Antiviral Treatment of Chronic Hepatitis B Virus (HBV) Infections †
Abstract
:Abbreviations
1. Introduction
2. HBV Replication Cycle
3. Currently Approved (Licensed) Anti-HBV Drugs for the Treatment of CHB
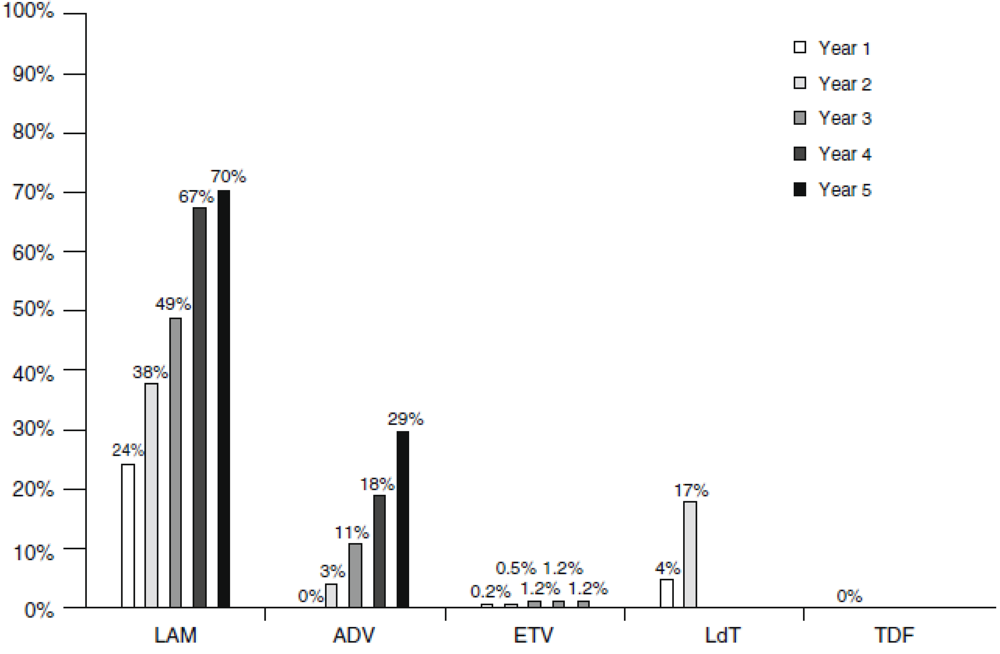
3.1. Lamivudine
3.2. Adefovir dipivoxil
| Agent | Mutation(s) | Cross-resistant to | Sensitive to | |
|---|---|---|---|---|
| Lamivudine | rtM204I/S/V | Other L-nucleoside analogs (Emtricitabine, Telbivudine) Adefovir | Adefovir, Tenofovir, Entecavir ± MPA | |
| ± rtL180M | ||||
| ± rtV173C | ||||
| rTA181S plus rTM204I | ||||
| rT181T | ||||
| Adefovir | rtA181V/T and/or rtN236T | Lamivudine, Entecavir, Emtricitabine, Tenofovir | ||
| Adefovir | rtI233V | Tenofovir | ||
| Entecavir | rtT184G | + Lamivudine- resistance mutations | Lamivudine | Adefovir |
| rtS202I | ||||
| rtM250V | ||||
| Telbivudine | rtM204I | Lamivudine | ||
| Tenofovir | rtA194T + Lamivudine resistance mutations (rtM204V + rtL180M) | |||
| Emtricitabine | rt204I/V ± rtL180M and rtV173C | Lamivudine | ||
3.3. Entecavir
3.4. Telbivudine
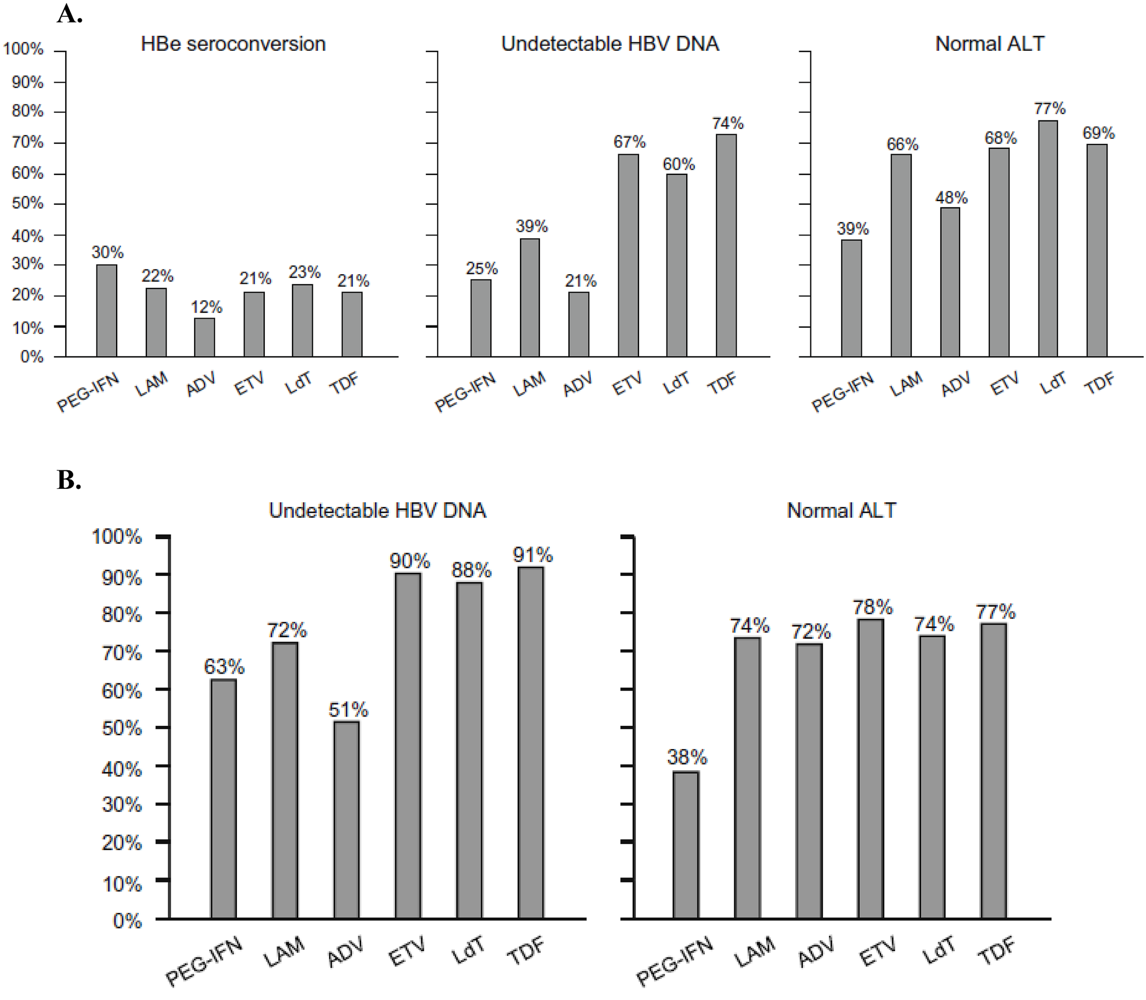
3.5. INF-α
3.6. Tenofovir disoproxil fumarate
4. Novel anti-HBV agents
4.1. Emtricitabine
4.2. Clevudine
5. Recommendations
| HBV variant | Level of susceptibility | ||||
|---|---|---|---|---|---|
| Lamivudine | Telbivudine | Entecavir | Adefovir | Tenofovir | |
| Wild-type | S | S | S | S | S |
| M204I | R | R | I | S | S |
| L180M + M204V | R | R | I | S | S |
| A181T/V | I | S | S | R | S |
| N236T | S | S | S | R | I |
| L180M + M204I/S/V ± I169T ± V173L ± M250V | R | R | R | S | S |
| L180M + M204I/S/V ± T184G ± S202I/G | R | R | R | S | S |
6. Concluding Remarks
| Generic name | Brand name | Manufacturer | Date of FDA approval |
|---|---|---|---|
| Anti-HBV drugs approved by the US Food and Drug Administration | |||
| Interferon alfa-2b | Intron A | Schering-Plough | 13 July 1992 |
| Lamivudine, 3TC | Epivir-HBV | GlaxoSmithKline | 9 December 1998 |
| Adefovir dipivoxil | Hepsera | Gilead Sciences | 20 September 2002 |
| Entecavir | Baraclude | Bristol-Myers Squibb | 30 March 2005 |
| Peginterferon alfa-2a | Pegasys | Roche | 13 May 2005 |
| Telbivudine | Tyzeka | Idenix | 25 October 2006 |
| Tenofovir disoproxil fumarate | Viread | Gilead Sciences | 11 August 2008 |
Acknowledgments
References
- Ganem, D; Prince, A.M. Hepatitis B virus infection - Natural history and clinical consequences . N. Engl. J. Med. 2004, 350, 1118–1129. [Google Scholar] [CrossRef] [PubMed]
- Beasley, R.P. Hepatitis B virus: the major etiology of hepatocellular carcinoma. Cancer 1988, 61, 1942–1956. [Google Scholar] [CrossRef] [PubMed]
- Chiaramonte, M.; Stroffolini, T.; Vian, A.; Stazi, M.A.; Floreani, A.; Lorenzoni, U.; Lobello, S.; Farinati, F.; Naccarato, R. Rate of incidence of hepatocellular carcinoma in patients with compensated viral cirrhosis. Cancer 1999, 85, 2132–2137. [Google Scholar] [CrossRef] [PubMed]
- Fattovich, G.; Pantalena, M.; Zagni, I.; Realdi, G.; Schalm, S.W.; Christensen, E. Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. Am. J. Gastroenterol. 2002, 97, 2886–2895. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.I.; Lu, S.N.; Liaw, Y.F.; You, S.L.; Sun, C.A.; Wang, L.Y.; Hsiao, C.K.; Chen, P.J.; Chen, D.S.; Chen, C.J. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N. Engl. J. Med. 2002, 347, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Zoulim, F. Antiviral therapy of chronic hepatitis B. Antiviral Res. 2006, 71, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Keeffe, E.B.; Marcellin, P. New and emerging treatment of chronic hepatitis B. Clin. Gastroenterol. Hepatol. 2007, 5, 285–294. [Google Scholar] [CrossRef]
- Palumbo, E. New drugs for chronic hepatitis B: a review. Am. J. Therapeut. 2008, 15, 167–172. [Google Scholar] [CrossRef]
- Peng, J.; Zhao, Y.; Mai, J.; Pang, W.K.; Wei, X.; Zhang, P.; Xu, Y. Inhibition of hepatitis B virus replication by various RNAi constructs and their pharmacodynamic properties. J. Gen. Virol. 2005, 86, 3227–3234. [Google Scholar] [CrossRef] [PubMed]
- Ying, C.; De Clercq, E.; Neyts, J. Selective inhibition of hepatitis B virus replication by RNA interference. Biochem. Biophys. Res. Commun. 2003, 309, 482–484. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, G. RNAi for treating hepatitis B viral infection . Pharm. Res. 2008, 25, 72–86. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, A.P.; Nakai, H.; Pandey, K.; Huang, Z.; Salazar, F.H.; Xu, H.; Wieland, S.F.; Marion, P.I.; Kay, M.A. Inhibition of hepatitis B virus in mice by RNA interference . Nat. Biotechnol. 2003, 21, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.C.; Ying, C.X.; Leung, C.H.; Li, Y. New targets and inhibitors of HBV replication to combat drug resistance . J. Clin. Virol. 2005, 34 Suppl. 1, S147–S150. [Google Scholar] [CrossRef] [PubMed]
- Ying, C.; Li, Y.; Leung, C.H.; Robek, M.D.; Cheng, Y.C. Unique antiviral mechanism discovered in anti-hepatitis B virus research with a natural product analogue. Proc. Natl. Acad. Sci. USA 2007, 104, 8526–8531. [Google Scholar] [CrossRef]
- Deres, K.; Schröder, C.H.; Paessens, A.; Goldmann, S.; Hacker, H.J.; Weber, O.; Krämer, T.; Niewöhner, U.; Pleiss, U.; Stoltefuss, J.; Graef, E.; Koletzki, D.; Masantschek, R.N.; Reimann, A.; Jaeger, R.; Gross, R.; Beckermann, B.; Schlemmer, K.H.; Haebich, D.; Rübsamen-Waigmann, H. Inhibition of hepatitis B virus replication by drug-induced depletion of nucleocapsids. Science 2003, 299, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Block, T.M.; Lu, X.; Mehta, A.S.; Blumberg, B.S.; Tennant, B.; Ebling, M.; Korba, B.; Lansky, D.M.; Jacob, G.S. Treatment of chronic hepadnavirus infection in a woodchuck animal model with an inhibitor of protein folding and trafficking . Nat. Med. 1998, 4, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Korba, B.E.; Montero, A.B.; Farrar, K.; Gaye, K.; Mukerjee, S.; Ayers, M.S.; Rossignol, J.F. Nitazoxanide, tizoxanide and other thiazolides are potent inhibitors of hepatitis B virus and hepatitis C virus replication. Antiviral Res. 2008, 77, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, A.M.; Guo, H.; Westby, G.; Liu, Y.; Simsek, E.; Guo, J.T.; Mehta, A.; Norton, P.; Gu, B.; Block, T.; Cuconati, A. A substituted tetrahydro-tetrazolo-pyrimidine is a specific and novel inhibitor of hepatitis B virus surface antigen secretion. Antimicrob. Agents Chemother. 2007, 51, 4427–4437. [Google Scholar] [CrossRef] [PubMed]
