Analysis of Sequence Polymorphism and Population Structure of Tomato chlorotic dwarf viroid and Potato spindle tuber viroid in Viroid-Infected Tomato Plants
Abstract
:1. Introduction
2. Results and Discussion

| Sequence variant | Size (nt) | Occurrence frequency (%) | Nucleotide difference from the reference sequence a | |
|---|---|---|---|---|
| Plant 1 | Plant 2 | |||
| AF162131 | 360 | n/a | n/a | n/a |
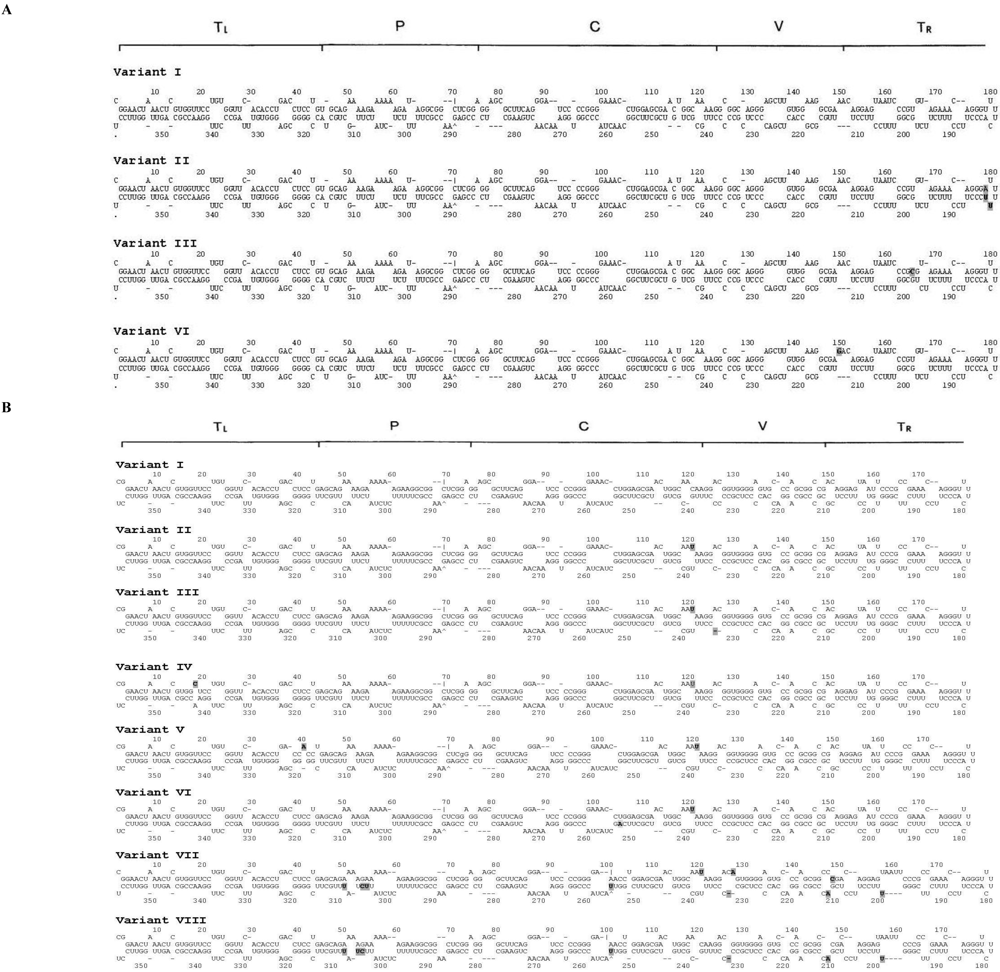
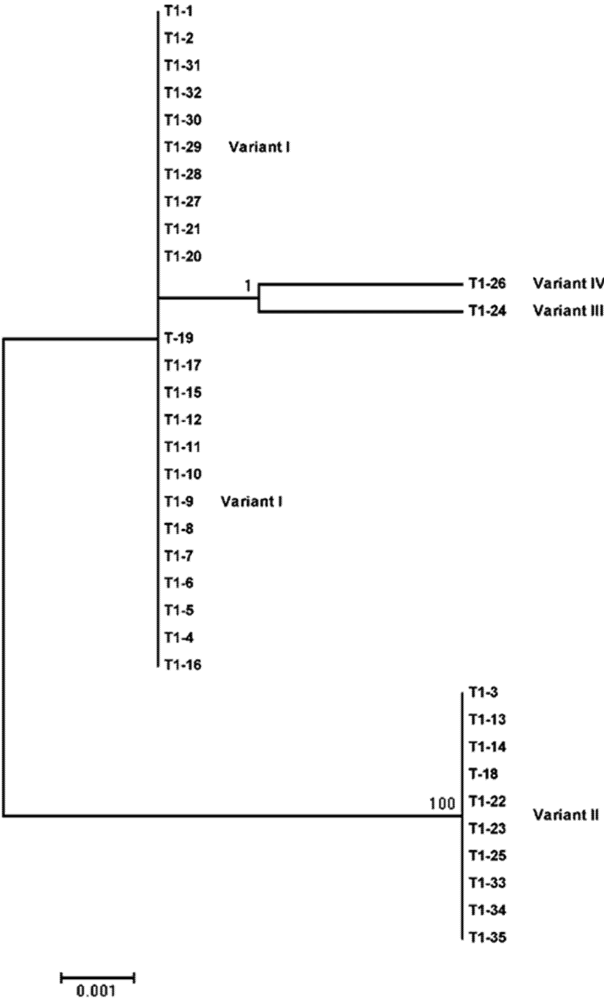
| I | 360 | 66.7 | 65.7 | No variation |
| II | 360 | 33.3 | 28.6 | 178 (U to A); 182 (C to U); 183 (A to U) |
| III | 360 | 0 | 2.9 | 165 (U to C) |
| IV | 360 | 0 | 2.9 | 149 (A to G) |
| Haplotype diversity b | 0.500 ± 0.128 | 0.499 ± 0.071 | ||
| Nucleotide diversity c | 0.00417 ± 0.00107 | 0.00382 ± 0.00057 | ||
| Sequence variant | Size (nt) | Occurrence frequency (%) | Nucleotide difference from the reference sequence a | |
|---|---|---|---|---|
| Plant 1 | Plant 2 | |||
| EF044303 | 358 | n/a | n/a | n/a |
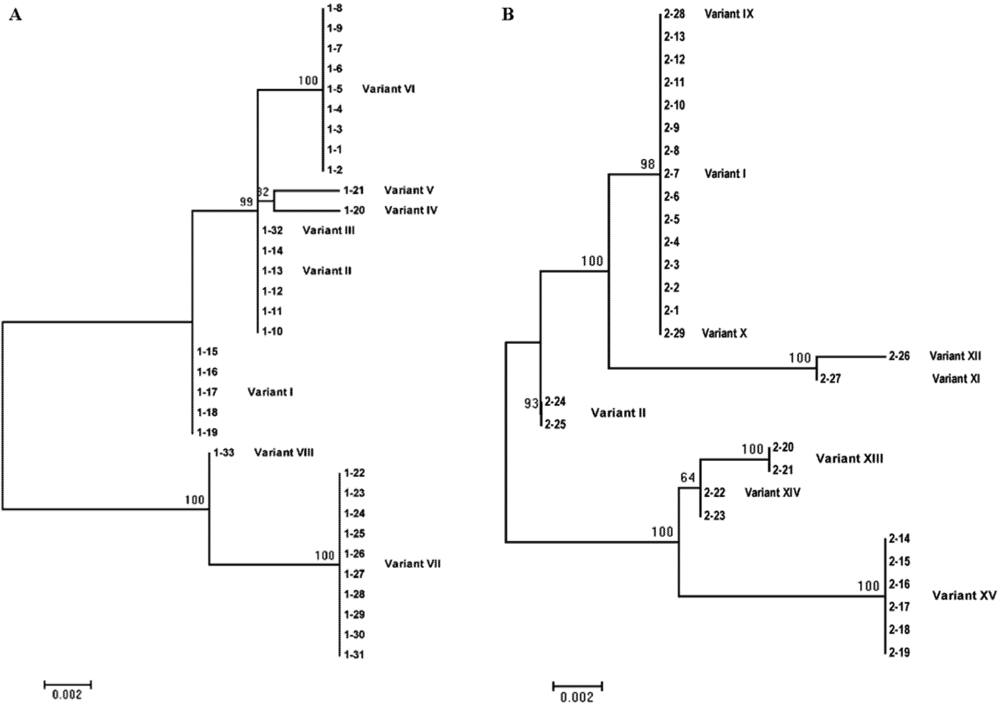
| I | 358 | 15.2 | 44.8 | No variation |
| II | 358 | 15.2 | 6.9 | 120 (C to U) |
| III | 357 | 3.0 | 0 | 120 (C to U), 236 (C to deletion) |
| IV | 358 | 3.0 | 0 | 18 (U to C), 120 (C to U) |
| V | 358 | 3.0 | 0 | 40 (U to A), 120 (C to U) |
| VI | 358 | 27.3 | 0 | 120 (C to U), 253 (G to A) |
| VII | 357 | 30.3 | 0 | 120 (C to U), 127 (G to A), between 145 and 146 (insertion of C), 200 (G to U), 211 (C to deletion), 212 (G to A), 232 (C to deletion), 255 (C to U), 306 (C to U), 307 (U to C), 310 (C to U) |
| VIII | 356 | 3.0 | 0 | 200 (G to U), 211 (C to deletion), 212 (G to A), 232 (C to deletion), 255 (C to U), 306 (C to U), 307 (U to C), 310 ( C to U) |
| IX | 357 | 0 | 3.4 | 277 (G to deletion) |
