Construction and characterization of a replication-competent human adenovirus type 3-based vector as a live-vaccine candidate and a viral delivery vector. Vaccine 2009, 27, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Nian, Q.G.; Zhang, Y.; Xu, L.J.; Hu, Y.; Li, J.; Deng, Y.Q.; Zhu, S.Y.; Wu, X.Y.; Qin, E.D.; et al. In vitro characterization of human adenovirus type 55 in comparison with its parental adenoviruses, types 11 and 14. PLoS ONE 2014, 9, e100665. [Google Scholar] [CrossRef] [PubMed]
- Matthews, Q.L. Capsid-incorporation of antigens into adenovirus capsid proteins for a vaccine approach. Mol. Pharm. 2011, 8, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Su, X.; Li, X.; Li, H.; Li, T.; Zhou, Z.; Zhong, T.; Zhou, R. Protection against enterovirus 71 with neutralizing epitope incorporation within adenovirus type 3 hexon. PLoS ONE 2012, 7, e41381. [Google Scholar] [CrossRef] [PubMed]
- Zhong, T.; Li, X.; Zhou, Z.; Li, T.; Tian, X.; Zhou, R. Characterization of malleability and immunological properties of human adenovirus type 3 hexon hypervariable region 1. Arch. Virol. 2012, 157, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, K.; Matsuzaki, Y.; Hongo, S.; Ohmi, A.; Okamoto, M.; Nishimura, H.; Itagaki, T.; Katsushima, N.; Oshitani, H.; Suzuki, A.; et al. Stability of the seven hexon hypervariable region sequences of adenovirus types 1–6 isolated in Yamagata, Japan between 1988 and 2007. Virus Res. 2009, 140, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Biere, B.; Schweiger, B. Human adenoviruses in respiratory infections: Sequencing of the hexon hypervariable region reveals high sequence variability. J. Clin. Virol. 2010, 47, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Seto, D.; Zhao, S.; Zhu, L.; Zhao, W.; Wan, C. Genome sequence of the first human adenovirus type 14 isolated in China. J. Virol. 2012, 86, 7019–7020. [Google Scholar] [CrossRef] [PubMed]
- Blast: Basic Local Alignment Search Tool. Available online: http://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 1 September 2015).
- RCSB PDB-Advanced Search. Available online: http://www.rcsb.org/pdb/search/advSearch.do?st=SequenceQuery. (accessed on 1 September 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, X.; Ma, Q.; Jiang, Z.; Huang, J.; Liu, Q.; Lu, X.; Luo, Q.; Zhou, R. Identification and Application of Neutralizing Epitopes of Human Adenovirus Type 55 Hexon Protein. Viruses 2015, 7, 5632-5642. https://doi.org/10.3390/v7102896
Tian X, Ma Q, Jiang Z, Huang J, Liu Q, Lu X, Luo Q, Zhou R. Identification and Application of Neutralizing Epitopes of Human Adenovirus Type 55 Hexon Protein. Viruses. 2015; 7(10):5632-5642. https://doi.org/10.3390/v7102896
Chicago/Turabian StyleTian, Xingui, Qiang Ma, Zaixue Jiang, Junfeng Huang, Qian Liu, Xiaomei Lu, Qingming Luo, and Rong Zhou. 2015. "Identification and Application of Neutralizing Epitopes of Human Adenovirus Type 55 Hexon Protein" Viruses 7, no. 10: 5632-5642. https://doi.org/10.3390/v7102896