- He, X.X.; Chen, T.; Lin, J.S.; Chang, Y.; Ye, B.X. Inhibition of the replication of hepatitis B virus in vitro by a novel 2,6-diaminopurine analog, beta-LPA. Biochem. Biophys. Res. Commun. 2008, 369, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Sharon, A.; Bal, C.; Wang, J.; Allu, M.; Huang, Z.; Murray, M.G.; Bassit, L.; Schinazi, R.F.; Korba, B.; Chu, C.K. Synthesis and anti-hepatitis B virus and anti-hepatitis C virus activities of 7-deazaneplanocin A analogues in vitro. J. Med. Chem. 2009, 52, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kuang, E.; Dai, W.; Zhou, B.; Yang, F. Efficient inhibition of hepatitis B virus replication by hammerhead ribozymes delivered by hepatitis delta virus. Virus. Res. 2005, 114, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Dane, D.S.; Cameron, C.H.; Briggs, M. Virus-like particles in serum of patients with Australia-antigen-associated hepatitis. Lancet 1970, 1, 695–698. [Google Scholar] [CrossRef]
- Robinson, W.S.; Lutwick, L.I. The virus of hepatitis, type B (first of two parts). N. Engl. J. Med. 1976, 295, 1168–1175. [Google Scholar] [PubMed]
- Gavilanes, F.; Gonzalez-Ros, J.M.; Peterson, D.L. Structure of hepatitis B surface antigen: Characterization of the lipid components and their association with the viral proteins. J. Biol. Chem. 1982, 257, 7770–7777. [Google Scholar] [PubMed]
- Peterson, D.L. Isolation and characterization of the major protein and glycoprotein of hepatitis B surface antigen. J. Biol. Chem. 1981, 256, 6975–6983. [Google Scholar] [PubMed]
- Peterson, D.L. The structure of hepatitis B surface antigen and its antigenic sites. Bioessays 1987, 6, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, P.; Gray, P.; Quiroga, M.; Zaldivar, J.; Goodman, H.M.; Rutter, W.J. Nucleotide sequence of the gene coding for the major protein of hepatitis B virus surface antigen. Nature 1979, 280, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Bartenschlager, R.; Schaller, H. The amino-terminal domain of the hepadnaviral P-gene encodes the terminal protein (genome linked protein) believed to prime reverse transcription. EMBO J. 1988, 7, 4185–4192. [Google Scholar] [PubMed]
- Chang, L.J.; Hirsch, R.C.; Ganem, D.; Varmus, H.E. Effects of insertional and point mutations on the functions of the duck hepatitis B virus polymerase. J. Virol. 1990, 11, 5553–5558. [Google Scholar]
- Radziwill, G.; Tucker, W.; Schaller, H. Mutational analysis of the hepatitis B virus P gene product: domain structure and RNase H activity. J. Virol. 1990, 2, 613–620. [Google Scholar]
- Poch, O.; Sauvaget, I.; Delarue, M.; Tordo, N. Identification of four conserved motifs among the RNA-dependent polymerase encoding elements. EMBO J. 1989, 8, 3867–3874. [Google Scholar] [PubMed]
- Tipples, G.A.; Ma, M.M.; Fischer, K.P.; Bain, V.G.; Kneteman, N.M.; Tyrrell, D.L.J. Mutation in HBV RNA-dependent DNA polymerase confers resistant to lamivudine in vivo. Hepatology 1996, 24, 714–717. [Google Scholar] [PubMed]
- Ou, J.H.; Laub, O.; Rutter, W.J. Hepatitis B virus gene function: the pre-core region targets the core antigen to cellular membranes and causes the secretion of the e entigen. Proc. Natl. Acad. Sci. USA 1986, 83, 1578–1582. [Google Scholar] [CrossRef]
- Pasek, M.; Goto, T.; Gilbert, W.; Zink, B.; Schaller, H.; MacKay, P.; Leadbetter, G.; Murray, K. Hepatitis B virus genes and their expression in E. coli. Nature 1979, 282, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Tuttleman, J.S.; Pourcel, C.; Summers, J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell 1986, 47, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Will, H.; Reiser, W.; Weimer, T.; Pfaff, E.; Büscher, M.; Sprengel, R.; Cattaneo, R.; Schaller, H. Replication strategy of human hepatitis B virus. J. Virol. 1987, 61, 904–911. [Google Scholar] [PubMed]
- Wong, D.K.; Yuen, M.F.; Poon, R.T.; Yuen, J.C.; Fung, J.; Lai, C.L. Quantification of hepatitis B virus covalently closed circular DNA in patients with hepatocellular carcinoma. J. Hepatol. 2006, 45, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Pollack, J.R.; Ganem, D. Site-specific RNA binding by a hepatitis B virus reverse transcriptase initiates two distinct reactions: RNA packaging and DNA synthesis. J. Virol. 1994, 68, 5579–5587. [Google Scholar] [PubMed]
- Hu, J.; Seeger, C. Hsp90 is required for the activity of a hepatitis B virus reverse transcriptase. Proc. Natl. Acad. Sci. USA 1996, 93, 1060–1064. [Google Scholar] [CrossRef]
- Seeger, C.; Mason, W.S. Hepatitis B virus biology. Microbiol. Mol. Biol. Rev. 2000, 64, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Staprans, S.; Loeb, D.D.; Ganem, D. Mutations affecting hepadnavirus plus-strand DNA synthesis dissociate primer cleavage from translocation and reveal the origin of linear viral DNA. J. Virol. 1991, 65, 1255–1262. [Google Scholar] [PubMed]
- Yang, W.; Mason, W.S.; Summers, J. Covalently closed circular viral DNA formed from two types of linear DNA in woodchuck hepatitis virus-infected liver. J. Virol. 1996, 70, 4567–4575. [Google Scholar] [PubMed]
- Yang, W.; Summers, J. Illegitimate replication of linear hepadnavirus DNA through nonhomologous recombination. J. Virol. 1995, 69, 4029–4036. [Google Scholar] [PubMed]
