Molecular Characterization and Phylogenetic Analysis of New Variants of the Porcine Epidemic Diarrhea Virus in Gansu, China in 2012
Abstract
:1. Introduction
2. Results and Discussion
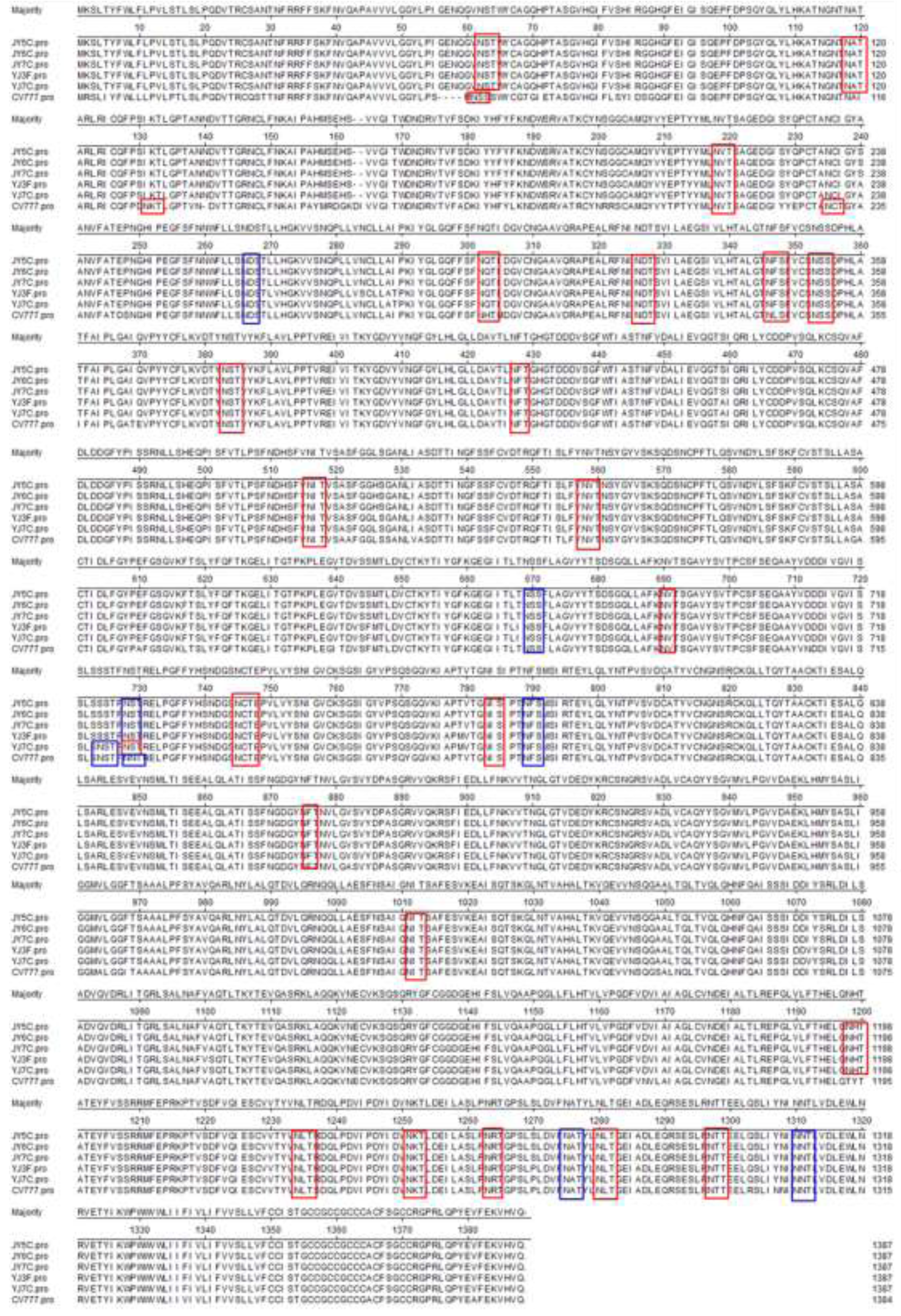
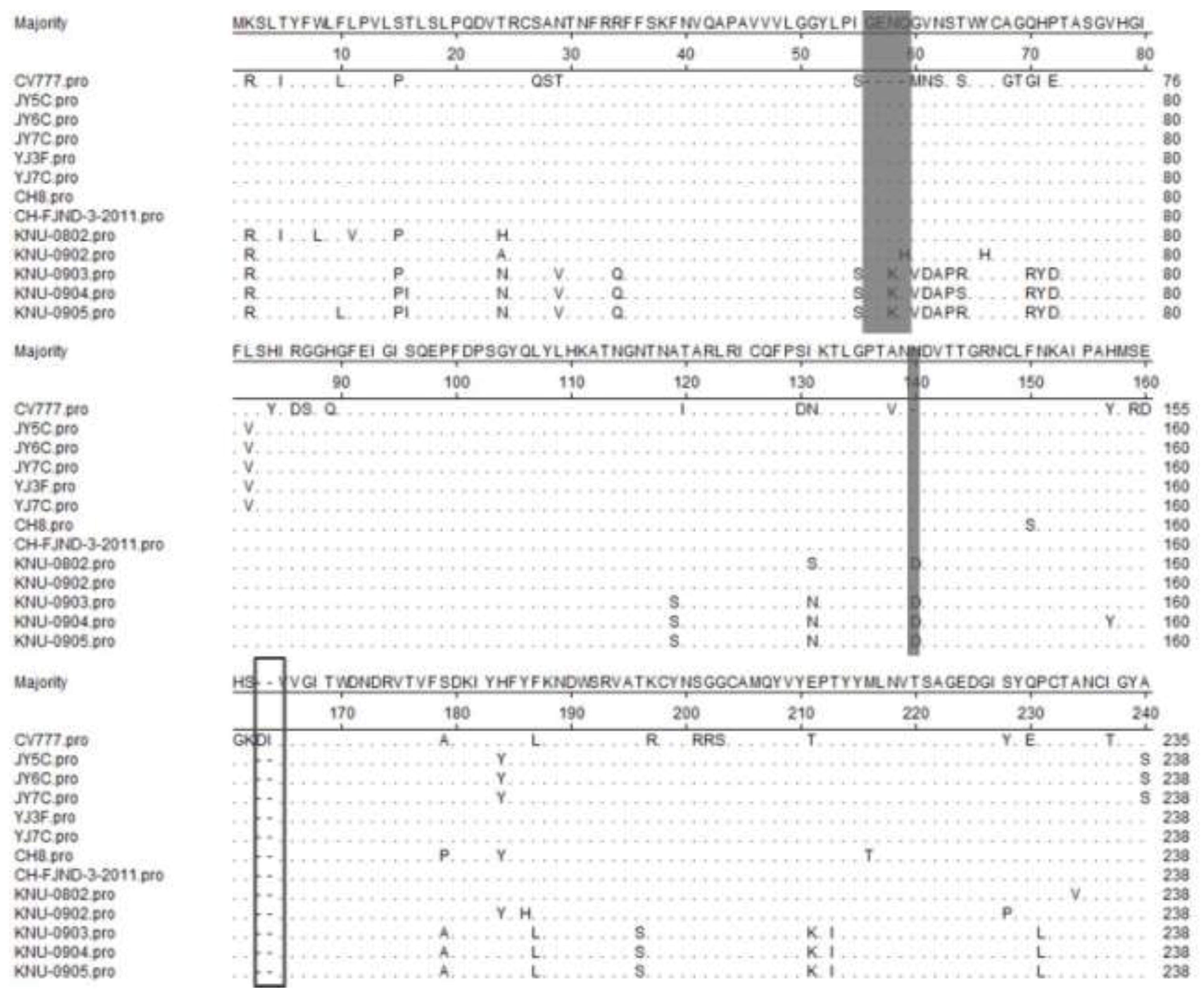
2.1. Sequence Analysis of the S Gene
2.2. Nucleotide and Deduced Amino Acid Sequence Homology
| Virus strian | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 JY5C | 100.0 | 100.0 | 99.3 | 99.3 | 97.6 | 95.1 | 93.4 | 93.4 | 95.1 | 95.2 | 94.4 | 98.4 | 97.7 | 94.6 | 95.3 | 97.5 | 95.0 | 93.3 | 94.9 | 95.7 | 93.4 | 95.1 | 97.1 | 95.9 | 95.0 | 95.8 | 94.7 | 96.5 | 93.8 | 93.2 | 93.6 | 93.2 | 94.5 | 94.3 | 93.8 | 93.7 | |
| 2 JY6C | 100.0 | 100.0 | 99.3 | 99.3 | 97.6 | 95.1 | 93.4 | 93.4 | 95.1 | 95.2 | 94.4 | 98.4 | 97.7 | 94.6 | 95.3 | 97.5 | 95.0 | 93.3 | 94.9 | 95.7 | 93.4 | 95.1 | 97.1 | 95.9 | 95.0 | 95.8 | 94.7 | 96.5 | 93.8 | 93.2 | 93.6 | 93.2 | 94.5 | 94.3 | 93.8 | 93.7 | |
| 3 JY7C | 100.0 | 100.0 | 99.3 | 99.3 | 97.6 | 95.1 | 93.4 | 93.4 | 95.1 | 95.2 | 94.4 | 98.4 | 97.7 | 94.6 | 95.3 | 97.5 | 95.0 | 93.3 | 94.9 | 95.7 | 93.4 | 95.1 | 97.1 | 95.9 | 95.0 | 95.8 | 94.7 | 96.5 | 93.8 | 93.2 | 93.6 | 93.2 | 94.5 | 94.3 | 93.8 | 93.7 | |
| 4 YJ3F | 99.0 | 99.0 | 99.0 | 100.0 | 97.6 | 95.1 | 93.5 | 93.5 | 95.1 | 95.2 | 94.4 | 98.4 | 97.7 | 94.7 | 95.3 | 97.5 | 95.0 | 93.3 | 94.9 | 95.6 | 93.5 | 95.2 | 97.0 | 95.8 | 94.9 | 95.7 | 94.7 | 96.7 | 93.8 | 93.3 | 93.7 | 93.2 | 94.6 | 94.2 | 93.8 | 93.8 | |
| 5 YJ7C | 99.0 | 99.0 | 99.0 | 99.9 | 97.6 | 95.1 | 93.6 | 93.5 | 95.1 | 95.2 | 94.4 | 98.4 | 97.7 | 94.7 | 95.4 | 97.6 | 95.0 | 93.4 | 94.9 | 95.6 | 93.6 | 95.2 | 97.0 | 95.9 | 95.0 | 95.8 | 94.8 | 96.7 | 93.9 | 93.4 | 93.7 | 93.2 | 94.6 | 94.3 | 93.9 | 93.8 | |
| 6 CH1 | 97.9 | 97.9 | 97.9 | 97.6 | 97.6 | 94.5 | 93.4 | 93.3 | 94.5 | 94.6 | 95.8 | 98.4 | 98.5 | 94.2 | 94.9 | 97.1 | 94.6 | 92.9 | 94.5 | 94.9 | 93.0 | 94.8 | 96.5 | 95.4 | 94.8 | 95.3 | 94.5 | 96.2 | 93.5 | 93.2 | 93.3 | 93.1 | 94.3 | 94.1 | 93.4 | 93.4 | |
| 7 CH2 | 95.1 | 95.1 | 95.1 | 94.9 | 94.9 | 94.6 | 95.8 | 95.8 | 99.5 | 99.6 | 97.6 | 95.3 | 94.0 | 96.8 | 95.9 | 94.4 | 97.3 | 95.2 | 97.2 | 98.4 | 95.6 | 93.1 | 93.9 | 93.8 | 92.9 | 93.8 | 92.8 | 93.6 | 95.8 | 95.6 | 92.6 | 95.2 | 93.0 | 92.7 | 95.7 | 95.6 | |
