PEGylated Adenoviruses: From Mice to Monkeys
Abstract
:1. Introduction
2. PEGylation: The Early Years
2.1. The Chemistry
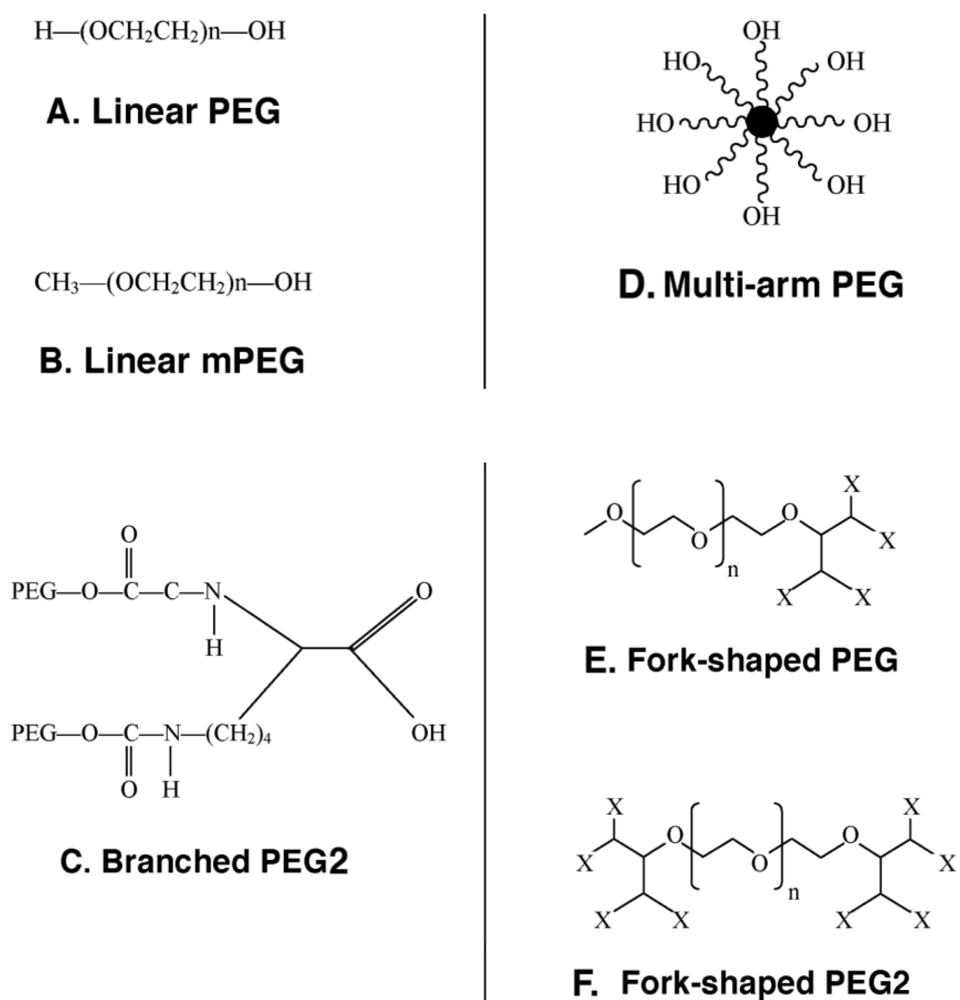
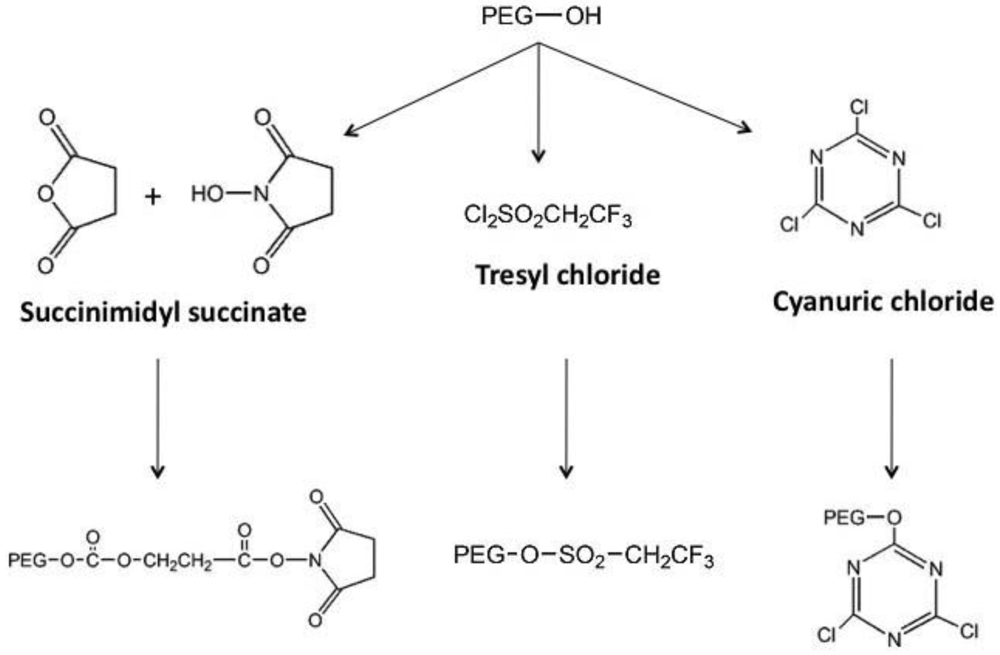
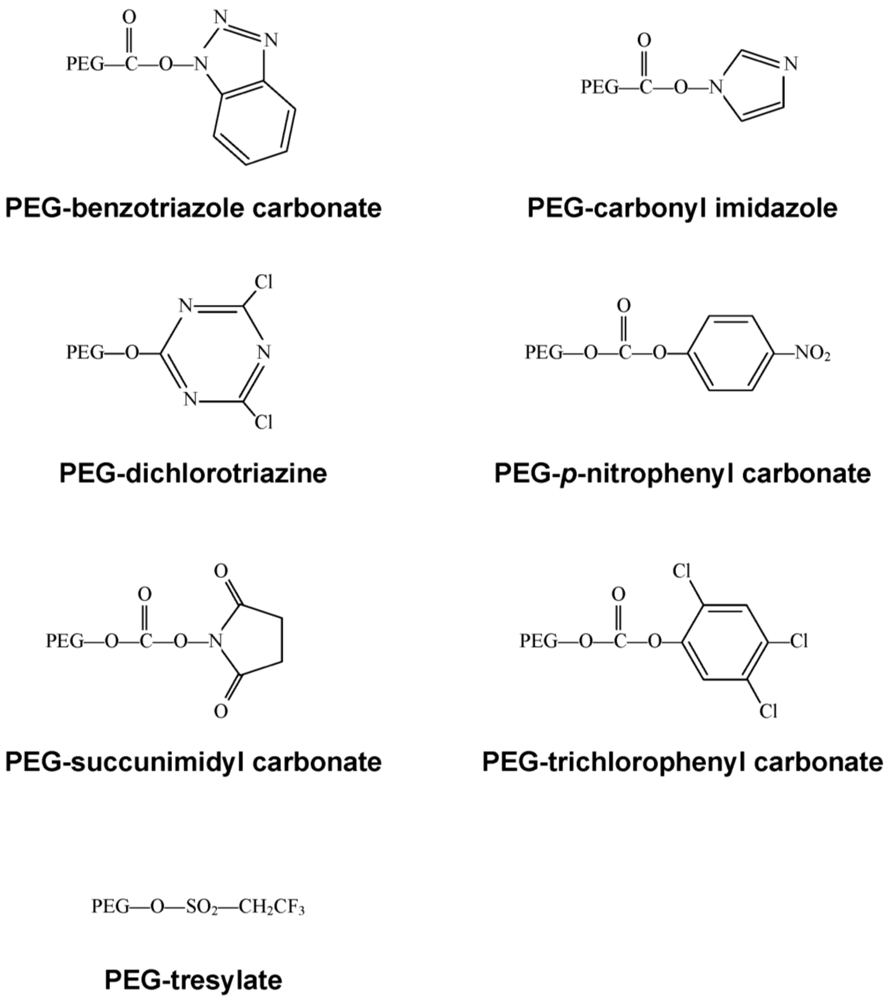
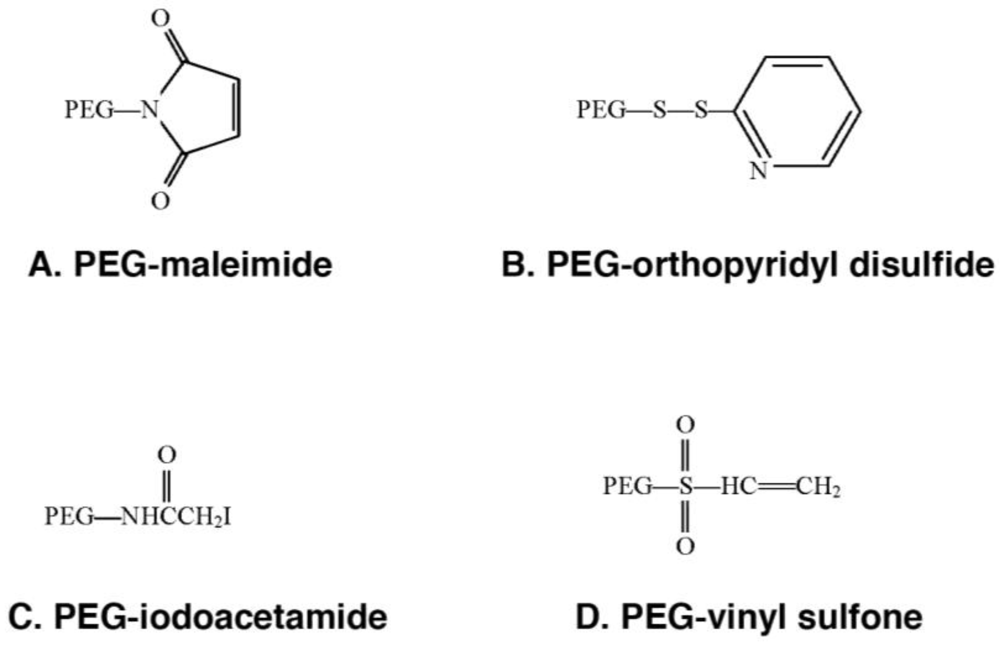
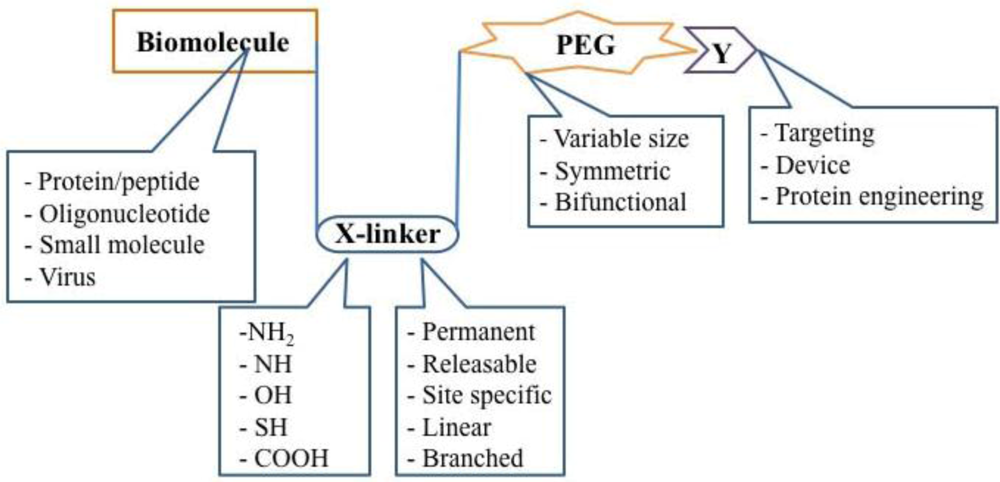
2.2. Tolerization to Antigens, Tissues and Cells
3. PEGylation in the Pharmaceutical Industry
| Parent Molecule | Generic name | Trade name (company) | Size of PEG moiety (kDa) | Indication | Year of approval |
|---|---|---|---|---|---|
| Adenosine deaminase | Pegademase bovine | AdagenÒ (Enzon) | 5 | Severe combined immunodeficiency disease (SCID) | 1990 |
| L-asparaginase | Pegaspargase | OncasparÒ (Enzon) | 5 | Acute lymphoblastic leukemia | 1994 |
| Interferon α-2b | Peginterferon α-2b | PegIntronÒ (Schering-Plough) | 12 | Hepatitis C | 2000 |
| Interferon α-2a | Peginterferon α-2a | PegasysÒ (Genetech) | 40 | Hepatitis C | 2001 |
| G-CSF | Pegfilgrastim | NeulastaTM (Amgen) | 20 | Neutropenia | 2002 |
| hGH | Pegvisomant | SomavertTM (Pfizer Pharmacia) | 5 | Acromegaly | 2003 |
| Erythropoietin | Methoxy polyethylene glycol-epoetin beta | Mircera (Roche) | 40 | Anemia | 2007 |
| Anti-TNF α Fab | Certolizumab pegol | Cimzia (UCB) | 40 | Rheumatoid arthritis and Crohn’s disease | 2008 |
| Pharmacokinetic Parameter | IFN-α | PegIntron (PEG-IFN α-2b) | Pegasys (PEG-IFN α-2a) |
|---|---|---|---|
| Elimination half life (hours) | 6-9 | 32-40 | 72-96 |
| Clearance (ml/hour) | 6,000 | 725 | 60-100 |
| Volume of distribution (L) | 25-30 | 20-40 | 8 |
| Tmax (hours) | 7-12 | 20 | 80 |
4. PEGylation and Gene Therapy in the 21st Century
4.1. Non-Viral Vectors.