- Yang, W.; Summers, J. Integration of hepadnavirus DNA in infected liver: evidence for a linear precursor . J. Virol. 1999, 73, 9710–9717. [Google Scholar] [PubMed]
- Gong, S.S.; Jensen, A.D.; Chang, C.J.; Rogler, C.E. Double-stranded linear duck hepatitis B virus (DHBV) stably integrates at a higher frequency than wild-type DHBV in LMH chicken hepatoma cells. J. Virol. 1999, 73, 1492–1502. [Google Scholar] [PubMed]
- Gong, S.S.; Jensen, A.D.; Wang, H.; Rogler, C.E. Duck hepatitis B virus integrations in LMH chicken hepatoma cells: identification and characterization of new episomally derived integrations. J. Virol. 1995, 69, 8102–8108. [Google Scholar] [PubMed]
- Kamimura, T.; Yoshikawa, A.; Ichida, F.; Sasaki, H. Electron microscopic studies of Dane particles in hepatocytes with special reference to intracellular development of Dane particles and their relation with HBeAg in serum. Hepatology 1981, 1, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Roingeard, P.; Lu, S.L.; Sureau, C.; Freschlin, M.; Arbeille, B.; Essex, M.; Romet-Lemonne, J.L. Immunocytochemical and electron microscopic study of hepatitis B virus antigen and complete particle production in hepatitis B virus DNA transfected HepG2 cells. Hepatology 1990, 11, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Ganem, D.; Schneider, R.J. Hepadnaviridae: The viruses and their replication. In Fields Virology, 4th edition. Lippincott Williams & Wi. 2001; Wilkins: Philadelphia, PA, USA. [Google Scholar]
- Cammack, N.; Rouse, P.; Marr, C.L.; Reid, P.J.; Boehme, R.E.; Coates, J.A.; Penn, C.R.; Cameron, J.M. Cellular metabolism of (-) enantiomeric 2'-deoxy-3'-thiacytidine. Biochem. Pharmacol. 1992, 43, 2059–2064. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.N.; Skalski, V.; Zhou, J.H.; Cheng, Y.C. Biochemical pharmacology of (+)- and (-)-2',3'-dideoxy-3'-thiacytidine as anti-hepatitis B virus agents. J. Biol. Chem. 1992, 267, 22414–22420. [Google Scholar] [PubMed]
- Lai, C.L.; Yuen, M.F. Profound suppression of hepatitis B virus replication with lamivudine. J. Med. Virol. 2000, 61, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Dienstag, J.L.; Schiff, E.R.; Wright, T.L.; Perrillo, R.P.; Hann, H.W.; Goodman, Z.; Crowther, L.; Condreay, L.D.; Woessner, M.; Rubin, M.; Brown, N.A. Lamivudine as initial treatment for chronic hepatitis B in the United States . N. Engl. J. Med. 1999, 341, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.L.; Chine, R.W.; Leung, N.W.Y.; Chang, T.T.; Guan, R.; Tai, D.I.; Ng, K.Y.; Wu, P.C.; Dent, J.C.; Barber, J.; Stephenson, S.L.; Gray, F. A one year trial of lamivudine for chronic hepatitis B. N. Engl. J. Med. 1998, 339, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Yuen, M.F.; Fong, D.Y.; Wong, D.K.; Yuen, J.C.; Fung, J.; Lai, C.L. Hepatitis B virus DNA levels at week 4 of lamivudine treatment predict the 5-year ideal response. Hepatology 2007, 46, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Fung, S.K.; Wong, F.; Hussain, M.; Lok, A.S.F. Sustained response after a 2-year course of lamivudine treatment of hepatitis B e antigen-negative chronic hepatitis B. J. Viral. Hepatol. 2004, 11, 432–438. [Google Scholar] [CrossRef]
- Perry, C.M.; Faulds, D. Lamivudine. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in the management of HIV infection. Drugs 1997, 53, 657–680. [Google Scholar] [CrossRef] [PubMed]
- Schalm, S.W.; Heathcote, J.; Cianciara, J.; Farrell, G.; Sherman, M.; Willems, B.; Dhillon, A.; Moorat, A. Lamivudine and alpha interferon combination treatment of patients with chronic hepatitis B infection: a randomised trial. Gut 2000, 46, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Yuen, M.F.; Seto, W.K.; Chow, D.H.; Tsui, K.; Wong, D.K.; Ngai, V.W.; Wong, B.C.; Fung, J.; Yuen, J.C.; Lai, C.L. Long-term lamivudine therapy reduces the risk of long-term complications of chronic hepatitis B infection even in patients without advanced disease. Antiviral Ther. 2007, 12, 1295–1303. [Google Scholar]
- Lok, A.S.; Lai, C.L.; Leung, N.; Yao, G.B.; Cui, Z.Y.; Schiff, E.R.; Dienstag, J.L.; Heathcote, E.J.; Little, N.R.; Griffiths, D.A.; Gardner, S.D.; Castiglia, M. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology 2003, 125, 1714–1722. [Google Scholar] [CrossRef] [PubMed]
- Pawlotsky, J.M.; Dusheiko, G.; Hatzakis, A.; Lau, D.; Lau, G.; Liang, T.J.; Locarnini, S.; Martin, P.; Richman, D.D.; Zoulim, F. Virologic monitoring of hepatitis B virus therapy in clinical trials and practice: recommendations for a standardized approach. Gastroenterology 2008, 134, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Xiong, X.; Yang, H.; Westland, C.E.; Gibbs, C.S.; Sarafianos, S.G.; Arnold, E. Molecular modeling and biochemical characterization reveal the mechanism of hepatitis B virus polymerase resistance to lamivudine (3TC) and emtricitabine (FTC). J. Virol. 2001, 75, 4771–4779. [Google Scholar] [CrossRef] [PubMed]
- Yatsuji, H.; Noguchi, C.; Hiraga, N.; Mori, N.; Tsuge, M.; Imamura, M.; Takahashi, S.; Iwao, E.; Fujimoto, Y.; Ochi, H.; Abe, H.; Maekawa, T.; Tateno, C.; Yoshizato, K.; Suzuki, F.; Kumada, H.; Chayama, K. Emergence of a novel lamivudine-resistant hepatitis B virus variant with a substitution outside the YMDD motif. Antimicrob. Agents Chemother. 2006, 50, 3867–3874. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Qi, X.; Sabogal, A.; Miller, M.; Xiong, S.; Delaney, W.E. Cross-resistance testing of next-generation nucleoside and nucleotide analogues against lamivudine-resistant HBV. Antiviral Ther. 2005, 10, 625–633. [Google Scholar]
- Karatayli, E.; Karayalçin, S.; Karaaslan, H.; Kayhan, H.; Türkyilmaz, A.R.; Sahin, F.; Yurdaydin, C.; Bozdayi, A.M. A novel mutation pattern emerging during lamivudine treatment shows cross-resistance to adefovir dipivoxil treatment. Antiviral Ther. 2007, 12, 761–768. [Google Scholar]