| 8 CH3 | 92.8 | 92.8 | 92.8 | 92.8 | 93.0 | 92.9 | 95.3 | 99.7 | 95.8 | 96.0 | 95.8 | 93.7 | 93.1 | 97.6 | 96.6 | 94.4 | 96.0 | 96.2 | 95.9 | 96.3 | 96.6 | 94.0 | 93.4 | 93.5 | 93.0 | 93.4 | 94.2 | 93.6 | 96.8 | 99.5 | 93.8 | 97.6 | 94.2 | 94.2 | 96.8 | 96.7 | |
| 9 CH4 | 92.8 | 92.8 | 92.8 | 92.8 | 93.0 | 92.9 | 95.3 | 99.4 | 95.8 | 95.9 | 95.7 | 93.6 | 93.0 | 97.5 | 96.6 | 94.3 | 95.9 | 96.2 | 95.8 | 96.2 | 96.6 | 94.0 | 93.3 | 93.4 | 92.9 | 93.3 | 94.1 | 93.5 | 96.8 | 99.4 | 93.7 | 97.5 | 94.2 | 94.1 | 96.8 | 96.7 | |
| 10 CH5 | 94.6 | 94.6 | 94.6 | 94.5 | 94.5 | 94.1 | 99.1 | 94.9 | 94.9 | 99.6 | 97.6 | 95.3 | 94.0 | 96.7 | 95.9 | 94.3 | 97.3 | 95.2 | 97.2 | 98.4 | 95.6 | 93.1 | 93.9 | 93.8 | 92.9 | 93.8 | 92.8 | 93.6 | 95.8 | 95.6 | 92.6 | 95.2 | 93.0 | 92.7 | 95.7 | 95.6 | |
| 11 CH6 | 95.1 | 95.1 | 95.1 | 94.9 | 94.9 | 94.6 | 99.6 | 95.4 | 95.4 | 99.1 | 97.7 | 95.4 | 94.1 | 96.9 | 96.1 | 94.5 | 97.4 | 95.4 | 97.3 | 98.5 | 95.7 | 93.3 | 94.0 | 93.9 | 93.0 | 93.9 | 93.0 | 93.8 | 96.0 | 95.7 | 92.8 | 95.3 | 93.2 | 92.9 | 95.9 | 95.8 | |
| 12 CH7 | 94.6 | 94.6 | 94.6 | 94.3 | 94.3 | 95.6 | 97.5 | 95.3 | 95.3 | 97.0 | 97.5 | 94.7 | 95.1 | 96.5 | 95.6 | 94.0 | 97.2 | 94.9 | 97.0 | 97.8 | 95.3 | 92.8 | 93.5 | 93.5 | 92.9 | 93.5 | 92.6 | 93.4 | 95.4 | 95.5 | 92.3 | 95.1 | 92.7 | 92.4 | 95.4 | 95.3 | |
| 13 CH8 | 98.1 | 98.1 | 98.1 | 98.0 | 98.0 | 98.6 | 95.0 | 92.9 | 92.9 | 94.6 | 95.0 | 94.6 | 97.5 | 94.6 | 95.3 | 97.4 | 94.9 | 93.3 | 94.8 | 95.6 | 93.4 | 95.2 | 96.9 | 95.8 | 95.0 | 95.7 | 94.8 | 96.5 | 93.8 | 93.5 | 93.5 | 93.4 | 94.7 | 94.5 | 93.8 | 93.7 | |
| 14 CHGD01 | 98.1 | 98.1 | 98.1 | 97.9 | 97.9 | 98.2 | 93.9 | 92.7 | 92.7 | 93.5 | 93.9 | 94.6 | 97.4 | 94.3 | 95.0 | 97.1 | 94.4 | 92.9 | 94.4 | 94.7 | 92.9 | 94.6 | 96.5 | 95.3 | 94.5 | 95.3 | 94.4 | 96.3 | 93.4 | 92.9 | 93.4 | 92.9 | 94.2 | 93.9 | 93.4 | 93.4 | |
| 15 CH-FJND-1-2011 | 93.8 | 93.8 | 93.8 | 94.0 | 94.1 | 93.6 | 96.0 | 97.3 | 97.2 | 95.6 | 96.1 | 95.7 | 93.8 | 93.7 | 98.9 | 96.7 | 96.8 | 95.9 | 96.7 | 97.3 | 96.0 | 93.2 | 94.2 | 94.2 | 93.2 | 94.1 | 93.3 | 94.1 | 96.4 | 97.3 | 93.1 | 96.1 | 93.4 | 93.3 | 96.4 | 96.3 | |
| 16 CH-FJND-2-2011 | 95.1 | 95.1 | 95.1 | 95.2 | 95.4 | 94.9 | 95.0 | 96.1 | 96.0 | 94.6 | 95.1 | 94.7 | 94.8 | 94.9 | 98.6 | 97.5 | 96.1 | 95.2 | 96.0 | 96.5 | 95.3 | 93.6 | 94.9 | 94.5 | 93.6 | 94.5 | 93.7 | 94.7 | 95.7 | 96.4 | 93.4 | 95.3 | 93.8 | 93.5 | 95.8 | 95.7 | |