| X | 359 | 0 | 3.4 | Between 145 and 146 (insertion of C) |
| XI | 358 | 0 | 3.4 | 173 (G to A), 306 (C to U), 307 (U to C), 310 (C to U) |
| XII | 358 | 0 | 3.4 | 306 (C to U), 307 (U to C), 310 (C to U) |
| XIII | 357 | 0 | 6.9 | 120 (C to U), 127 (G to A), between 145 and 146 (insertion of C), 200 (G to U), 211 (C to deletion), 212 (G to A), 232 (C to deletion), 343 (A to G) |
| XIV | 357 | 0 | 6.9 | 120 (C to U), 127 (G to A), between 145 and 146 (insertion of C), 200 (G to U), 211 (C to deletion), 212 (G to A), 232 (C to deletion) |
| XV | 357 | 0 | 20.7 | 120 (C to U), 127 (G to A), between 145 and 146 (insertion of C), 200 (G to U), 211 (C to deletion), 212 (G to A), 232 (C to deletion), 306 (C to U), 307 (U to C), 310 ( C to U) |
| Haplotype diversity b | 0.799 ± 0.034 | 0.697 ± 0.078 | ||
| Nucleotide diversity c | 0.01130 ± 0.00103 | 0.00944 ± 0.00115 | ||
3. Experimental Section
3.1. Viroid Sources
3.2. Total RNA Isolation and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
3.3. Complementary cDNA Cloning and Sequencing
3.4. Phylogeny, Secondary Structure and Nucleotide Diversity Analysis
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Hadidi, A.; Flores, R.; Randles, J.W.; Semancik, J.S. Viroids; CSIRO Publishing: Collingwood, Victoria, Australia, 2003; pp. 1–370. [Google Scholar]
- Hammond, R.W.; Owens, R.A. Viroids: New and continuing risks for horticultural and agricultural crops. APSnet. 2006. Available online: http://www.apsnet.org/publications/apsnetfeatures/Pages/Viroids.aspx (accessed on 14 May 2012).
- Keese, P.; Symons, R.H. Domains in viroids: evidence of intermolecular RNA rearrangements and their contribution to viroid evolution. Proc. Natl. Acad. Sci. U. S. A. 1985, 82, 4582–4586. [Google Scholar]