- De Clercq, E. A novel selective broad-spectrum anti-DNA virus agent. Nature 1986, 323, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Merta, A.; Votruba, I.; Jindrich, J.; Holý, A.; Cihlár, T.; Rosenberg, I.; Otmar, M.; Herve, T.Y. Phosphorylation of 9-(2-phosphonomethoxyethyl)adenine and 9-(S)-(3-hydroxy-2-phosphonomethoxypropyl)adenine by AMP(dAMP) kinase from L1210 cells. Biochem. Pharmacol. 1992, 44, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Antivirals and antiviral strategies. Nat. Rev. Microbiol. 2004, 2, 704–720. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhukovskaya, N.L.; Zimmer, M.I.; Soelaiman, S.; Bergson, P.; Wang, C.R.; Gibbs, C.S.; Tang, W.J. Selective inhibition of anthrax edema factor by adefovir, a drug for chronic hepatitis B virus infection. Proc. Natl. Acad. Sci. U S A 2004, 101, 3242–3247. [Google Scholar] [CrossRef] [PubMed]
- Werle-Lapostolle, B.; Bowden, S.; Locarnini, S.; Wursthorn, K.; Petersen, J.; Lau, G.; Trepo, C.; Marcellin, P.; Goodman, Z.; Delaney 4th, W.E.; Xiong, S.; Brosgart, C.L.; Chen, S.S.; Gibbs, C.S.; Zoulim, F. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology 2004, 126, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Hadziyannis, S.J.; Tassopoulos, N.C.; Heathcote, E.J.; Chang, T.T.; Kitis, G.; Rizzetto, M.; Marcellin, P.; Lim, S.G.; Goodman, Z.; Wulfsohn, M.S.; Xiong, S.; Fry, J.; Brosgart, C.L. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N. Engl. J. Med. 2003, 348, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Marcellin, P.; Chang, T.T.; Lim, S.G.; Tong, M.J.; Sievert, W.; Shiffman, M.L.; Jeffers, L.; Goodman, Z.; Wulfsohn, M.S.; Xiong, S.; Fry, J.; Brosgart, C.L. Adefovir dipivoxil 437 study group. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N. Engl. J. Med. 2003, 348, 848–850. [Google Scholar] [CrossRef] [PubMed]
- Hadziyannis, S.J.; Tassopoulos, N.C.; Heathcote, E.J.; Chang, T.T.; Kitis, G.; Rizzetto, M.; Marcellin, P.; Lim, S.G.; Goodman, Z.; Ma, J.; Arterburn, S.; Xiong, S.; Currie, G.; Brosgart, C.L. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. N. Engl. J. Med. 2005, 352, 2673–2681. [Google Scholar] [CrossRef] [PubMed]
- Carrouée-Durantel, S.; Durantel, D.; Werle-Lapostolle, B.; Pichoud, C.; Naesens, L.; Neyts, J.; Trépo, C.; Zoulim, F. Suboptimal response to adefovir dipivoxil therapy for chronic hepatitis B in nucleoside-naive patients is not due to pre-existing drug-resistant mutants. Antiviral Ther. 2008, 13, 381–388. [Google Scholar]
- Hadziyannis, S.J.; Tassopoulos, N.C.; Heathcote, E.J.; Chang, T.T.; Kitis, G.; Rizzetto, M.; Marcellin, P.; Lim, S.G.; Goodman, Z.; Ma, J.; Brosgart, C.L.; Borroto-Esoda, K.; Arterburn, S.; Chuck, S.L. Adefovir Dipivoxil 438 Study Group. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years . Gastroenterology 2006, 131, 1743–1751. [Google Scholar] [CrossRef] [PubMed]
- Schiff, E.; Lai, C.L.; Hadziyannis, S.; Neuhaus, P.; Terrault, N.; Colombo, M.; Tillmann, H.; Samuel, D.; Zeuzem, S.; Villeneuve, J.P.; Arterburn, S.; Borroto-Esoda, K.; Brosgart, C.; Chuck, S. Adefovir dipivoxil for wait-listed and post-liver transplantation patients with lamivudine-resistant hepatitis B: final long-term results. Liver Transpl. 2007, 13, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Marcellin, P.; Asselah, T. Resistance to adefovir: a new challenge in the treatment of chronic hepatitis B. J. Hepatol. 2005, 43, 920–923. [Google Scholar] [PubMed]
- Borroto-Esoda, K.; Miller, M.D.; Arterburn, S. Pooled analysis of amino acid changes in the HBV polymerase in patients from four major adefovir dipivoxil clinical trials . J. Hepatol. 2007, 47, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Gallego, A.; Sheldon, J.; García-Samaniego, J.; Margall, N.; Romero, M.; Hornillos, P.; Soriano, V.; Enrĺquez, J. Evaluation of initial virological response to adefovir and development of adefovir-resistant mutations in patients with chronic hepatitis B. J. Viral Hepat. 2008, 15, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, K.; Ollivet, A.; Gozlan, J.; Durantel, S.; Tran, N.; Girard, P.M.; Zoulim, F. A novel hepatitis B virus mutation with resistance to adefovir but not to tenofovir in an HIV-hepatitis B virus-co-infected patient. AIDS 2006, 20, 2229–2231. [Google Scholar] [CrossRef] [PubMed]
- Osiowy, C.; Villeneuve, J.P.; Heathcote, E.J.; Giles, E.; Borlang, J. Detection of rtN236T and rtA181V/T mutations associated with resistance to adefovir dipivoxil in samples from patients with chronic hepatitis B virus infection by the INNO-LiPA HBV DR Line Probe Assay (version 2). J. Clin. Microbiol. 2006, 44, 1994–1997. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Suh, D.J.; Lim, Y.S.; Jung, S.W.; Kim, K.M.; Lee, H.C.; Chung, Y.H.; Lee, Y.S.; Yoo, W.; Kim, S.O. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology 2006, 43, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Schildgen, O.; Sirma, H.; Funk, A.; Olotu, C.; Wend, U.C.; Hartmann, H.; Helm, M.; Rockstroh, J.K.; Willems, W.R.; Will, H.; Gerlich, W.H. Variant of hepatitis B virus with primary resistance to adefovir. N. Engl. J. Med. 2006, 354, 1807–1812. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, J.P.; Durantel, D.; Durantel, S.; Westland, C.; Xiong, S.; Brosgart, C.L.; Gibbs, C.S.; Parvaz, P.; Werle, B.; Trépo, C.; Zoulim, F. Selection of a hepatitis B virus strain resistant to adefovir in a liver transplantation patient. J. Hepatol. 2003, 39, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Fung, S.K.; Andreone, P.; Han, S.H.; Reddy, K.R.; Regev, A.; Keeffe, E.B.; Hussain, M.; Cursaro, C.; Richtmyer, P.; Marrero, J.A.; Lok, A.S.F. Adefovir-resistant hepatitis B can be associated with viral rebound and hepatic decompensation. J. Hepatol. 2005, 43, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Fung, S.K.; Chae, H.B.; Fontana, R.J.; Conjeevaral, H.; Marrero, J.; Oberhelman, K.; Hussain, M.; Lok, A.S.F. Virologic response and resistance to adefovir in patients with chronic hepatitis B. J. Hepatol. 2006, 44, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Zoulim F, Parvaz P; Benhamou Y, Bailly F. Adefovir dipivoxil is effective for the treatment of cirrhotic patients with lamivudine failure . Liver Int. 2009, 29, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, G.; Wilson, T.; Innaimo, S.; Bisacchi, G.S.; Egli, P.; Rinehart, J.K.; Zahler, R.; Colonno, R.J. Metabolic studies on BMS-200475, a new antiviral compound active against hepatitis B virus. Antimicrob. Agents Chemother. 1999, 43, 190–193. [Google Scholar] [PubMed]