| 17 CH-FJND-3-2011 | 97.0 | 97.0 | 97.0 | 97.1 | 97.3 | 96.8 | 93.8 | 93.8 | 93.8 | 93.3 | 93.8 | 93.4 | 96.7 | 96.6 | 96.3 | 97.5 | 94.5 | 93.6 | 94.5 | 94.9 | 93.7 | 95.1 | 96.9 | 95.9 | 95.0 | 95.8 | 95.1 | 96.5 | 94.1 | 94.2 | 93.9 | 93.7 | 94.9 | 94.8 | 94.1 | 94.1 | |
| 18 DX | 95.3 | 95.3 | 95.3 | 95.2 | 95.2 | 94.8 | 97.2 | 95.4 | 95.4 | 96.9 | 97.2 | 97.0 | 95.0 | 94.6 | 96.5 | 95.5 | 94.4 | 95.5 | 99.1 | 98.1 | 95.9 | 93.2 | 94.2 | 94.3 | 93.4 | 94.2 | 93.1 | 94.0 | 96.0 | 95.7 | 93.0 | 95.3 | 93.2 | 93.0 | 96.0 | 95.9 | |
| 19 LZC | 92.8 | 92.8 | 92.8 | 92.6 | 92.8 | 92.3 | 94.8 | 94.9 | 94.9 | 94.4 | 95.0 | 94.6 | 92.3 | 92.6 | 94.9 | 94.2 | 92.7 | 94.8 | 95.4 | 95.9 | 95.8 | 93.4 | 93.1 | 93.2 | 93.0 | 93.2 | 93.5 | 93.5 | 99.4 | 96.0 | 93.8 | 95.5 | 93.7 | 93.3 | 99.5 | 99.4 | |
| 20 LJB-03 | 94.9 | 94.9 | 94.9 | 94.8 | 94.8 | 94.4 | 96.9 | 95.2 | 95.2 | 96.6 | 96.9 | 96.7 | 94.6 | 94.3 | 96.0 | 95.0 | 93.8 | 98.6 | 94.5 | 98.0 | 95.8 | 93.2 | 94.2 | 94.1 | 93.3 | 94.1 | 93.1 | 93.9 | 96.0 | 95.6 | 92.9 | 95.2 | 93.1 | 92.9 | 96.0 | 95.9 | |
| 21 JS-2004-2 | 95.7 | 95.7 | 95.7 | 95.5 | 95.5 | 94.9 | 98.0 | 95.8 | 95.8 | 97.6 | 98.0 | 97.6 | 95.3 | 94.7 | 96.7 | 95.7 | 94.4 | 98.0 | 95.4 | 97.8 | 96.2 | 93.6 | 94.7 | 94.6 | 93.7 | 94.6 | 93.3 | 94.4 | 96.4 | 96.1 | 93.1 | 95.7 | 93.4 | 93.2 | 96.4 | 96.3 | |
| 22 CHS | 93.3 | 93.3 | 93.3 | 93.3 | 93.5 | 93.0 | 95.4 | 96.1 | 96.1 | 95.2 | 95.5 | 95.2 | 93.1 | 93.0 | 95.6 | 94.6 | 93.3 | 95.7 | 95.2 | 95.4 | 96.1 | 94.0 | 93.9 | 93.9 | 93.4 | 93.8 | 93.7 | 93.7 | 96.3 | 96.3 | 93.5 | 95.8 | 94.0 | 93.4 | 96.4 | 96.3 | |
| 23 KUN-0901 | 94.7 | 94.7 | 94.7 | 94.7 | 94.8 | 94.4 | 92.9 | 93.4 | 93.3 | 92.5 | 93.1 | 92.5 | 94.7 | 94.4 | 92.7 | 93.5 | 94.5 | 93.0 | 92.8 | 92.7 | 93.3 | 93.6 | 95.4 | 95.5 | 95.7 | 95.4 | 97.9 | 95.8 | 93.9 | 93.7 | 96.0 | 93.4 | 96.7 | 94.7 | 93.9 | 93.9 | |
| 24 KUN-0902 | 96.4 | 96.4 | 96.4 | 96.1 | 96.3 | 95.9 | 93.1 | 92.4 | 92.4 | 92.9 | 93.1 | 92.8 | 96.0 | 95.9 | 93.0 | 94.2 | 96.0 | 93.6 | 92.3 | 93.2 | 94.1 | 93.5 | 94.5 | 97.8 | 95.3 | 97.6 | 94.9 | 96.5 | 93.6 | 93.1 | 93.8 | 92.9 | 94.6 | 94.3 | 93.6 | 93.6 | |