| Virus | Model | Biological effects | Ref. |
|---|---|---|---|
| Adeno-associated virus | in vitro | Conjugation of AAV with monomethoxy poly(ethylene) glycols activated by tresyl chloride (TMPEG) and succinimidyl succinate (SSPEG) chemistries did not compromise transduction efficiency. | 66 |
| PEGylation with either 2 kDa or 5 kDa PEG at 1:1, 10:1, 100:1 and 1000:1 PEG:lysine ratios did not compromise transduction efficiency. Conjugation of rAAV with 2 kDa PEG at the 1000:1 PEG lysine ratio protected from serum neutralization. | 67 | ||
| in vivo | SSPEG and TMPEG improved gene transduction in the lung without compromising transduction efficiency in the liver and muscle. TMPEG reduced Th1-type response. Successful readministration of virus after iv injection was achieved by modification with TMPEG. | 66 | |
| Lentivirus | in vitro | Transduction efficiency of PEGylated virus was not compromised in the presence of neutralizing antibodies PEGylation provided a 20 fold resistance to antiserum and extended circulatory half-life by a factor of 5 with no observable loss in titer and prevented interaction with antibodies and inactivation of virus by complement in human and mouse sera. | 68 |
| in vivo | PEGylation extended the circulation half-life by a factor of 5 PEGylation improved transduction efficiency in the bone marrow and in the spleen 14 days after systemic administration of virus. | 68 | |
| Retrovirus | in vitro | Coating of retrovirus with PEG-poly(L-lysine)(PLL) block copolymer improved transduction efficiency 3 to 7 fold without increasing cytotoxicity. | 69 |
| Conjugation of a (1,2-distearoyl-sn-glycero-3-phosphoethanolamine), polyethylene glycol and biotin complex[DSPE-PEG-biotin] increased the number of viruses that bound to streptavidin coated plates by more than three-fold. | 70 | ||