- Zoulim, F. Entecavir: a new treatment option for chronic hepatitis B. J. Clin. Virol. 2006, 36, 8–12. [Google Scholar] [CrossRef]
- Seifer, M.; Hamatake, R.K.; Colonno, R.J.; Standring, D.N. In vitro inhibition of hepadnavirus polymerases by the triphosphates of BMS-200475 and lobucavir. Antimicrob. Agents Chemother. 1998, 42, 3200–3208. [Google Scholar] [PubMed]
- Tchesnokov, E.P.; Obikhod, A.; Schinazi, R.F.; Götte, M. Delayed chain termination protects the anti-hepatitis B virus drug entecavir from excision by HIV-1 reverse transcriptase. J. Biol. Chem. 2008, 283, 34218–34228. [Google Scholar] [CrossRef] [PubMed]
- Colonno, R.J.; Genovesi, E.V.; Medina, I.; Lamb, L.; Durham, S.K.; Huang, M.L.; Corey, L.; Littlejohn, M.; Locarnini, S.; Tennant, B.C.; Rose, B.; Clark, J.M. Long-term entecavir treatment results in sustained antiviral efficacy and prolonged life span in the woodchuck model of chronic hepatitis infection. J. Infect. Dis. 2001, 184, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.T.; Gish, R.G.; de Man, R.; Gadano, A.; Sollano, J.; Chao, Y.C.; Lok, A.S.; Han, K.H.; Goodman, Z.; Zhu, J.; Cross, A.; DeHertogh, D.; Wilber, R.; Colonno, R.; Apelian, D. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N. Engl. J. Med. 2006, 354, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.L.; Shouval, D.; Lok, A.S.; Chang, T.T.; Cheinquer, H.; Goodman, Z.; DeHertogh, D.; Wilber, R.; Zink, R.C.; Cross, A.; Colonno, R.; Fernandes, L. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N. Engl. J. Med. 2006, 354, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.; Yurdaydin, C.; Simsek, H.; Silva, M.; Liaw, Y.F.; Rustgi, V.K.; Sette, H.; Tsai, N.; Tenney, D.J.; Vaughan, J.; Kreter, B.; Hindes, R. Entecavir therapy for lamivudine-refractory chronic hepatitis B: improved virologic, biochemical, and serology outcomes through 96 weeks. Hepatology 2008, 48, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Yao, G. Entecavir is a potent anti-HBV drug superior to lamivudine: experience from clinical trials in China. J. Antimicrob. Chemother. 2007, 60, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Reijnders, J.G.P.; Pas, S.D.; Schutten, M.; de Man, R.A.; Janssen, H.L.A. Entecavir shows limited efficacy in HBeAg-positive hepatitis B patients with a partial virologic response to adefovir therapy . J. Hepatol. 2009, 50, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Tenney, D.J.; Levine, S.M.; Rose, R.E.; Walsh, A.W.; Weinheimer, S.P.; Discotto, L.; Plym, M.; Pokornowski, K.; Yu, C.F.; Angus, P.; Ayres, A.; Bartholomeusz, A.; Sievert, W.; Thompson, G.; Warner, N.; Locarnini, S.; Colonno, R.J. Clinical emergence of entecavir-resistant hepatitis B virus requires additional substitutions in virus already resistant to lamivudine. Antimicrob. Agents Chemother. 2004, 48, 3498–3507. [Google Scholar] [CrossRef] [PubMed]
- Baldick, C.J.; Eggers, B.J.; Fang, J.; Levine, S.M.; Pokornowski, K.A.; Rose, R.E.; Yu, C.-F.; Tenney, D.J.; Colonno, R.J. Comprehensive evaluation of hepatitis B virus reverse transcriptase substitutions associated with entecavir resistance . Hepatology 2008, 47, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Baldick, C.J.; Eggers, B.J.; Fang, J.; Levine, S.M.; Pokornowski, K.A.; Rose, R.E.; Yu, C.-F.; Tenney, D.J.; Colonno, R.J. Hepatitis B virus quasispecies susceptibility to entecavir confirms the relationship between genotypic resistance and patient virologic response . J. Hepatol. 2008, 48, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Nagasaki, F.; Niitsuma, H.; Ueno, Y.; Inoue, J.; Kogure, T.; Fukushima, K.; Shimosegawa, T. The high incidence of the emergence of entecavir-resistant mutants among patients infected with lamivudine-resistant hepatitis B virus. Tohoku J. Exp. Med. 2007, 213, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Tenney, D.J.; Rose, R.E.; Baldick, C.J.; Pokornowski, K.A.; Eggers, B.J.; Fang, J.; Wichroski, M.J.; Xu, D.; Yang, J.; Wilber, R.B.; Colonno, R.J. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology 2009, 49, 1503–1514. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.A.; Jilek, B.L.; Brennan, T.P.; Shen, L.; Zhou, Y.; Wind-Rotolo, M.; Xing, S.; Bhat, S.; Hale, B.; Hegarty, R.; Chong, C.R.; Liu, J.O.; Siliciano, R.F.; Thio, C.L. The HBV drug entecavir - effects on HIV-1 replication and resistance. N. Engl. J. Med. 2007, 356, 2614–2621. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.K.; Zoellner, C.L. Entecavir can select for M184V of HIV-1: a case of an HIV/hepatitis B (HBV) naïve patient treated for chronic HBV. AIDS 2007, 21, 2365–2366. [Google Scholar] [CrossRef] [PubMed]