- Diener, T.O. Potato spindle tuber "virus". IV. A replicating, low molecular weight RNA. Virology 1971, 45, 411–428. [Google Scholar] [CrossRef]
- Singh, R.P.; Finnie, R.F.; Bagnall, R.H. Losses due to the potato spindle tuber virus. Am. Potato J. 1971, 48, 262–267. [Google Scholar]
- Singh, R.P.; Nie, X.; Singh, M. Tomato chlorotic dwarf viroid: An evolutionary link in the origin of pospiviroids. J. Gen. Virol. 1999, 80, 2823–2828. [Google Scholar]
- Singh, R.P.; Ready, K.F.M.; Nie, X. Viroids of solanaceous species. In Viroids; Hadidi A. Flores, R., Randles J.W. Semancik, J.S., Eds.; CSIRO Publishing: Collingwood, Victoria, Australia, 2003; pp. 125–133. [Google Scholar]
- Verhoeven, J.Th.J.; Jansen, C.C.C.; Willemen, T.M.; Kox, L.F.F.; Owens, R.A.; Roenhorst, J.W. Natural infections of tomato by Citrus exocortis viroid, Columnea latent viroid, Potato spindle tuber viroid and Tomato chlorotic dwarf viroid. Eur. J. Plant Pathol. 2004, 110, 823–831. [Google Scholar] [CrossRef]
- Slack, S. A.; Singh, R. P. Control of viruses affecting potatoes through seed potato certification programs. In Plant Virus Disease Control; Hadidi A. Khetarpal, R.K., Koganezawa, H., Eds.; American Phytopathological Society: St. Paul, MN, USA, 1998; pp. 249–260. [Google Scholar]
- Elliott, D.R.; Alexander, B.J.R.; Smales, T.E.; Tang, Z.; Clover, G.R.G. First report of Potato spindle tuber viroid in tomato in New Zealand. Plant Dis. 2001, 85, 1027. [Google Scholar]
- Mackie, A.E.; McKirdy, S.J.; Rodoni, B.; Kumar, S. Potato spindle tuber viroid eradicated in Western Australia. Australas. Plant Pathol. 2002, 31, 311–312. [Google Scholar] [CrossRef]
- EPPO Reporting Service. 2004/006: Occurrence of Potato Spindle tuber pospiviroid (PSTVd) in tomato plants in Germany. EPPO, 5 01 2004.