| 25 KUN-0903 | 95.0 | 95.0 | 95.0 | 94.8 | 95.0 | 94.4 | 93.1 | 92.6 | 92.5 | 92.8 | 93.3 | 93.1 | 94.7 | 94.4 | 93.1 | 94.1 | 94.8 | 93.6 | 92.3 | 93.1 | 94.1 | 93.5 | 95.1 | 96.5 | 96.8 | 99.8 | 95.1 | 95.8 | 93.7 | 93.2 | 94.1 | 92.9 | 95.1 | 94.6 | 93.8 | 93.7 | |
| 26 KUN-0904 | 94.1 | 94.1 | 94.1 | 94.0 | 94.2 | 93.9 | 92.5 | 92.0 | 92.0 | 92.1 | 92.8 | 92.4 | 93.9 | 93.8 | 92.3 | 93.3 | 93.9 | 92.8 | 91.9 | 92.5 | 93.1 | 92.8 | 95.3 | 94.0 | 96.5 | 96.7 | 95.1 | 96.2 | 93.5 | 92.7 | 93.9 | 92.3 | 95.0 | 94.2 | 93.5 | 93.5 | |
| 27 KUN-0905 | 94.7 | 94.7 | 94.7 | 94.6 | 94.7 | 94.2 | 93.1 | 92.4 | 92.3 | 92.8 | 93.2 | 93.0 | 94.4 | 94.2 | 92.9 | 93.8 | 94.6 | 93.4 | 92.2 | 92.9 | 94.0 | 93.3 | 95.0 | 96.2 | 99.6 | 96.4 | 95.0 | 95.7 | 93.6 | 93.1 | 94.0 | 92.9 | 94.9 | 94.5 | 93.7 | 93.6 | |
| 28 KUN-0801 | 94.1 | 94.1 | 94.1 | 94.1 | 94.2 | 94.2 | 92.3 | 93.6 | 93.5 | 91.9 | 92.5 | 92.2 | 94.2 | 94.2 | 92.8 | 93.6 | 94.5 | 92.8 | 92.7 | 92.4 | 93.0 | 93.3 | 97.9 | 94.1 | 94.7 | 94.5 | 94.4 | 95.4 | 94.1 | 93.9 | 97.8 | 93.6 | 97.9 | 95.0 | 94.1 | 94.0 | |
| 29 KUN-0802 | 96.6 | 96.6 | 96.6 | 96.6 | 96.8 | 96.2 | 93.5 | 92.8 | 92.8 | 93.1 | 93.6 | 93.2 | 96.3 | 96.5 | 93.6 | 94.7 | 96.2 | 93.8 | 92.7 | 93.4 | 94.2 | 93.4 | 95.4 | 96.4 | 95.5 | 94.9 | 95.2 | 95.5 | 94.0 | 93.3 | 94.2 | 93.0 | 95.1 | 94.6 | 94.1 | 94.0 | |
| 30 Parent DR13 | 93.7 | 93.7 | 93.7 | 93.6 | 93.7 | 93.3 | 95.7 | 95.9 | 95.9 | 95.4 | 96.0 | 95.4 | 93.3 | 93.5 | 95.7 | 95.0 | 93.5 | 95.7 | 98.9 | 95.4 | 96.2 | 96.0 | 93.6 | 93.1 | 93.1 | 92.7 | 93.0 | 93.6 | 93.5 | 96.6 | 94.3 | 96.0 | 94.2 | 93.9 | 99.9 | 99.8 | |
| 31 Attenuated DR13 | 92.8 | 92.8 | 92.8 | 92.8 | 93.0 | 92.8 | 95.2 | 99.2 | 99.1 | 94.8 | 95.3 | 95.2 | 92.8 | 92.6 | 97.0 | 95.9 | 93.7 | 95.3 | 94.8 | 95.0 | 95.7 | 95.8 | 93.1 | 92.3 | 92.5 | 92.0 | 92.3 | 93.3 | 92.8 | 95.7 | 93.5 | 97.3 | 94.0 | 93.9 | 96.5 | 96.4 | |
| 32 Chinju99 | 92.5 | 92.5 | 92.5 | 92.5 | 92.6 | 92.3 | 90.9 | 92.0 | 92.0 | 90.5 | 91.2 | 90.9 | 92.3 | 92.5 | 91.6 | 92.2 | 92.8 | 91.5 | 92.0 | 91.2 | 91.8 | 92.0 | 95.4 | 92.5 | 92.8 | 92.7 | 92.6 | 97.3 | 93.6 | 92.8 | 91.9 | 93.3 | 96.3 | 93.8 | 94.4 | 94.4 | |
| 33 MK | 93.4 | 93.4 | 93.4 | 93.1 | 93.3 | 93.5 | 94.9 | 96.6 | 96.5 | 94.5 | 94.9 | 95.1 | 93.3 | 93.3 | 95.4 | 94.7 | 93.5 | 95.2 | 94.8 | 94.9 | 95.5 | 95.5 | 93.3 | 93.1 | 92.9 | 92.2 | 92.7 | 93.8 | 93.4 | 95.6 | 96.3 | 92.5 | 93.8 | 93.7 | 96.1 | 96.0 | |