- Sasadeusz, J. The anti-HIV antiviral activity of entecavir: the loss of a trusted friend? J. Hepatol. 2007, 47, 872–874. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.F.; Nowicka-Sans, B.; Terry, B.; Zhang, S.; Wang, C.; Fan, L.; Dicker, I.; Gali, V.; Higley, H.; Parkin, N.; Tenney, D.; Krystal, M.; Colonno, R. Entecavir exhibits inhibitory activity against human immunodeficiency virus under conditions of reduced viral challenge. Antimicrob. Agents Chemother. 2008, 52, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Bifano, M.; Xu, X.; Wang, Y.; LaCreta, F.; Grasela, D,; Pfister, M. Lack of an effect of human immunodeficiency virus coinfection on the pharmacokinetics of entecavir in hepatitis B virus-infected patients . Antimicrob. Agents Chemother. 2008, 52, 2836–2841. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Telbivudine. Drugs 2007, 67, 1917–1929. [Google Scholar] [CrossRef] [PubMed]
- Nash, K. Telbivudine in the treatment of chronic hepatitis B. Adv. Ther. 2009, 26, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Sancho, A.; Sheldon, J.; Soriano, V. Telbivudine: a new option for the treatment of chronic hepatitis B. Exp. Opin. Biol. Ther. 2007, 7, 751–761. [Google Scholar] [CrossRef]
- Bryant, M.L.; Bridges, E.G.; Placidi, L.; Faraj, A.; Loi, A.G.; Pierra, C.; Dukhan, D.; Gosselin, G.; Imbach, J.L.; Hernandez, B.; Juodawlkis, A.; Tennant, B.; Korba, B.; Cote, P.; Marion, P.; Cretton-Scott, E.; Schinazi, R.F.; Sommadossi, J.P. Antiviral L-nucleosides specific for hepatitis B virus infection. Antimicrob Agents Chemother 2001, 45, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Santiago, B.; Placidi, L.; Cretton-Scott, E.; Faraj, A.; Bridges, E.G.; Bryant, M.L.; Rodriguez-Orengo, J.; Imbach, J.L.; Gosselin, G.; Pierra, C.; Dukhan, D.; Sommadossi, J.P. Pharmacology of beta-L-thymidine and beta-L-2'-deoxycytidine in HepG2 cells and primary human hepatocytes: relevance to chemotherapeutic efficacy against hepatitis B virus. Antimicrob Agents Chemother 2002, 46, 1728–1733. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Park, S.H.; Louie, S.G. Telbivudine: A novel nucleoside analog for chronic hepatitis B. Ann Pharmacother 2006, 40, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.L.; Lim, S.G.; Brown, N.A.; Zhou, X.J.; Lloyd, D.M.; Lee, Y.M.; Yuen, M.F.; Chao, G.C.; Myers, M.W. A dose-finding study of once-daily oral telbivudine in HBeAg-positive patients with chronic hepatitis B virus infection. Hepatology 2004, 40, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.L.; Leung, N.; Teo, E.K.; Tong, M.; Wong, F.; Hann, H.W.; Han, S.; Poynard, T.; Myers, M.; Chao, G.; Lloyd, D.; Brown, N.A. A 1-year trial of telbivudine, lamivudine, and the combination in patients with hepatitis B e antigen-positive chronic hepatitis B. Gastroenterology 2005, 129, 528–536. [Google Scholar] [PubMed]
- Chan, H.L.; Heathcote, E.J.; Marcellin, P.; Lai, C.L.; Cho, M.; Moon, Y.M.; Chao, Y.C.; Myers, R.P.; Minuk, G.Y.; Jeffers, L.; Sievert, W.; Bzowej, N.; Harb, G.; Kaiser, R.; Qiao, X.J.; Brown, N.A. Treatment of hepatitis B e antigen positive chronic hepatitis with telbivudine or adefovir: a randomized trial. Ann. Intern. Med. 2007, 147, 745–754. [Google Scholar] [PubMed]
- Lai, C.L.; Gane, E.; Liaw, Y.F.; Hsu, C.W.; Thongsawat, S.; Wang, Y.; Chen, Y.; Heathcote, E.J.; Rasenack, J.; Bzowej, N.; Naoumov, N.V.; Di Bisceglie, A.M.; Zeuzem, S.; Moon, Y.M.; Goodman, Z.; Chao, G.; Constance, B.F.; Brown, N.A. Telbivudine versus lamivudine in patients with chronic hepatitis B. N. Engl. J. Med. 2007, 357, 2576–2588. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Yin, Y.K.; Xu, D.; Tan, D.; Niu, J.; Zhou, X.; Wang, Y.; Zhu, L.; He, Y.; Ren, H.; Wan, M.; Chen, C.; Wu, S.; Chen, Y.; Xu, J.; Wang, Q.; Wei, L.; Chao, G.; Constance, B.F.; Harb, G.; Brown, N.A.; Jia, J. Telbivudine versus lamivudine in Chinese patients with chronic hepatitis B: Results at 1 year of a randomized, double-blind trial. Hepatology 2008, 47, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Zeuzem, S.; Gane, E.; Liaw, Y.F.; Lim, S.G.; DiBisceglie, A.; Buti, M.; Chutaputti, A.; Rasenack, J.; Hou, J.; O'Brien, C.; Nguyen, T.T.; Jia, J.; Poynard, T.; Belanger, B.; Bao, W.; Naoumov, N.V. Baseline characteristics and early on-treatment response predict the outcomes of 2 years of telbivudine treatment of chronic hepatitis B. J. Hepatol. 2009, 51, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Liaw, Y.F.; Gane, E.; Leung, N.; Zeuzem, S.; Wang, Y.; Lai, C.L.; Heathcote, E.J.; Manns, M.; Bzowej, N.; Niu, J.; Han, S.H.; Hwang, S.G.; Cakaloglu, Y.; Tong, M.J.; Papatheodoridis, G.; Chen, Y.; Brown, N.A.; Albanis, E.; Galil, K.; Naoumov, N.V. 2-Year GLOBE trial results: telbivudine Is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology 2009, 136, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.J.; Fielman, B.A.; Lloyd, D.M.; Chao, G.C.; Brown, N.A. Pharmacokinetics of telbivudine in healthy subjects and absence of drug interaction with lamivudine or adefovir dipivoxil. Antimicrob. Agents Chemother. 2006, 50, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Seifer, M.; Patty, A.; Serra, I.; Li, B.; Standring, D.N. Telbivudine, a nucleoside analog inhibitor of HBV polymerase, has a different in vitro cross-resistance profile than the nucleotide analog inhibitors adefovir and tenofovir. Antiviral Res. 2009, 81, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.; Foster, G.; Platis, D. Mechanisms of action of interferon and nucleoside analogues . J. Hepatol. 2003, 39(S1), S93–S98. [Google Scholar] [CrossRef]
- Lok, A.S.F.; McMahon, B.J. Chronic hepatitis B. Hepatology 2007, 45, 507–539. [Google Scholar] [CrossRef] [PubMed]
- Lau, G.K.; Piratvisuth, T.; Luo, K.X.; Marcellin, P.; Thongsawat, S.; Cooksley, G.; Gane, E.; Fried, M.W.; Chow, W.C.; Paik, S.W.; Chang, W.Y.; Berg, T.; Flisiak, R.; McCloud, P.; Pluck, N. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N. Engl. J. Med. 2005, 352, 2682–2695. [Google Scholar] [CrossRef] [PubMed]