| Baculovirus | in vitro | Transduction efficiency was deceased with an increased amount of PEG added to the virus surface. | 71 |
| in vivo | PEGylation improved transduction efficiency in the lung and brain. | 71 | |
| Influenza virosomes | in vitro | Reconstituted viral membranes containing 3 mol% poly(ethylene –glycol) grafted phosphatidylethanolamine retained 40% of their fusion activity. | 72, 73 |
4.2. The Adenovirus
4.3. Characterization of PEGylated Adenoviruses
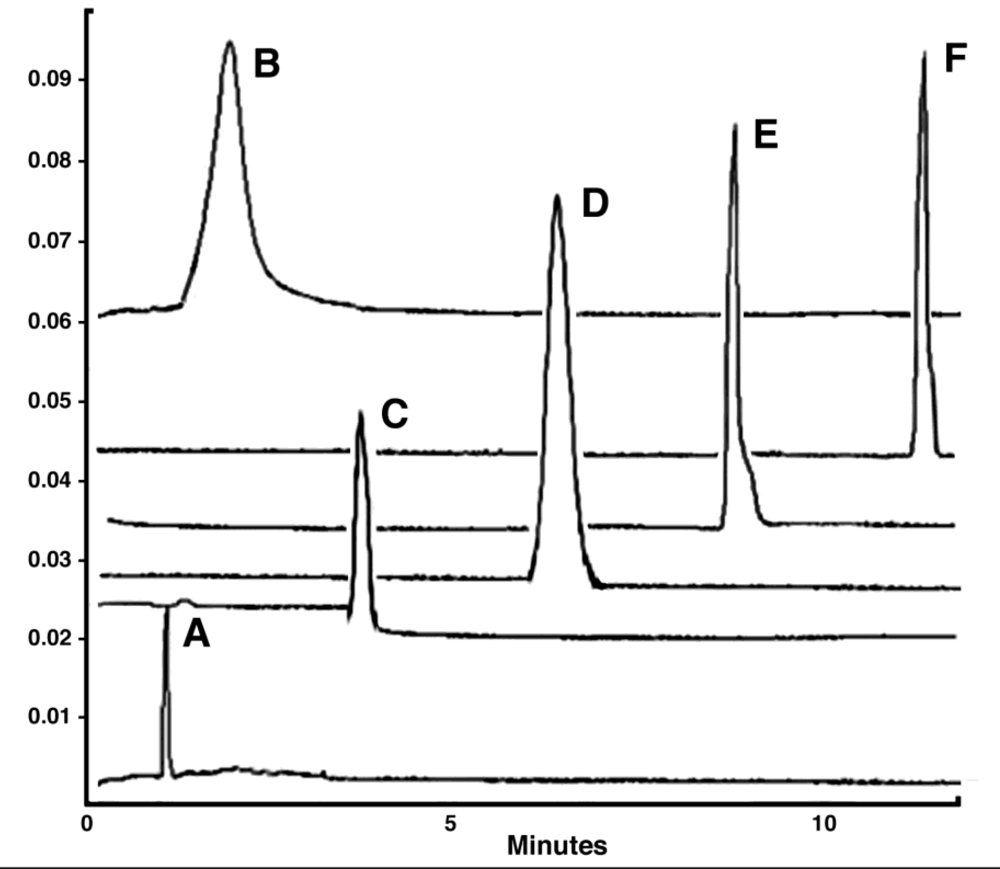
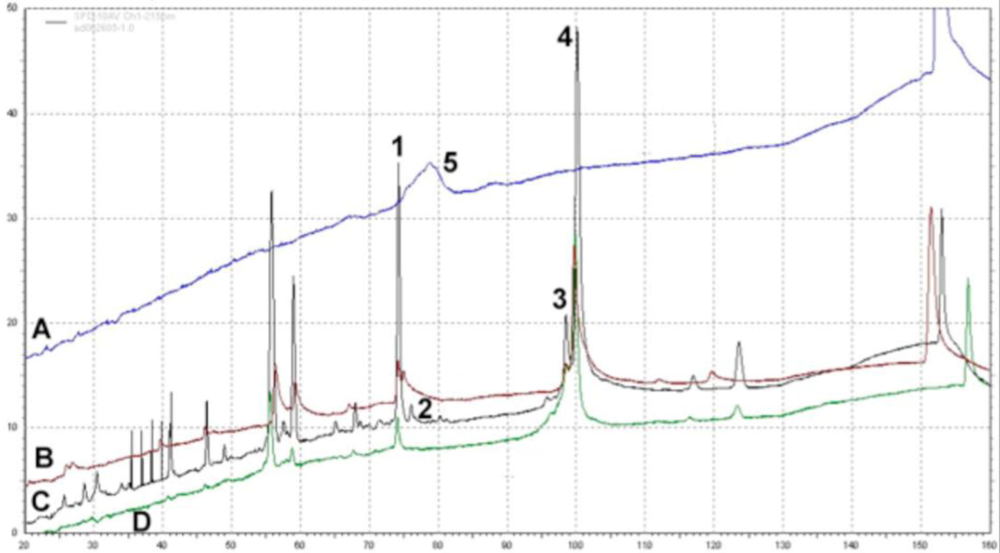
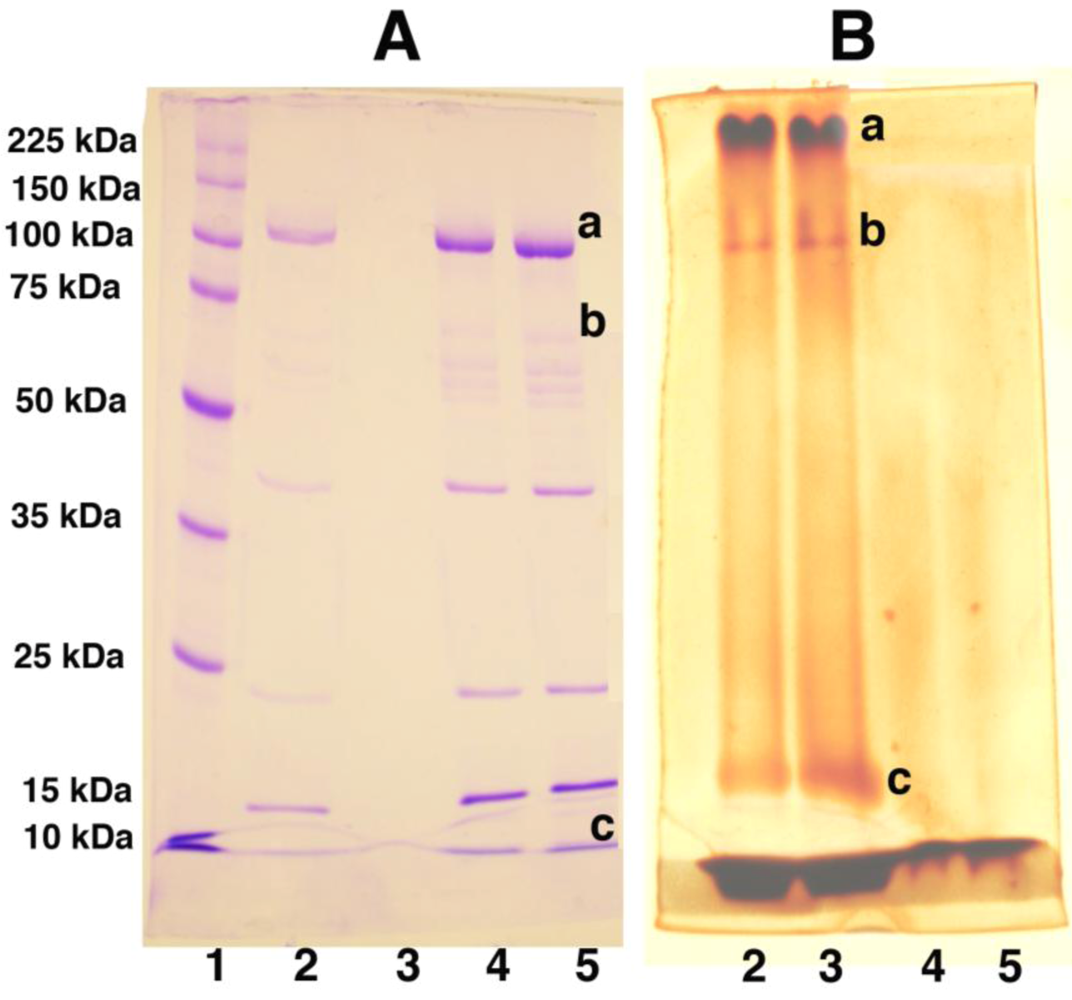
4.4. Pharmacology of Adenovirus-Pharmacokinetics and Biodistribution
| ↑AUC | ↓CL | ↑Half-life | |||||
|---|---|---|---|---|---|---|---|
| PEG size | 3.4 kDa | 5 kDa | 5 kDa | 20 kDa | |||
| Degree of modification | - | - | 30% | 60% | 90% | 100% | 45% |
| Fold change in Pharmacokinetic parameter | 12 | 4 | 1.3 | 2 | 3 | 22 | 25 |
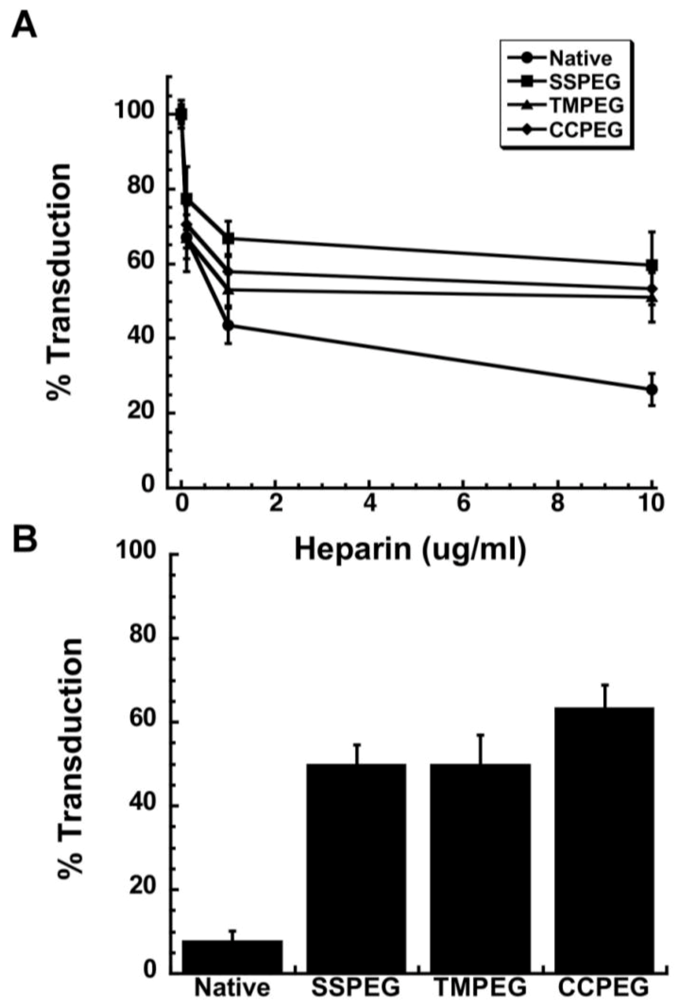
4.5. Toxicology of PEGylated Adenovirus
4.6. The Immune Response
5. Re-Directing Adenovirus by Physical Means: Effect of Molecular Size and Degree of PEGylation
Re-Directing Adenovirus by Chemical Means: Use of PEG as a “Linker” for Attachment of Receptor-Specific Conjugates
6. PEGylated Adenovirus-Based Vaccines
7. The Future of PEGylation of Viruses for Gene Transfer and Vaccine Applications
Acknowledgments
References
- Roberts, M.J.; Bentley, M.D.; Harris, J.M. Chemistry for peptide and protein PEGylation. Adv. Drug Deliv. Rev. 2002, 54, 459–476. [Google Scholar] [CrossRef] [PubMed]
- Smolinske, S.C. CRC Press: Boca Raton, FL, USA, 1992.