| 34 NK | 93.9 | 93.9 | 93.9 | 94.1 | 94.2 | 93.9 | 92.8 | 93.7 | 93.6 | 92.3 | 93.0 | 92.6 | 94.0 | 93.8 | 93.0 | 93.7 | 94.2 | 93.1 | 92.9 | 92.7 | 93.5 | 93.8 | 96.3 | 93.7 | 94.4 | 94.2 | 94.2 | 97.5 | 94.7 | 93.8 | 93.5 | 95.5 | 94.1 | 95.2 | 94.2 | 94.2 | |
| 35 KH | 93.9 | 93.9 | 93.9 | 93.7 | 93.9 | 93.8 | 92.7 | 93.1 | 93.0 | 92.3 | 92.8 | 92.3 | 94.0 | 93.7 | 92.3 | 93.2 | 93.8 | 92.7 | 92.3 | 92.3 | 92.9 | 93.1 | 94.2 | 93.4 | 94.1 | 93.4 | 93.9 | 94.6 | 94.1 | 93.3 | 92.9 | 92.8 | 93.7 | 94.9 | 93.8 | 93.8 | |
| 36 CV777 | 93.7 | 93.7 | 93.7 | 93.6 | 93.7 | 93.3 | 95.7 | 95.9 | 95.9 | 95.3 | 95.9 | 95.4 | 93.3 | 93.6 | 95.9 | 95.2 | 93.6 | 95.7 | 99.1 | 95.4 | 96.2 | 96.2 | 93.7 | 93.2 | 93.2 | 92.8 | 93.1 | 93.6 | 93.6 | 99.7 | 95.7 | 93.0 | 95.7 | 93.8 | 93.2 | 99.9 | |
| 37 Br1-87 | 93.6 | 93.6 | 93.6 | 93.4 | 93.6 | 93.1 | 95.4 | 95.6 | 95.6 | 95.1 | 95.6 | 95.2 | 93.1 | 93.4 | 95.6 | 94.9 | 93.5 | 95.4 | 98.8 | 95.2 | 96.0 | 95.9 | 93.6 | 93.1 | 93.1 | 92.7 | 93.0 | 93.5 | 93.5 | 99.4 | 95.4 | 93.0 | 95.4 | 93.8 | 93.1 | 99.7 |
| The other strains | Nucleotide identities | Deduced amino acid identities |
|---|---|---|
| CH8 (one Chinese PEDV strain) | 98.40% | 98.0%-98.1% |
| CV777 vaccine strain | 93.8%–93.9% | 93.6%–93.7% |
| Previous domestic strains (DX, LZC, LJB-03, JS-2004-2, and CHS) | 93.3%–95.7% | 92.6%–95.7% |
| Japanese strains (MK, NK, and KH) | 93.2%–94.6% | 93.1%–94.2% |
| European strain (Br1-87) | 93.7%–93.8% | 93.4%–93.6% |
| South Korean strains (KNU-0801, KNU-0802, KNU-0901, KNU-0902, KNU-0903, KNU-0904, and KNU-0905) | 94.7%–97.1% | 94.1%–96.8% |
2.3. Phylogenetic Analysis of the S Gene
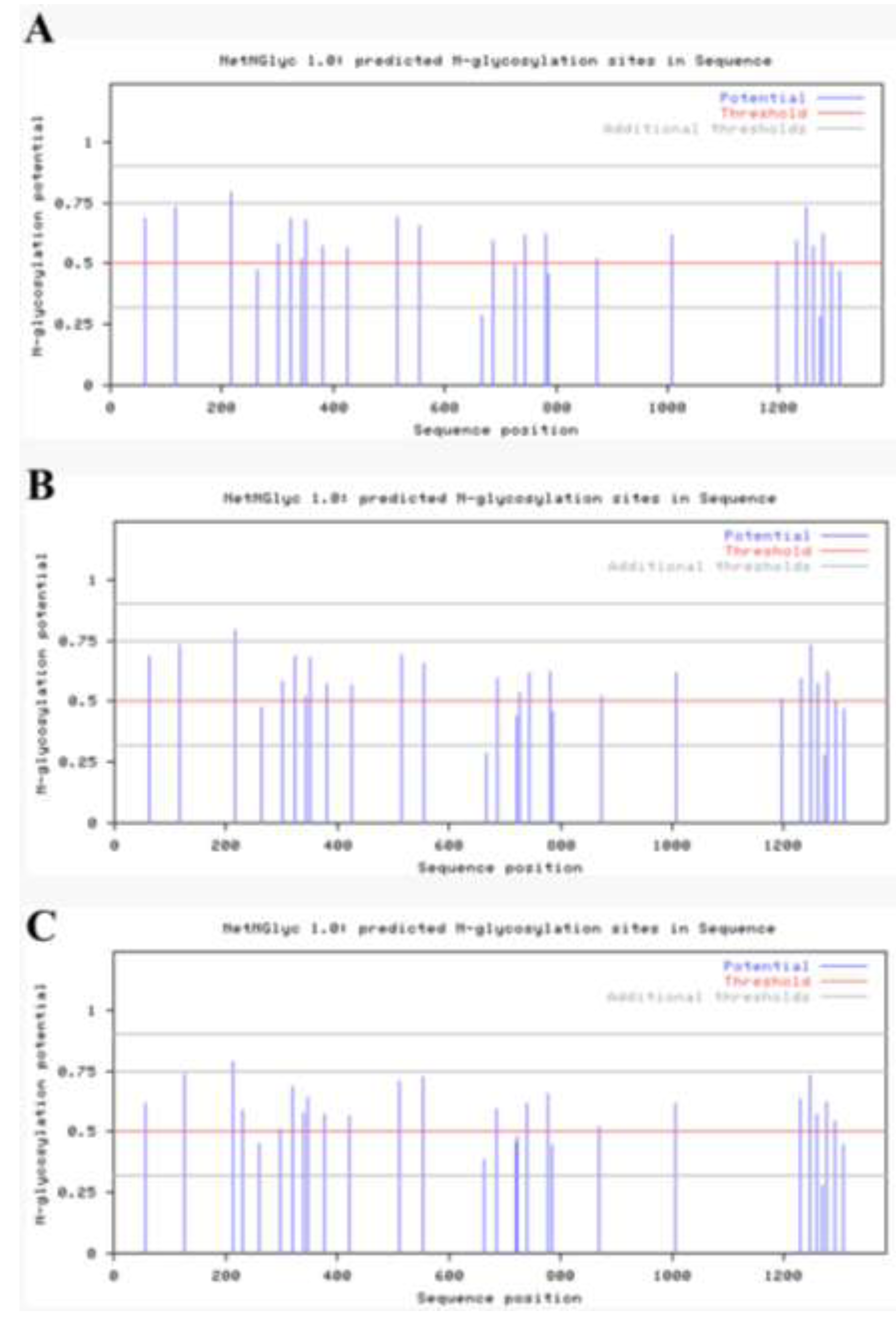
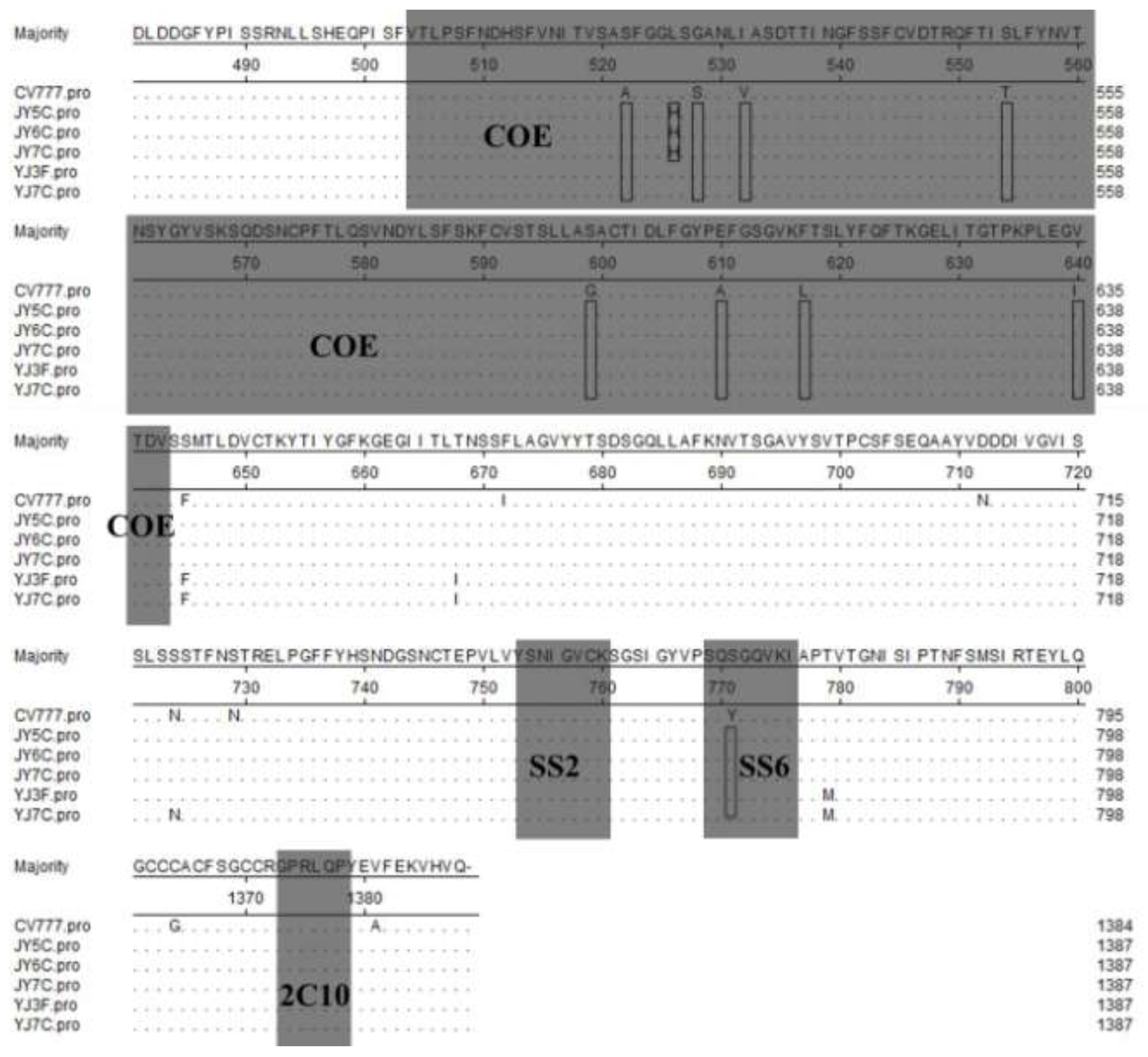
2.4. Antigenicity Analysis of the S Gene

2.5. Discussion
3. Experimental
3.1. Sample Collection
| Farm | No. of sows | Vaccination a | Illness rate (%/y) | Mortality rate (%) |
|---|---|---|---|---|
| (Yongjing Tai Chi Breed Co., Ltd.) YJ | 400 | Yes | 80 | 60 |
| (Hoggery of Science and Technology Breed Park of Jiugang Hongfeng Company) JY | 2000 | Yes | 80 | 60 |
3.2. RNA Extraction and Reverse Transcription
| Primers | Nucleotide sequence, 5'→3' | Primer location b | Length (bp) c |