- Brunetto, M.R.; Moriconi, F.; Bonino, F.; Lau, G.K.; Farci, P.; Yurdaydin, C.; Piratvisuth, T.; Luo, K.; Wang, Y.; Hadziyannis, S.; Wolf, E.; McCloud, P.; Batrla, R.; Marcellin, P. Hepatitis B virus surface antigen levels: a guide to sustained response to peginterferon alfa-2a in HBeAg-negative chronic hepatitis B. Hepatology 2009, 49, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Janssen, H.L.; van Zonneveld, M.; Senturk, H.; Zeuzem, S.; Akarca, U.S.; Cakaloglu, Y.; Simon, C.; So, T.M.; Gerken, G.; de Man, R.A.; Niesters, H.G; Hansen, B.; Schalm, S.W. Pegylated interferon alpha-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial . Lancet 2005, 365, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Lai, M.Y.; Chao, Y.C.; Liao, L.Y.; Yang, S.S.; Hsiao, T.J.; Hsieh, T.Y.; Lin, C.L.; Hu, J.T.; Chen, C.L.; Chen, P.J.; Kao, J.H.; Chen, D.S. Interferon alpha-2b with and without ribavirin in the treatment of hepatitis B e antigen-positive chronic hepatitis B: a randomized study . Hepatology 2006, 43, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Wursthorn, K.; Lutgehetmann, M.; Dandri, M.; Volz, T.; Buggisch, P.; Zollner, B.; Longerich, T.; Schirmacher, P.; Metzler, F.; Zankel, M.; Fischer, C.; Currie, G.; Brosgart, C.; Petersen, J. Peginterferon alpha-2b plus adefovir induce strong cccDNA decline and HBsAg reduction in patients with chronic hepatitis B. Hepatology 2006, 44, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Buster, E.H.; Hansen, B.E.; Buti, M.; Delwaide, J.; Niederau, C.; Michielsen, P.P.; Flisiak, R.; Zondervan, P.E.; Schalm, S.W.; Janssen, H.L. Peginterferon alpha-2b is safe and effective in HBeAg-positive chronic hepatitis B patients with advanced fibrosis. Hepatology 2007, 46, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Benhamou, Y.; Fleury, H.; Trimoulet, P.; Pellegrin, I.; Urbinelli, R.; Katlama, C.; Rozenbaum, W.; Le Teuff, G.; Trylesinski, A.; Piketty, C. Anti-hepatitis B virus efficacy of tenofovir disoproxil fumarate in HIV-infected patients. Hepatology 2006, 43, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Curtis, M.; Qi, X.; Miller, M.D.; Borroto-Esoda, K. Anti-hepatitis B virus activity in vitro of combinations of tenofovir with nucleoside/nucleotide analogues. Antiviral Chem. Chemother. 2009, 19, 165–176. [Google Scholar]
- Delaney 4th, W.E.; Ray, A.S.; Yang, H.; Qi, X.; Xiong, S.; Zhu, Y.; Miller, M.D. Intracellular metabolism and in vitro activity of tenofovir against hepatitis B virus. Antimicrob. Agents Chemother. 2006, 50, 2471–2477. [Google Scholar] [CrossRef] [PubMed]
- Birkus, G.; Hajek, M.; Kramata, P.; Votruba, I.; Holý, A.; Otova, B. Tenofovir diphosphate is a poor substrate and a weak inhibitor of rat DNA polymerases alpha, delta, and epsilon*. Antimicrob. Agents Chemother. 2002, 46, 1610–1613. [Google Scholar] [CrossRef] [PubMed]
- Delaney, W.E. 4th; Borroto-Esoda, K. Therapy of chronic hepatitis B: trends and developments. Curr. Opin. Pharmacol. 2008, 8, 532–540. [Google Scholar] [CrossRef]
- Marcellin, P.; Heathcote, E.J.; Buti, M.; Gane, E.; de Man, R.A.; Krastev, Z.; Germanidis, G.; Lee, S.S.; Flisiak, R.; Kaita, K.; Manns, M.; Kotzev, I.; Tchernev, K.; Buggisch, P.; Weilert, F.; Kurdas, O.O.; Shiffman, M.L.; Trinh, H.; Washington, M.K.; Sorbel, J.; Anderson, J.; Snow-Lampart, A.; Mondou, E.; Quinn, J.; Rousseau, F. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N. Engl. J. Med. 2008, 359, 2442–2455. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, K.; Gozlan, J.; Boyd, A.; Boelle, P.Y.; Bonnard, P.; Molina, J.M.; Miailhes, P.; Lascoux-Combe, C.; Serfaty, L.; Zoulim, F.; Girard, P.M. Comparison of the antiviral activity of adefovir and tenofovir on hepatitis B virus in HIV-HBV-coinfected patients. Antiviral Ther. 2008, 13, 705–713. [Google Scholar]
- Del Poggio, P.; Zaccanelli, M.; Oggionni, M.; Colombo, S.; Jamoletti, C.; Puhalo, V. Low-dose tenofovir is more potent than adefovir and is effective in controlling HBV viremia in chronic HBeAg-negative hepatitis B. World J. Gastroenterol. 2007, 13, 4096–4099. [Google Scholar] [PubMed]
- Tan, J.; Degertekin, B.; Wong, S.N.; Husain, M.; Oberhelman, K.; Lok, A.S. Tenofovir monotherapy is effective in hepatitis B patients with antiviral treatment failure to adefovir in the absence of adefovir-resistant mutations. J. Hepatol. 2008, 48, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Leemans, W.F.; Janssen, H.L.; Niesters, H.G.; de Man, R.A. Switching patients with lamivudine resistant chronic hepatitis B virus from tenofovir to adefovir results in less potent HBV-DNA suppression. J. Viral Hepat. 2008, 15, 108–114. [Google Scholar] [PubMed]
- Gutiérrez, S.; Guillemi, S.; Jahnke, N.; Montessori, V.; Harrigan, P.R.; Montaner, J.S. Tenofovir-based rescue therapy for advanced liver disease in 6 patients coinfected with HIV and hepatitis B virus and receiving lamivudine . Clin. Infect. Dis. 2008, 46, e28–30. [Google Scholar] [CrossRef] [PubMed]
- Menne, S.; Cote, P.J.; Korba, B.E.; Butler, S.D.; George, A.L.; Tochkov, I.A.; Delaney 4th, W.E.; Xiong, S ; Gerin, J.L.; Tennant, B.C. Antiviral effect of oral administration of tenofovir disoproxil fumarate in woodchucks with chronic woodchuck hepatitis virus infection. Antimicrob. Agents Chemother. 2005, 49, 2720–2728. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, J.; Camino, N.; Rodés, B.; Bartholomeusz, A.; Kuiper, M.; Tacke, F.; Núñez, M.; Mauss, S.; Lutz, T.; Klausen, G.; Locarnini, S.; Soriano, V. Selection of hepatitis B virus polymerase mutations in HIV-coinfected patients treated with tenofovir. Antiviral Ther. 2005, 10, 727–734. [Google Scholar]