- Pasut, G.; Veronese, F.M. Polymer-drug conjugation, recent achievements and general strategies. Prog. Polym. Sci. 2007, 32, 933–961. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Pharmacokinetic consequences of pegylation. Drug Deliv. 2006, 13, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.M.; Mantovani, G.; Wang, X.; Haddleton, D.M.; Brayden, D.J. Advances in PEGylation of important biotech molecules: delivery aspects. Expert Opin. Drug Deliv. 2008, 5, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Fishburn, C.S. The pharmacology of PEGylation: balancing PD with PK to generate novel therapeutics. J. Pharm. Sci. 2008, 97, 4167–4183. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Jain, S.K. PEGylation: an approach for drug delivery. A review. Crit. Rev. Ther. Drug Carrier Syst. 2008, 25, 403–447. [Google Scholar] [PubMed]
- Harris, J.M.; Martin, N.E.; Modi, M. Pegylation: a novel process for modifying pharmacokinetics. Clin. Pharmacokinet. 2001, 40, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.M. PEGylation - A "Sunset" technology? - Recent advances and new applications suggest a favorable future for PEGylation technology Biopharm. Int. 2004, 17, 82–82. [Google Scholar]
- Veronese, F.M.; Pasut, G. PEGylation, successful approach to drug delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.M.; Chess, R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Bailon, P.; Won, C.Y. PEG-modified biopharmaceuticals. Expert Opin. Drug Deliv. 2009, 6, 1–16. [Google Scholar] [CrossRef]
- Abuchowski, A.; McCoy, J.R.; Palczuk, N.C.; van Es, T.; Davis, F.F. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J. Biol. Chem. 1977, 252, 3582–3586. [Google Scholar] [PubMed]
- Abuchowski, A.; van Es, T.; Palczuk, N.C.; Davis, F.F. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J. Biol. Chem. 1977, 252, 3578–3581. [Google Scholar] [PubMed]
- Lee, W.Y.; Sehon, A.H. Abrogation of reaginic antibodies with modified allergens. Nature 1977, 267, 618–619. [Google Scholar] [CrossRef] [PubMed]
- King, T.P.; Kochoumian, L.; Lichtenstein, L.M. Preparation and immunochemical properties of methoxypolyethylene glycol-coupled and N-carboxymethylated derivatives of ragweed pollen allergen, antigen E. Arch. Biochem. Biophys. 1977, 178, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Savoca, K.V.; Davis, F.F.; Palczuk, N.C. Induction of tolerance in mice by uricase and monomethoxypolyethylene glycol-modified uricase. Int. Arch. Allergy Appl. Immunol. 1984, 75, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Igarashi, T.; Fujii, T.; Kamisaki, Y.; Wada, H.; Kishimoto, S. Immune responses to polyethylene glycol modified L-asparaginase in mice. Int. Arch. Allergy Appl. Immunol. 1985, 76, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, I.; Jackson, C.J.; Lang, G.M.; Holford-Strevens, V.; Sehon, A.H. Tolerogenic polyethylene glycol derivatives of xenogeneic monoclonal immunoglobulins. Immunol. Lett. 1987, 15, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, I.; Jackson, C.J.; Lang, G.M.; Holford-Strevens, V.; Sehon, A.H. Tolerance induction in mice by conjugates of monoclonal immunoglobulins and monomethoxypolyethylene glycol. Transfer of tolerance by T cells and by T cell extracts. J. Immunol. 1987, 139, 326–331. [Google Scholar] [PubMed]
- Maiti, P.K.; Lang, G.M.; Sehon, A.H. Tolerogenic conjugates of xenogeneic monoclonal antibodies with monomethoxypolyethylene glycol I. Induction of long-lasting tolerance to xenogeneic monoclonal antibodies. Int. J. Cancer Suppl. 1988, 3, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.O.; So, T.; Ueda, T.; Imoto, T.; Koga, T. Prevention of collagen-induced arthritis (CIA) by treatment with polyethylene glycol-conjugated type II collagen; distinct tolerogenic property of the conjugated collagen from the native one. Clin. Exp. Immunol. 1997, 108, 213–219. [Google Scholar] [PubMed]
- Ito, H.O.; So, T.; Hirata, M.; Koga, T.; Ueda, T.; Imoto, T. Tolerogenic activity of polyethylene glycol-conjugated lysozyme distinct from that of the native counterpart. Immunology 1998, 93, 200–207. [Google Scholar] [CrossRef] [PubMed]
- So, T.; Ito, H.O.; Hirata, M.; Ueda, T.; Imoto, T. Extended blood half-life of monomethoxypolyethylene glycol-conjugated hen lysozyme is a key parameter controlling immunological tolerogenicity. Cell. Mol. Life Sci. 1999, 55, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- So, T.; Ito, H.O.; Tsujihata, Y.; Hirata, M.; Ueda, T.; Imoto, T. The molecular weight ratio of monomethoxypolyethylene glycol (mPEG) to protein determines the immunotolerogenicity of mPEG proteins. Protein Eng. 1999, 12, 701–705. [Google Scholar] [CrossRef] [PubMed]
- King, T.P.; Kochoumian, L.; Chiorazzi, N. Immunological properties of conjugates of ragweed pollen antigen E with methoxypolyethylene glycol or a copolymer of D-glutamic acid and D-lysine. J. Exp. Med. 1979, 149, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Sehon, A.H. Modulation of antibody responses by conjugates of antigens with monomethoxypolyethylene glycol. Adv. Exp. Med. Biol. 1989, 251, 341–351. [Google Scholar] [PubMed]
- Chen, Y.; Takata, M.; Maiti, P.K.; Rector, E.S.; Sehon, A.H. Characterization of suppressor T cell clones derived from a mouse tolerized with conjugates of ovalbumin and monomethoxypolyethylene glycol. Cell Immunol. 1992, 142, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.T.; Byun, S.M. Decreased agglutinability of methoxy-polyethylene glycol attached red blood cells: significance as a blood substitute. Artif. Cells Blood Substit. Immobil. Biotechnol. 1996, 24, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.D.; Murad, K.L.; Koumpouras, F.; Talbot, M.; Eaton, J.W. Chemical camouflage of antigenic determinants: stealth erythrocytes. Proc. Natl. Acad. Sci. U S A 1997, 94, 7566–7571. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.D.; Murad, K.L. Cellular camouflage: Fooling the immune system with polymers. Curr. Pharm. Design 1998, 4, 423–438. [Google Scholar]
- Murad, K.L.; Gosselin, E.J.; Eaton, J.W.; Scott, M.D. Stealth cells: prevention of major histocompatibility complex class II-mediated T-cell activation by cell surface modification. Blood 1999, 94, 2135–2141. [Google Scholar] [PubMed]
- Murad, K.L.; Mahany, K.L.; Brugnara, C.; Kuypers, F.A.; Eaton, J.W.; Scott, M.D. Structural and functional consequences of antigenic modulation of red blood cells with methoxypoly(ethylene glycol). Blood 1999, 93, 2121–2127. [Google Scholar] [PubMed]