|---|---|---|---|
| PEDVS1-P1 | CCATTAGTGATGTTGTGTTAG | 20, 535–20, 555 | 1031 |
| PEDVS1-P2 | GCACAGCAGCTCCATT | 21, 565–21, 550 | |
| PEDVS2-P1 | CCACATACCAGAAGGTTTTAG | 21, 372–21, 392 | 1146 |
| PEDVS2-P2 | CCAGTAATCAACTCACCCTT | 22, 517–22, 498 | |
| PEDVS3-P1 | CCCTGAGTTTGGTAGTGG | 22, 446–22463 | 1154 |
| PEDVS3-P2 | CATCCGTCTGTAGAGCAAG | 23, 599–23, 581 | |
| PEDVS4-P1 | CTCATCGGTGGTATGGTGCT | 23, 497–23, 516 | 1355 |
| PEDVS4-P2 | AGCAGACTTTGAGACATCTTTGAC | 24, 851–24, 828 |
3.3. Sequence Analysis
| Virus strain | GenBank accession No. | Country and year of isolation |
|---|---|---|
| JY5C | KF177254 | China 2012 |
| JY6C | KF177255 | China 2012 |
| JY7C | KF177256 | China 2012 |
| YJ3F | KF177257 | China 2012 |
| YJ7C | KF177258 | China 2012 |
| CH1 | JQ239429 | China 2011 |
| CH2 | JQ239430 | China 2011 |
| CH3 | JQ239431 | China 2011 |
| CH4 | JQ239432 | China 2011 |
| CH5 | JQ239433 | China 2011 |
| CH6 | JQ239434 | China 2011 |
| CH7 | JQ239435 | China 2011 |
| CH8 | JQ239436 | China 2011 |
| CHGD01 | JN980698 | China 2011 |
| CH-FJND-1-2011 | JN543367.1 | China 2011 |
| CH-FJND-2-2011 | JN315706.1 | China 2011 |
| CH-FJND-3-2011 | JN381492.1 | China 2011 |
| DX | EU031893 | China 2007 |
| LZC | EF185992 | China 2006 |
| LJB-03 | DQ985739 | China 2006 |
| JS-2004-2 | AY653204 | China 2004 |
| CHS | JN547228.1 | China 1986 |
| KUN-0901 | GU180144 | South Korea 2009 |
| KUN-0902 | GU180145 | South Korea 2009 |
| KUN-0903 | GU180146 | South Korea 2009 |
| KUN-0904 | GU180147 | South Korea 2009 |
| KUN-0905 | GU180148 | South Korea 2009 |
| KUN-0801 | GU180142 | South Korea 2008 |
| KUN-0802 | GU180143 | South Korea 2008 |
| Parent DR13 | DQ862099 | South Korea 2006 |
| Attenuated DR13 | DQ462404.2 | South Korea 2006 |
| Chinju99 | AY167585 | South Korea 1999 |
| MK | AB548624.1 | Japan 1996 |
| NK | AB548623.1 | Japan |
| KH | AB548622.1 | Japan |
| CV777 | AF353511.1 | Belgium 1988 |
| Br1-87 | Z25483 | Great Britain 1993 |
| PUR46-MAD | M94101 | USA 1992 |
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Chen, J.; Wang, C.; Shi, H.; Qiu, H.; Liu, S.; Chen, X.; Zhang, Z.; Feng, L. Molecular epidemiology of porcine epidemic diarrhea virus in China. Arch. Virol. 2010, 155, 1471–1476. [Google Scholar] [CrossRef]