- Amini-Bavil-Olyaee, S.; Herbers, U.; Sheldon, J.; Luedde, T.; Trautwein, C.; Tacke, F. The rtA194T polymerase mutation impacts viral replication and susceptibility to tenofovir in hepatitis B e antigen-positive and hepatitis B e antigen-negative hepatitis B virus strains. Hepatology 2009, 49, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- van Bömmel, F.; Zöllner, B.; Sarrazin, C.; Spengler, U.; Hüppe, D.; Möller, B.; Feucht, H.H.; Wiedenmann, B.; Berg, T. Tenofovir for patients with lamivudine-resistant hepatitis B virus (HBV) infection and high HBV DNA level during adefovir therapy. Hepatology 2006, 44, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Korba, B.E.; Schinazi, R.F.; Cote, P.; Tennant, B.C.; Gerin, J.L. Effect of oral administration of emtricitabine on woodchuck hepatitis virus replication in chronically infected woodchucks. Antimicrob. Agents Chemother. 2000, 44, 1757–1760. [Google Scholar] [CrossRef] [PubMed]
- Gish, R.G.; Trinh, H.; Leung, N.; Chan, F.K.; Fried, M.W.; Wright, T.L.; Wang, C.; Anderson, J.; Mondou, E.; Snow, A.; Sorbel, J.; Rousseau, F.; Corey, L. Safety and antiviral activity of emtricitabine (FTC) for the treatment of chronic hepatitis B infection: a two-year study. J. Hepatol. 2005, 43, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.G.; Krastev, Z.; Ng, T.M.; Mechkov, G.; Kotzev, I.A.; Chan, S.; Mondou, E.; Snow, A.; Sorbel, J.; Rousseau, F. Randomized, double-blind study of emtricitabine (FTC) plus clevudine versus FTC alone in treatment of chronic hepatitis B. Antimicrob. Agents Chemother. 2006, 50, 1642–1648. [Google Scholar] [CrossRef] [PubMed]
- Marcellin, P.; Mommeja-Marin, H.; Sacks, S.L.; Lau, G.K.; Sereni, D.; Bronowicki, J.P.; Conway, B.; Trepo, C.; Blum, M.R.; Yoo, B.C.; Mondou, E.; Sorbel, J.; Snow, A.; Rousseau, F.; Lee, H.S. A phase II dose-escalating trial of clevudine in patients with chronic hepatitis B. Hepatology 2004, 40, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Goulis, I.; Dalekos, G.N. Entecavir monotherapy for lamivudine-refractory chronic hepatitis B. Expert Rev. Anti Infect. Ther. 2008, 6, 855–859. [Google Scholar] [CrossRef]
- Yoo, B.C.; Kim, J.H.; Chung, Y.H.; Lee, K.S.; Paik, S.W.; Ryu, S.H.; Han, B.H.; Han, J.Y.; Byun, K.S.; Cho, M.; Lee, H.J.; Kim, T.H.; Cho, S.H.; Park, J.W.; Um, SH.; Hwang, S.G.; Kim, Y.S.; Lee, Y.J.; Chon, C.Y.; Kim, B.I.; Lee, Y.S.; Yang, J.M.; Kim, H.C.; Hwang, J.S.; Choi, S.K.; Kweon, Y.O.; Jeong, S.H.; Lee, M.S.; Choi, J.Y.; Kim, D.G.; Kim, Y.S.; Lee, H.Y.; Yoo, K.; Yoo, H.W.; Lee, H.S. Twenty-four-week clevudine therapy showed potent and sustained antiviral activity in HBeAg-positive chronic hepatitis B. Hepatology 2007, 45, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.C.; Kim, J.H.; Kim, T.H.; Koh, K.C.; Um, S.H.; Kim, Y.S.; Lee, K.S.; Han, B.H.; Chon, C.Y.; Han, J.Y.; Ryu, S.H.; Kim, H.C.; Byun, K.S.; Hwang, S.G.; Kim, B.I.; Cho, M.; Yoo, K.; Lee, H.J.; Hwang, J.S.; Kim, Y.S.; Lee, Y.S.; Choi, S.K.; Lee, Y.J.; Yang, J.M.; Park, J.W.; Lee, M.S.; Kim, D.G.; Chung, Y.H.; Cho, S.H.; Choi, J.Y.; Kweon, Y.O.; Lee, H.Y.; Jeong, S.H.; Yoo, H.W.; Lee, H.S. Clevudine is highly efficacious in hepatitis B e antigen-negative chronic hepatitis B with durable off-therapy viral suppression. Hepatology 2007, 46, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Oh, J.; Kwon, S.Y.; Choe, W.H.; Ko, S.Y.; Rhee, K.H.; Seo, T.H.; Lim, S.D.; Lee, C.H. Clevudine myopathy in patients with chronic hepatitis B . J. Hepatol. 2009, 51, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.I.; Lee, D.K.; Lee, C.H.; Park, M.S.; Kim, S.Y.; Kim, H.S.; Jo, H.Y.; Lee, C.H.; Kim, D.S. Long-term therapy with clevudine for chronic hepatitis B can be associated with myopathy characterized by depletion of mitochondrial DNA . Hepatology 2009, 49, 2080–2086. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical practice guidelines: management of chronic hepatitis B . J. Hepatol. 2009, 50, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Lok, A.S.F.; McMahon, B.J. AASLD Practice guidelines. Chronic hepatitis B: update 2009. Hepatology 2009, 50, 1–36. [Google Scholar] [PubMed]
- Liaw, Y.-F.; Suh, D.J.; Omata, M. APASL guidelines for HBV management . the APASL Annual Meeting: Seoul, Korea, 2008. [Google Scholar]
- Piratvisuth, T. Reviews for APASL guidelines: immunomodulatory therapy of chronic hepatitis B. Hepatol. Int. 2008, 2, 140–146. [Google Scholar] [CrossRef]
- Zoulim F.; Perrillo, R. Hepatitis B reflections on the current approach to antiviral therapy . J. Hepatol. 2008, 48 (Suppl. 1), S2–S19. [Google Scholar] [CrossRef] [PubMed]
- Lampertico, P.; Vigano, M.; Manenti, E.; Iavarone, M.; Colombo, M. Add-on adefovir prevents the emergence of adefovir resistance in lamivudine-resistant patients: a 4-year study . J. Hepatol. 2008, 48, S259. [Google Scholar] [CrossRef]
- Fournier, C.; Zoulim, F. Antiviral therapy of chronic hepatitis B prevention of drug resistance. Clin. Liver Dis. 2007, 11, 869–892. [Google Scholar] [CrossRef] [PubMed]
- Papatheodoridis, G.V.; Manolakopoulos, S.; Dusheiko, G.; Archimandritis, A.J. Therapeutic strategies in the management of patients with chronic hepatitis B virus infection. Lancet Infect. Dis. 2008, 8, 167–178. [Google Scholar] [CrossRef]
- Lampertico, P.; Colombo, M. HBeAg-negative chronic hepatitis B: why do I treat my patients with nucleos(t)ide analogues. Liver Int. 2009, 29, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Liaw, Y.F. On-treatment outcome prediction and adjustment during chronic hepatitis B therapy: now and future. Antiviral Ther. 2009, 14, 13–22. [Google Scholar]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Share and Cite
De Clercq, E.; Férir, G.; Kaptein, S.; Neyts, J. Antiviral Treatment of Chronic Hepatitis B Virus (HBV) Infections. Viruses 2010, 2, 1279-1305. https://doi.org/10.3390/v2061279
De Clercq E, Férir G, Kaptein S, Neyts J. Antiviral Treatment of Chronic Hepatitis B Virus (HBV) Infections. Viruses. 2010; 2(6):1279-1305. https://doi.org/10.3390/v2061279
Chicago/Turabian StyleDe Clercq, Erik, Geoffrey Férir, Suzanne Kaptein, and Johan Neyts. 2010. "Antiviral Treatment of Chronic Hepatitis B Virus (HBV) Infections" Viruses 2, no. 6: 1279-1305. https://doi.org/10.3390/v2061279