- Chen, A.M.; Scott, M.D. Immunocamouflage: prevention of transfusion-induced graft-versus-host disease via polymer grafting of donor cells. J. Biomed. Mater. Res. A 2003, 67, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Nam, J.H.; Byun, Y. Effect of polyethylene glycol grafted onto islet capsules on prevention of splenocyte and cytokine attacks. J. Biomater. Sci. Polym. Ed. 2004, 15, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Park, S.J.; Nam, J.H.; Byun, Y. A combination therapy of PEGylation and immunosuppressive agent for successful islet transplantation. J. Control Release 2006, 110, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Park, S.J.; Nam, J.H.; Byun, Y. A new strategy toward improving immunoprotection in cell therapy for diabetes mellitus: long-functioning PEGylated islets in vivo. Tissue Eng. 2006, 12, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Park, S.J.; Lee, S.; Nam, J.H.; Byun, Y. Highly poly(ethylene) glycolylated islets improve long-term islet allograft survival without immunosuppressive medication. Tissue Eng. 2007, 13, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.; Abuchowski, A.; Park, Y.K.; Davis, F.F. Alteration of the circulating life and antigenic properties of bovine adenosine deaminase in mice by attachment of polyethylene glycol. Clin. Exp. Immunol. 1981, 46, 649–652. [Google Scholar] [PubMed]
- Hershfield, M.S. PEG-ADA replacement therapy for adenosine deaminase deficiency: an update after 8.5 years . Clin. Immunol. Immunopathol. 1995, 76, S228–S232. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.L. Pegaspargase: a review of clinical studies. Adv. Drug Deliv. Rev. 2003, 55, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Ashihara, Y.; Kono, T.; Yamazaki, S.; Inada, Y. Modification of E. coli L-asparaginase with polyethylene glycol: disappearance of binding ability to anti-asparaginase serum. Biochem. Biophys. Res. Commun. 1978, 83, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Abuchowski, A.; Davis, S.; Davis, F. Pharmacology of Escherichia coli-L-asparaginase polyethylene glycol adduct. Anticancer Res. 1981, 1, 373–376. [Google Scholar] [PubMed]
- Holle, L.M. Pegaspargase: an alternative? Ann. Pharmacother. 1997, 31, 616–624. [Google Scholar] [PubMed]
- Youngster, S.; Wang, Y.S.; Grace, M.; Bausch, J.; Bordens, R.; Wyss, D.F. Structure, biology, and therapeutic implications of pegylated interferon alpha-2b. Curr. Pharm. Des. 2002, 8, 2139–2157. [Google Scholar] [CrossRef]
- Veronese, F.M.; Mero, A. The impact of PEGylation on biological therapies. BioDrugs 2008, 22, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.R. Review article: pegylated interferons: chemical and clinical differences. Aliment Pharmacol. Ther. 2004, 20, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Monkarsh, S.P.; Ma, Y.; Aglione, A.; Bailon, P.; Ciolek, D.; DeBarbieri, B.; Graves, M.C.; Hollfelder, K.; Michel, H.; Palleroni, A.; Porter, J.E.; Russoman, E.; Roy, S.; Pan, Y.C. Positional isomers of monopegylated interferon alpha-2a: isolation, characterization, and biological activity. Anal. Biochem. 1997, 247, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Glue, P.; Fang, J.W.; Rouzier-Panis, R.; Raffanel, C.; Sabo, R.; Gupta, S.K.; Salfi, M.; Jacobs, S. Pegylated interferon-alpha2b: pharmacokinetics, pharmacodynamics, safety, and preliminary efficacy data. Hepatitis C Intervention Therapy Group. Clin. Pharmacol. Ther. 2000, 68, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Rajender Reddy, K.; Modi, M.W.; Pedder, S. Use of peginterferon alfa-2a (40 KD) (Pegasys) for the treatment of hepatitis C. Adv. Drug Deliv. Rev. 2002, 54, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Molineux, G. The design and development of pegfilgrastim (PEG-rmetHuG-CSF, Neulasta). Curr. Pharm. Des. 2004, 10, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Mintzer, M.A.; Simanek, E.E. Nonviral vectors for gene delivery. Chem. Rev. 2009, 109, 259–302. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.B.; Koehler, D.R.; Hu, J. Adenoviral vectors for gene replacement therapy. Viral Immunology 2004, 17, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Kim, K.S.; Liu, D. Nonviral gene delivery: what we know and what is next . Aaps. J. 2007, 9, E92–E104. [Google Scholar] [CrossRef] [PubMed]
- Senior, J.H. Fate and behavior of liposomes in vivo: a review of controlling factors. Crit. Rev. Ther. Drug Carrier Syst. 1987, 3, 123–193. [Google Scholar] [PubMed]
- Sakurai, H.; Kawabata, K.; Sakurai, F.; Nakagawa, S.; Mizuguchi, H. Innate immune response induced by gene delivery vectors. Int. J. Pharm. 2008, 354, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kichler, A.; Chillon, M.; Leborgne, C.; Danos, O.; Frisch, B. Intranasal gene delivery with a polyethylenimine-PEG conjugate. J. Control Release 2002, 81, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Webster, P.; Davis, M.E. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur. J. Cell Biol. 2004, 83, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, J.; Zhang, Y.; Pan, Y.; Zhao, J.; Ren, L.; Liao, M.; Hu, Z.; Kong, L.; Wang, J. A novel PEGylation of chitosan nanoparticles for gene delivery. Biotechnol. Appl. Biochem. 2007, 46, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Klibanov, A.L.; Maruyama, K.; Torchilin, V.P.; Huang, L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990, 268, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Papisov, M.I. Theoretical considerations of RES-avoiding liposomes: Molecular mechanics and chemistry of liposome interactions. Adv. Drug Deliv. Rev. 1998, 32, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Fenske, D.B.; MacLachlan, I.; Cullis, P.R. Long-circulating vectors for the systemic delivery of genes. Curr. Opin. Mol. Ther. 2001, 3, 153–158. [Google Scholar] [PubMed]
- Song, L.Y.; Ahkong, Q.F.; Rong, Q.; Wang, Z.; Ansell, S.; Hope, M.J.; Mui, B. Characterization of the inhibitory effect of PEG-lipid conjugates on the intracellular delivery of plasmid and antisense DNA mediated by cationic lipid liposomes. Biochim. Biophys. Acta. 2002, 1558, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kichler, A. Gene transfer with modified polyethylenimines . J. Gene Med. 2004, 6, S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, J.K.; Park, J.S.; Byun, Y.; Kim, C.K. The use of PEGylated liposomes to prolong circulation lifetimes of tissue plasminogen activator. Biomaterials 2009, 30, 5751–5756. [Google Scholar] [CrossRef] [PubMed]
- Le, H.T.; Yu, Q.C.; Wilson, J.M.; Croyle, M.A. Utility of PEGylated recombinant adeno-associated viruses for gene transfer. J. Control Release 2005, 108, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.K.; Maheshri, N.; Kaspar, B.; Schaffer, D.V. PEG conjugation moderately protects adeno-associated viral vectors against antibody neutralization. Biotechnol. Bioeng. 2005, 92, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Croyle, M.A.; Callahan, S.M.; Auricchio, A.; Schumer, G.; Linse, K.D.; Wilson, J.M.; Brunner, L.J.; Kobinger, G.P. PEGylation of a vesicular stomatitis virus G pseudotyped lentivirus vector prevents inactivation in serum. J. Virol. 2004, 78, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Katakura, H.; Harada, A.; Kataoka, K.; Furusho, M.; Tanaka, F.; Wada, H.; Ikenaka, K. Improvement of retroviral vectors by coating with poly(ethylene glycol)-poly(L-lysine) block copolymer (PEG-PLL). J. Gene Med. 2004, 6, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, N.G.; Lyon, L.A.; Le Doux, J.M. Rapid modification of retroviruses using lipid conjugates. Nanotechnology 2009, 20, 65103. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Park, I.K.; Jiang, H.L.; Choi, J.Y.; Je, Y.H.; Jin, H.; Kim, H.W.; Cho, M.H.; Cho, C.S. Regulation of transduction efficiency by pegylation of baculovirus vector in vitro and in vivo. J. Biotechnol. 