- Song, D.; Park, B. Porcine epidemic diarrhoea virus: A comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes 2012, 44, 167–175. [Google Scholar] [CrossRef]
- Chang, S.H.; Bae, J.L.; Kang, T.J.; Kim, J.; Chung, G.H.; Lim, C.W.; Laude, H; Yang, M.S.; Jang, Y.S. Identification of the epitope region capable of inducing neutralizing antibodies against the porcine epidemic diarrhea virus. Mol. Cells 2002, 14, 295–299. [Google Scholar]
- Cruz, D.J.; Kim, C.J.; Shin, H.J. The GPRLQPY motif located at the carboxy-terminal of the spike protein induces antibodies that neutralize Porcine epidemic diarrhea virus. Virus Res. 2008, 132, 192–196. [Google Scholar] [CrossRef]
- Sun, D.; Feng, L.; Shi, H.; Chen, J.; Cui, X.; Chen, H.; Liu, S.; Tong, Y.; Wang, Y.; Tong, G. Identification of two novel B cell epitopes on porcine epidemic diarrhea virus spike protein. Vet. Microbiol. 2008, 131, 73–81. [Google Scholar] [CrossRef]
- Bosch, B.J.; van der Zee, R.; de Haan, C.A.; Rottier, P.J. The coronavirus spike protein is a class I virus fusion protein: Structural and functional characterization of the fusion core complex. J. Virol. 2003, 77, 8801–8811. [Google Scholar] [CrossRef]
- Chen, X.; Yang, J.; Yu, F.; Ge, J.; Lin, T.; Song, T. Molecular characterization and phylogenetic analysis of porcine epidemic diarrhea virus (PEDV) samples from field cases in Fujian, China. Virus Genes 2012, 36, 366–364. [Google Scholar]
- Li, Z.L.; Zhu, L.; Ma, J.Y.; Zhou, Q.F.; Song, Y.H.; Sun, B.L.; Chen, R.A.; Xie, Q.M.; Bee, Y.Z. Molecular characterization and phylogenetic analysis of porcine epidemic diarrhea virus (PEDV) field strains in south China. Virus Genes 2012, 45, 181–185. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Liu, Y.; Pan, Y.; Deng, F.; Song, Y.; Tang, X.; He, Q. New variants of porcine epidemic diarrhea virus, China, 2011. Emerg. Infect. Dis. 2012, 18, 1350–1353. [Google Scholar]
- Wood, E.N. An apparently new syndrome of porcine epidemic diarrhoea. Vet. Rec. 1977, 100, 243–244. [Google Scholar]
- Debouck, P.; Pensaert, M. Experimental infection of pigs with a new porcine enteric coronavirus, CV 777. Am. J. Vet. Res. 1980, 41, 219–223. [Google Scholar]
- Park, S.J.; Kim, H.K.; Song, D.S.; Moon, H.J.; Park, B.K. Molecular characterization and phylogenetic analysis of porcine epidemic diarrhea virus (PEDV) field isolates in Korea. Arch. Virol. 2011, 156, 577–585. [Google Scholar] [CrossRef]