- Ling, K.S.; Verhoeven, J.Th.J.; Singh, R.P.; Brown, J.K. First report of Tomato chlorotic dwarf viroid in greenhouse tomatoes in Arizona. Plant Dis. 2009, 93, 1075. [Google Scholar]
- James, T.; Mulholland, V.; Jeffries, C.; Chard, J. First report of Tomato chlorotic dwarf viroid infecting commercial Petunia stocks in the United Kingdom. Plant Pathol. 2008, 57, 400. [Google Scholar]
- Matsushita, Y.; Kanda, A.; Usugi, T.; Tsuda, S. First report of a Tomato chlorotic dwarf viroid disease on tomato plants in Japan. J. Gen. Plant Pathol. 2008, 74, 182–184. [Google Scholar] [CrossRef]
- Góra, A.; Candresse, T.; Zagórski, W. Analysis of the population structure of three phenotypically distinct PSTVd isolates. Arch. Virol. 1994, 138, 233–245. [Google Scholar]
- Góra-Sochacka, A.; Kierzek, A.; Candresse, T.; Zagórski, W. The genetic stability of potato spindle tuber viroid (PSTVd) molecular variants. RNA 1997, 3, 68–74. [Google Scholar]
- Wiesyk, A.; Candresse, T.; Zagórski, W.; Góra-Sochacka, A. Use of randomly mutagenized genomic cDNA banks of potato spindle tuber viroid to screen for viable versions of the viroid genome. J. Gen. Virol. 2011, 92, 457–466. [Google Scholar] [CrossRef] [Green Version]
- Visvader, J.E.; Symons, R.H. Eleven new sequence variants of citrus exocortis viroid and the correlation of sequence with pathogenicity. Nucleic Acids Res. 1985, 13, 2907–2920. [Google Scholar]
- Gandía, M.; Rubio, L.; Palacio, A.; Duran-Vila, N. Genetic variation and population structure of a Citrus exocortis viroid (CEVd) isolate and the progenies of infectious haplotypes. Arch. Virol. 2005, 150, 1945–1957. [Google Scholar] [CrossRef]
- Palacio-Bielsa, A.; Romero-Durban, J.; Duran-Vila, N. Characterization of citrus HSVd isolates. Arch. Virol. 2004, 149, 537–552. [Google Scholar]
- Jiang, D.; Peng, S.; Wu, Z.; Cheng, Z.; Li, S. Genetic diversity and phylogenetic analysis of Australian grapevine viroid (AGVd) isolated from different grapevines in China. Virus Genes 2009, 38, 178–183. [Google Scholar]
- Ambrós, S.; Desvignes, J.C.; Llácer, G.; Flores, R. Pear blister canker viroid: Sequence variability and causal role in pear blister canker disease. J. Gen. Virol. 1995, 76, 2625–2629. [Google Scholar]
- Ambrós, S.; Hernandez, C.; Desvignes, J.C; Flores, R. Genomic structure of three phenotypically different isolates of peach latent mosaic viroid: implications of the existence of constraints limiting the heterogeneity of viroid quasispecies. J. Virol. 1998, 72, 7397–7406. [Google Scholar]
- Xu, W.X.; Hong, N.; Wang, G.P.; Fan, X. Population structure and genetic diversity within Peach latent mosaic viroid field isolates from peach showing three symptoms. J. Phytopathol. 2008, 156, 565–572. [Google Scholar] [CrossRef]
- Polívka, H.; Staub, U.; Gross, H.J. Variation of viroid profiles in individual grapevine plants: Novel grapevine yellow speckle viroid 1 mutants show alterations of hairpin I. J. Gen. Virol. 1996, 77, 155–161. [Google Scholar]