2006, 125, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Mastrobattista, E.; Schoen, P.; Wilschut, J.; Crommelin, D.J.; Storm, G. Targeting influenza virosomes to ovarian carcinoma cells. FEBS Lett. 2001, 509, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Khoshnejad, M.; Young, P.R.; Toth, I.; Minchin, R.F. Modified influenza virosomes: recent advances and potential in gene delivery. Curr. Med. Chem. 2007, 14, 3152–3156. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.T. Adenoviral vectors for gene therapy. Mol. Biotechnol. 2007, 36, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Schagen, F.H.E.; Ossevoort, M.; Toes, R.E.M.; Hoeben, R.C. Immune responses against adenoviral vectors and their transgene products: a review of strategies for evasion. Crit. Rev. Oncol. Hematol. 2004, 50, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Morral, N.; O'Neal, W.K.; Rice, K.; Leland, M.M.; Piedra, P.A.; Aguilar-Cordova, E.; Carey, K.D.; Beaudet, A.L.; Langston, C. Lethal toxicity, severe endothelial injury, and a threshold effect with high doses of an adenoviral vector in baboons. Hum. Gene Ther. 2002, 13, 143–154. [Google Scholar] [PubMed]
- McConnell, M.J.; Imperiale, M.J. Biology of adenovirus and its use as a vector for gene therapy. Hum. Gene Ther. 2004, 15, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bergelson, J.M. Adenovirus receptors. J. Virol. 2005, 79, 12125–12131. [Google Scholar] [CrossRef] [PubMed]
- O'Riordan, C.R.; Lachapelle, A.; Delgado, C.; Parkes, V.; Wadsworth, S.C.; Smith, A.E.; Francis, G.E. PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Hum. Gene Ther. 1999, 10, 1349–1358. [Google Scholar] [PubMed]
- Croyle, M.A.; Yu, Q.C.; Wilson, J.M. Development of a rapid method for the PEGylation of adenoviruses with enhanced transduction and improved stability under harsh storage conditions. Hum. Gene Ther. 2000, 11, 1713–1722. [Google Scholar] [PubMed]
- Croyle, M.A.; Chirmule, N.; Zhang, Y.; Wilson, J.M. "Stealth" adenoviruses blunt cell-mediated and humoral immune responses against the virus and allow for significant gene expression upon readministration in the lung. J. Virol. 2001, 75, 4792–4801. [Google Scholar] [CrossRef] [PubMed]
- Croyle, M.A.; Chirmule, N.; Zhang, Y.; Wilson, J.M. PEGylation of E1-deleted adenovirus vectors allows significant gene expression on readministration to liver. Hum. Gene Ther. 2002, 13, 1887–1900. [Google Scholar] [PubMed]
- Mok, H.; Park, J.W.; Park, T.G. Microencapsulation of PEGylated adenovirus within PLGA microspheres for enhanced stability and gene transfection efficiency. Pharm. Res. 2007, 24, 2263–2269. [Google Scholar] [CrossRef] [PubMed]
- Mok, H.; Palmer, D.J.; Ng, P.; Barry, M.A. Evaluation of polyethylene glycol modification of first-generation and helper-dependent adenoviral vectors to reduce innate immune responses. Mol. Ther. 2005, 11, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.K.; Mok, H.; Park, T.G. Folate immobilized and PEGylated adenovirus for retargeting to tumor cells. Bioconjug. Chem. 2006, 17, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.Q.; Eto, Y.; Yoshioka, Y.; Sekiguchi, F.; Kurachi, S.; Morishige, T.; Yao, X.; Watanabe, H.; Asavatanabodee, R.; Sakurai, F.; Mizuguchi, H.; Okada, Y.; Mukai, Y.; Tsutsumi, Y.; Mayumi, T.; Okada, N.; Nakagawa, S. Effective tumor targeted gene transfer using PEGylated adenovirus vector via systemic administration. J. Control Release 2007, 122, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Delgado, C.; Malik, F.; Selisko, B.; Fisher, D.; Francis, G.E. Quantitative analysis of polyethylene glycol (PEG) in PEG-modified proteins/cytokines by aqueous two-phase systems. J. Biochem. Biophys. Methods 1994, 29, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Kurfurst, M.M. Detection and molecular weight determination of polyethylene glycol-modified hirudin by staining after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal. Biochem. 1992, 200, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Alemany, R.; Suzuki, K.; Curiel, D.T. Blood clearance rates of adenovirus type 5 in mice. J. Gen. Virol. 2000, 81, 2605–2609. [Google Scholar] [PubMed]
- Wonganan, P.; Courtney, C.C.; Leggiero, E.; Brasky, K.M.; Astone, D.; Pastore, L.; Croyle, M.A. Pharmacology and toxicology of PEGylated helper-dependent adenovirus in non-human primates . Mol. Ther. 2009, submitted for publication. [Google Scholar]
- Ogawara, K.; Rots, M.G.; Kok, R.J.; Moorlag, H.E.; Van Loenen, A.M.; Meijer, D.K.; Haisma, H.J.; Molema, G. A novel strategy to modify adenovirus tropism and enhance transgene delivery to activated vascular endothelial cells in vitro and in vivo. Hum. Gene Ther. 2004, 15, 433–443. [Google Scholar] [PubMed]
- Yao, X.; Yoshioka, Y.; Morishige, T.; Eto, Y.; Watanabe, H.; Okada, Y.; Mizuguchi, H.; Mukai, Y.; Okada, N.; Nakagawa, S. Systemic administration of a PEGylated adenovirus vector with a cancer-specific promoter is effective in a mouse model of metastasis. Gene Ther. 2009. [Google Scholar]
- Hofherr, S.E.; Shashkova, E.V.; Weaver, E.A.; Khare, R.; Barry, M.A. Modification of adenoviral vectors with polyethylene glycol modulates in vivo tissue tropism and gene expression. Mol. Ther. 2008, 16, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.A.; Mehaffey, M.G.; Kayda, D.B.; Saunders, J.M.; Yei, S.; Trapnell, B.C.; McClelland, A.; Kaleko, M. Adenovirus mediated expression of therapeutic plasma levels of human factor IX in mice. Nat. Genet. 1993, 5, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Huard, J.; Lochmuller, H.; Acsadi, G.; Jani, A.; Massie, B.; Karpati, G. The route of administration is a major determinant of the transduction efficiency of rat tissues by adenoviral recombinants. Gene Ther. 1995, 2, 107–115. [Google Scholar] [PubMed]
- Sullivan, D.E.; Dash, S.; Du, H.; Hiramatsu, N.; Aydin, F.; Kolls, J.; Blanchard, J.; Baskin, G.; Gerber, M.A. Liver-directed gene transfer in non-human primates. Hum. Gene Ther. 1997, 8, 1195–1206. [Google Scholar] [CrossRef]
- Lanciotti, J.; Song, A.; Doukas, J.; Sosnowski, B.; Pierce, G.; Gregory, R.; Wadsworth, S.; O'Riordan, C. Targeting adenoviral vectors using heterofunctional polyethylene glycol FGF2 conjugates. Mol. Ther. 2003, 8, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Lievens, J.; Snoeys, J.; Vekemans, K.; Van Linthout, S.; de Zanger, R.; Collen, D.; Wisse, E.; De Geest, B. The size of sinusoidal fenestrae is a critical determinant of hepatocyte transduction after adenoviral gene transfer. Gene Ther. 2004, 11, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Croyle, M.A.; Le, H.T.; Linse, K.D.; Cerullo, V.; Toietta, G.; Beaudet, A.; Pastore, L. PEGylated helper-dependent adenoviral vectors: highly efficient vectors with an enhanced safety profile. Gene Ther. 2005, 12, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.H.; McVey, J.H.; Waddington, S.N.; Di Paolo, N.C.; Shayakhmetov, D.M. The influence of blood on in vivo adenovirus bio-distribution and transduction. Mol. Ther. 2007, 15, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Kalyuzhniy, O.; Di Paolo, N.C.; Silvestry, M.; Hofherr, S.E.; Barry, M.A.; Stewart, P.L.; Shayakhmetov, D.M. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 5483–5488. [Google Scholar] [CrossRef]