- Chen, J.F.; Sun, D.B.; Wang, C.B.; Shi, H.Y.; Cui, X.C.; Liu, S.W.; Qiu, H. J.; Feng, L. Molecular characterization and phylogenetic analysis of membrane protein genes of porcine epidemic diarrhea virus isolates in China. Virus Genes 2008, 36, 355–364. [Google Scholar] [CrossRef]
- Sueyoshi, M.; Tsuda, T.; Yamazaki, K.; Yoshida, K.; Nakazawa, M.; Sato, K.; Minami, T.; Iwashita, K.; Watanabe, M.; Suzuki, Y.; et al. An immunohistochemical investigation of porcine epidemic diarrhoea. J. Comp. Pathol. 1995, 113, 59–67. [Google Scholar] [CrossRef]
- Puranaveja, S.; Poolperm, P.; Lertwatcharasarakul, P.; Kesdaengsakonwut, S.; Boonsoongnern, A.; Urairong, K.; Kitikoon, P.; Choojai, P.; Kedkovid, R.; Teankum, K.; et al. Chinese-like strain of porcine epidemic diarrhea virus, Thailand. Emerg. Infect. Dis. 2009, 15, 1112–1115. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, J.; Deng, X.; Ye, Y.; Liao, M.; Fan, H. Complete genome sequence of a highly prevalent isolate of porcine epidemic diarrhea virus in South china. J. Virol. 2012, 86. [Google Scholar] [CrossRef]
- Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007, 24, 1596–1599. [Google Scholar] [CrossRef]
- Petersen, T.N.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tian, Y.; Yu, Z.; Cheng, K.; Liu, Y.; Huang, J.; Xin, Y.; Li, Y.; Fan, S.; Wang, T.; Huang, G.; et al. Molecular Characterization and Phylogenetic Analysis of New Variants of the Porcine Epidemic Diarrhea Virus in Gansu, China in 2012. Viruses 2013, 5, 1991-2004. https://doi.org/10.3390/v5081991
Tian Y, Yu Z, Cheng K, Liu Y, Huang J, Xin Y, Li Y, Fan S, Wang T, Huang G, et al. Molecular Characterization and Phylogenetic Analysis of New Variants of the Porcine Epidemic Diarrhea Virus in Gansu, China in 2012. Viruses. 2013; 5(8):1991-2004. https://doi.org/10.3390/v5081991
Chicago/Turabian StyleTian, Yufei, Zhijun Yu, Kaihui Cheng, Yuxiu Liu, Jing Huang, Yue Xin, Yuanguo Li, Shengtao Fan, Tiecheng Wang, Geng Huang, and et al. 2013. "Molecular Characterization and Phylogenetic Analysis of New Variants of the Porcine Epidemic Diarrhea Virus in Gansu, China in 2012" Viruses 5, no. 8: 1991-2004. https://doi.org/10.3390/v5081991