- Codoñer, F.M.; Darós, J.-A.; Solé, R.V.; Elena, S.F. The fittest versus the flattest: Experimental confirmation of the quasispecies effect with subviral pathogens. PLoS Pathog. 2006, 2, e136. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.P.; Dilworth, A.D. Tomato chlorotic dwarf viroid in the ornamental plant Vinca minor and its transmission through tomato seed. Eur. J. Plant Pathol. 2009, 123, 111–116. [Google Scholar] [CrossRef]
- Singh, R.P.; Dilworth, A.D.; Ao, X.P.; Singh, M.; Misra, S. Molecular and biological characterization of a severe isolate of Tomato chlorotic dwarf viroid containing a novel terminal right (T-R) domain sequence. Eur. J. Plant Pathol. 2010, 127, 63–72. [Google Scholar]
- Hu, X.; Nie, X.; Song, Y.; Xiong, X.; Tai, H. Ethylene is involved but plays a limited role in Tomato chlorotic dwarf viroid-Induced Symptom Development in Tomato. Agri. Sci. China 2011, 10, 544–552. [Google Scholar] [CrossRef]
- Verhoeven, J.Th.J.; Roenhorst, J.W. High stability of original predominant pospiviroid genotypes upon mechanical inoculation from ornamentals to potato and tomato. Arch. Virol. 2010, 155, 269–274. [Google Scholar] [CrossRef]
- Owens, R.A.; Girsova, N.V.; Kromina, K.A.; Lee, I.M.; Mozhaeva, K.A.; Kastalyeva, T.B. Russian isolates of Potato spindle tuber viroid exhibit low sequence diversity. Plant Dis. 2009, 93, 752–759. [Google Scholar] [CrossRef]
- Nie, X.; Singh, R.P.; Bostan, H. Molecular cloning, secondary structure, and phylogeny of three pospiviroids from ornamental plants. Can. J. Plant Pathol. 2005, 27, 592–602. [Google Scholar] [CrossRef]
- Behjatnia, S.A.A.; Dry, I.B.; Krake, L.R.; Condé, B.D.; Connelly, M.L.; Randles, J.W.; Rezaian, M.A. New potato spindle tuber viroid and tomato leaf curl geminivirus strains from wild Solanum sp. Phytopathology 1996, 86, 880–886. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar]
- ClustalW2, version 2.21. EMBL-EBI: Cambridge, UK, 2007. Available online: http://www.ebi.ac.uk/Tools/msa/clustalw2/ (accessed on 4 June 2012).
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar]
- DnaSP, version 5. Universitat de Barcelona: Barcelona, Catalonia, Spain, 2009.
- Nei, M. Molecular Evolutionary Genetics; Columbia University Press: New York, NY, USA, 1987. [Google Scholar]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar]
- Mfold, Version 3.1. University at Albany, State University of New York: Albany, NY, USA, 2003. Available online: http://mfold.rna.albany.edu/?q=mfold/RNA-Folding-Form (accessed on 4 June 2012).
- Zuker, M.; Stiegler, P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981, 9, 133–148. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nie, X. Analysis of Sequence Polymorphism and Population Structure of Tomato chlorotic dwarf viroid and Potato spindle tuber viroid in Viroid-Infected Tomato Plants. Viruses 2012, 4, 940-953. https://doi.org/10.3390/v4060940
Nie X. Analysis of Sequence Polymorphism and Population Structure of Tomato chlorotic dwarf viroid and Potato spindle tuber viroid in Viroid-Infected Tomato Plants. Viruses. 2012; 4(6):940-953. https://doi.org/10.3390/v4060940
Chicago/Turabian StyleNie, Xianzhou. 2012. "Analysis of Sequence Polymorphism and Population Structure of Tomato chlorotic dwarf viroid and Potato spindle tuber viroid in Viroid-Infected Tomato Plants" Viruses 4, no. 6: 940-953. https://doi.org/10.3390/v4060940