- Waddington, S.N.; McVey, J.H.; Bhella, D.; Parker, A.L.; Barker, K.; Atoda, H.; Pink, R.; Buckley, S.M.; Greig, J.A.; Denby, L.; Custers, J.; Morita, T.; Francischetti, I.M.; Monteiro, R.Q.; Barouch, D.H.; van Rooijen, N.; Napoli, C.; Havenga, M.J.; Nicklin, S.A.; Baker, A.H. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 2008, 132, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.A.; Idamakanti, N.; Rollence, M.L.; Marshall-Neff, J.; Kim, J.; Mulgrew, K.; Nemerow, G.R.; Kaleko, M.; Stevenson, S.C. Adenovirus serotype 5 fiber shaft influences in vivo gene transfer in mice. Hum. Gene Ther. 2003, 14, 777–787. [Google Scholar] [PubMed]
- Smith, T.A. G.; Idamakanti, N.; Marshall-Neff, J.; Rollence, M.L.; Wright, P.; Kaloss, M.; King, L.; Mech, C.; Dinges, L.; Iverson, W.O.; Sherer, A.D.; Markovits, J.E.; Lyons, R.M.; Kaleko, M.; Stevenson, S.C. Receptor interactions involved in adenoviral-mediated gene delivery after systemic administration in non-human primates. Hum. Gene Ther. 2003, 14, 1595–1604. [Google Scholar] [PubMed]
- Nicol, C.G.; Graham, D.; Miller, W.H.; White, S.J.; Smith, T.A.; Nicklin, S.A.; Stevenson, S.C.; Baker, A.H. Effect of adenovirus serotype 5 fiber and penton modifications on in vivo tropism in rats. Mol. Ther. 2004, 10, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Braet, F.; Wisse, E. Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp. Hepatol. 2002, 1, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunetti-Pierri, N.; Palmer, D.J.; Beaudet, A.L.; Carey, K.D.; Finegold, M.; Ng, P. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum. Gene Ther. 2004, 15, 35–46. [Google Scholar] [PubMed]
- Zhang, Y.; Chirmule, N.; Gao, G.P.; Qian, R.; Croyle, M.; Joshi, B.; Tazelaar, J.; Wilson, J.M. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol. Ther. 2001, 3, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Higginbotham, J.N.; Seth, P.; Blaese, R.M.; Ramsey, W.J. The release of inflammatory Cytokines from human peripheral blood mononuclear cells in vitro following exposure to adenovirus variants and capsid. Hum. Gene Ther. 2002, 13, 129–141. [Google Scholar] [PubMed]
- Schnell, M.A.; Zhang, Y.; Tazelaar, J.; Gao, G.P.; Yu, Q.C.; Qian, R.; Chen, S.J.; Varnavski, A.N.; LeClair, C.; Raper, S.E.; Wilson, J.M. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol. Ther. 2001, 3, 708–722. [Google Scholar] [CrossRef] [PubMed]
- Lozier, J.N.; Csako, G.; Mondoro, T.H.; Krizek, D.M.; Metzger, M.E.; Costello, R.; Vostal, J.G.; Rick, M.E.; Donahue, R.E.; Morgan, R.A. Toxicity of a first-generation adenoviral vector in rhesus macaques. Hum. Gene Ther. 2002, 13, 113–124. [Google Scholar] [PubMed]
- Wolins, N.; Lozier, J.; Eggerman, T.L.; Jones, E.; Aguilar-Cordova, E.; Vostal, J.G. Intravenous administration of replication-incompetent adenovirus to rhesus monkeys induces thrombocytopenia by increasing in vivo platelet clearance. Br. J. Haematol. 2003, 123, 903–905. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, N.; Kawabata, K.; Sakurai, F.; Watanabe, Y.; Hayakawa, T.; Mizuguchi, H. Modified adenoviral vectors ablated for coxsackievirus-adenovirus receptor, alpha(v) integrin, and heparan sulfate binding reduce in vivo tissue transduction and toxicity. Hum. Gene Ther. 2006, 17, 264–279. [Google Scholar] [CrossRef] [PubMed]
- De Geest, B.; Snoeys, J.; Van Linthout, S.; Lievens, J.; Collen, D. Elimination of innate immune responses and liver inflammation by PEGylation of adenoviral vectors and methylprednisolone. Hum. Gene Ther. 2005, 16, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Hofherr, S.E.; Mok, H.; Gushiken, F.C.; Lopez, J.A.; Barry, M.A. Polyethylene glycol modification of adenovirus reduces platelet activation, endothelial cell activation, and thrombocytopenia. Hum. Gene Ther. 2007, 18, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Muruve, D.A.; Barnes, M.J.; Stillman, I.E.; Libermann, T.A. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum. Gene Ther. 1999, 10, 965–976. [Google Scholar] [PubMed]
- Jooss, K.; Ertl, H.C.J.; Wilson, J. M. Cytotoxic T-lymphocyte target proteins and their major histocompatibility complex class I restriction in response to adenovirus vectors delivered to mouse liver. J. Virol. 1998, 72, 2945–2954. [Google Scholar] [PubMed]
- Yang, Y.P.; Wilson, J.M. Clearance of Adenovirus-Infected Hepatocytes by Mhc Class I-Restricted Cd4(+) Ctls in-Vivo. J. Immunol. 1995, 155, 2564–2570. [Google Scholar] [PubMed]
- Kuriyama, S.; Tominaga, K.; Kikukawa, M.; Tsujimoto, T.; Nakatani, T.; Tsujinoue, H.; Okuda, H.; Nagao, S.; Mitoro, A.; Yoshiji, H.; Fukui, H. Transient cyclophosphamide treatment before intraportal readministration of an adenoviral vector can induce re-expression of the original gene construct in rat liver. Gene Ther. 1999, 6, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, S.; Tominaga, K.; Mitoro, A.; Tsujinoue, H.; Nakatani, T.; Yamazaki, M.; Nagao, S.; Toyokawa, Y.; Okamoto, S.; Fukui, H. Immunomodulation with FK506 around the time of intravenous re-administration of an adenoviral vector facilitates gene transfer into primed rat liver. Int. J. Cancer 2000, 85, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Jooss, K.; Yang, Y.; Wilson, J.M. Cyclophosphamide diminishes inflammation and prolongs transgene expression following delivery of adenoviral vectors to mouse liver and lung. Hum. Gene Ther. 1996, 7, 1555–1566. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Eisensmith, R.C.; Wang, H.; Kay, M.A.; Cross, R.E.; Landen, C.N.; Gordon, G.; Bellinger, D.A.; Read, M.S.; Hu, P.C. Gene therapy for hemophilia B: host immunosuppression prolongs the therapeutic effect of adenovirus-mediated factor IX expression. Hum. Gene Ther. 1995, 6, 1039–1044. [Google Scholar] [CrossRef]
- Sterman, D.H.; Molnar-Kimber, K.; Iyengar, T.; Chang, M.; Lanuti, M.; Amin, K.M.; Pierce, B.K.; Kang, E.; Treat, J.; Recio, A.; Litzky, L.; Wilson, J.M.; Kaiser, L.R.; Albelda, S.M. A pilot study of systemic corticosteroid administration in conjunction with intrapleural adenoviral vector administration in patients with malignant pleural mesothelioma. Cancer Gene Ther. 2000, 7, 1511–1518. [Google Scholar] [PubMed]
- Yang, Y.P.; Trinchieri, G.; Wilson, J.M. Recombinant Il-12 Prevents Formation of Blocking Iga Antibodies to Recombinant Adenovirus and Allows Repeated Gene-Therapy to Mouse Lung. Nat. Med. 1995, 1, 890–893. [Google Scholar] [CrossRef]
- Yang, Y.P.; Su, Q.; Grewal, I.S.; Schilz, R.; Flavell, R.A.; Wilson, J.M. Transient subversion of CD40 ligand function diminishes immune responses to adenovirus vectors in mouse liver and lung tissues. J. Virol. 1996, 70, 6370–6377. [Google Scholar] [PubMed]
- Kay, M.A.; Meuse, L.; Gown, A.M.; Linsley, P.; Hollenbaugh, D.; Aruffo, A.; Ochs, H.D.; Wilson, C.B. Transient immunomodulation with anti-CD40 ligand antibody and CTLA4Ig enhances persistence and secondary adenovirus-mediated gene transfer into mouse liver. Proc. Natl. Acad. of Sci. U. S. A. 1997, 94, 4686–4691. [Google Scholar] [CrossRef]
- Jooss, K.; Turka, L.A.; Wilson, J.M. Blunting of immune responses to adenoviral vectors in mouse liver and lung with CTLA4Ig. Gene Ther. 1998, 5, 309–319. [Google Scholar] [PubMed]
- Alba, R.; Bosch, A.; Chillon, M. Gutless adenovirus: last-generation adenovirus for gene therapy . Gene Ther. 2005, 12, S18–S27. [Google Scholar] [CrossRef] [PubMed]
- Seiler, M.P.; Cerullo, V.; Lee, B. Immune response to helper dependent adenoviral mediated liver gene therapy: Challenges and prospects. Curr. Gene Ther. 2007, 7, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Youil, R.; Toner, T.J.; Su, Q.; Chen, M.; Tang, A.; Bett, A.J.; Casimiro, D. Hexon gene switch strategy for the generation of chimeric recombinant adenovirus. Hum. Gene Ther. 2002, 13, 311–320. [Google Scholar] [PubMed]
- Roberts, D.M.; Nanda, A.; Havenga, M.J.; Abbink, P.; Lynch, D.M.; Ewald, B.A.; Liu, J.; Thorner, A.R.; Swanson, P.E.; Gorgone, D.A.; Lifton, M.A.; Lemckert, A.A.; Holterman, L.; Chen, B.; Dilraj, A.; Carville, A.; Mansfield, K.G.; Goudsmit, J.; Barouch, D.H. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 2006, 441, 239–243. [Google Scholar] [CrossRef] [PubMed]
- DiPaolo, N.; Ni, S.; Gaggar, A.; Strauss, R.; Tuve, S.; Li, Z.Y.; Stone, D.; Shayakhmetov, D.; Kiviat, N.; Toure, P.; Sow, S.; Horvat, B.; Lieber, A. Evaluation of adenovirus vectors containing serotype 35 fibers for vaccination. Mol. Ther. 2006, 13, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Bernt, K.; Gaggar, A.; Li, Z.Y.; Kiem, H.P.; Lieber, A. Evaluation of biodistribution and safety of adenovirus vectors containing group B fibers after intravenous injection into baboons. Hum. Gene Ther. 2005, 16, 664–677. [Google Scholar] [CrossRef] [PubMed]
- Romanczuk, H.; Galer, C.E.; Zabner, J.; Barsomian, G.; Wadsworth, S.C.; O'Riordan, C.R. Dressing up adenoviruses to modify their tropism. Interview by Florence Paillard. Hum. Gene Ther. 1999, 10, 2575–2576. [Google Scholar] [PubMed]
- Eto, Y.; Gao, J.Q.; Sekiguchi, F.; Kurachi, S.; Katayama, K.; Mizuguchi, H.; Hayakawa, T.; Tsutsumi, Y.; Mayumi, T.; Nakagawa, S. Neutralizing antibody evasion ability of adenovirus vector induced by the bioconjugation of methoxypolyethylene glycol succinimidyl propionate (MPEG-SPA). Biol. Pharm. Bull. 2004, 27, 936–938. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Kida, S.; Hojo, K.; Eto, Y.; Gaob, J. Q.; Kurachi, S.; Sekiguchi, F.; Mizuguchi, H.; Hayakawa, T.; Mayumi, T.; Nakagawa, S.; Kawasaki, K. Design and synthesis of a peptide-PEG transporter tool for carrying adenovirus vector into cells. Bioorg. Med. Chem. Lett. 2005, 15, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Wortmann, A.; Vohringer, S.; Engler, T.; Corjon, S.; Schirmbeck, R.; Reimann, J.; Kochanek, S.; Kreppel, F. Fully detargeted polyethylene glycol-coated adenovirus vectors are potent genetic vaccines and escape from pre-existing anti-adenovirus antibodies. Mol. Ther. 2008, 16, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Park, H.J.; Kim, P.H.; Lee, J.; Hyung, W.; Yang, J.; Ko, H.; Sohn, J.H.; Kim, J.H.; Huh, Y.M.; Yun, C.O.; Haam, S. Retargeting of adenoviral gene delivery via Herceptin-PEG-adenovirus conjugates to breast cancer cells. J. Control Release 2007, 123, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Bergelson, J.M.; Cunningham, J.A.; Droguett, G.; Kurt-Jones, E.A.; Krithivas, A.; Hong, J.S.; Horwitz, M.S.; Crowell, R.L.; Finberg, R.W. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 1997, 275, 1320–1323. [Google Scholar] [CrossRef] [PubMed]
- Wickham, T.J.; Mathias, P.; Cheresh, D.A.; Nemerow, G.R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 1993, 73, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Harfe, B.; Freimuth, P. Mutations that alter an Arg-Gly-Asp (RGD) sequence in the adenovirus type 2 penton base protein abolish its cell-rounding activity and delay virus reproduction in flat cells. J. Virol. 1993, 67, 5198–5205. [Google Scholar] [PubMed]
- Mizuguchi, H.; Hayakawa, T. Targeted adenovirus vectors. Hum. Gene Ther. 2004, 15, 1034–1044. [Google Scholar] [CrossRef] [PubMed]
- Volpers, C.; Kochanek, S. Adenoviral vectors for gene transfer and therapy . J. Gene Med. 2004, 6, S164–S171. [Google Scholar] [CrossRef] [PubMed]
- Doronin, K.; Shashkova, E. V.; May, S. M.; Hofherr, S. E.; Barry, M. A. Chemical Modification with High Molecular Weight Polyethylene Glycol Reduces Transduction of Hepatocytes and Increases Efficacy of Intravenously Delivered Oncolytic Adenovirus. Hum. Gene Ther. 2009, 20, 975–988. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J. T.; Rogers, B.E.; Rosenfeld, M.E.; Michael, S.I.; Feng, M.; Curiel, D.T. Targeted gene delivery by tropism-modified adenoviral vectors. Nat. Biotechnol. 1996, 14, 1574–1578. [Google Scholar] [CrossRef] [PubMed]
- Gall, J.; Kass-Eisler, A.; Leinwand, L.; Falck-Pedersen, E. Adenovirus type 5 and 7 capsid chimera: fiber replacement alters receptor tropism without affecting primary immune neutralization epitopes. J. Virol. 1996, 70, 2116–2123. [Google Scholar] [PubMed]
- Wickham, T.J.; Tzeng, E.; Shears 2nd., L.L.; Roelvink, P.W.; Li, Y.; Lee, G.M.; Brough, D.E.; Lizonova, A.; Kovesdi, I. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins . J. Virol. 1997, 71, 8221–8229. [Google Scholar] [PubMed]
- Barnett, B.G.; Crews, C.J.; Douglas, J.T. Targeted adenoviral vectors. Biochim. Biophys. Acta. 2002, 1575, 1–14. [Google Scholar] [PubMed]
- Romanczuk, H.; Galer, C.E.; Zabner, J.; Barsomian, G.; Wadsworth, S.C.; O'Riordan, C.R. Modification of an adenoviral vector with biologically selected peptides: a novel strategy for gene delivery to cells of choice. Hum. Gene Ther. 1999, 10, 2615–2626. [Google Scholar] [PubMed]
- Menezes, K.M.; Mok, H.S.; Barry, M.A. Increased transduction of skeletal muscle cells by fibroblast growth factor-modified adenoviral vectors. Hum. Gene Ther. 2006, 17, 314–320. [Google Scholar] [CrossRef] [PubMed]
- O'Riordan, C.R.; Song, A. PEGylated adenovirus for targeted gene therapy. Methods Mol. Biol. 2008, 434, 133–160. [Google Scholar] [PubMed]
- Eto, Y.; Gao, J.Q.; Sekiguchi, F.; Kurachi, S.; Katayama, K.; Maeda, M.; Kawasaki, K.; Mizuguchi, H.; Hayakawa, T.; Tsutsumi, Y.; Mayumi, T.; Nakagawa, S. PEGylated adenovirus vectors containing RGD peptides on the tip of PEG show high transduction efficiency and antibody evasion ability. J. Gene Med. 2005, 7, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Cheng, Z.; Zhang, X.; Patel, M.; Wu, J.C.; Gambhir, S.S.; Chen, X. Imaging chemically modified adenovirus for targeting tumors expressing integrin alphavbeta3 in living mice with mutant herpes simplex virus type 1 thymidine kinase PET reporter gene. J. Nucl. Med. 2006, 47, 130–139. [Google Scholar] [PubMed]
- Eto, Y.; Yoshioka, Y.; Mukai, Y.; Okada, N.; Nakagawa, S. Development of PEGylated adenovirus vector with targeting ligand . Int. J. Pharm. 2007. [Google Scholar]
- Park, J.W.; Mok, H.; Park, T.G. Epidermal growth factor (EGF) receptor targeted delivery of PEGylated adenovirus. Biochem. Biophys. Res. Commun. 2008, 366, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Lasaro, M.O.; Ertl, H.C.J. New Insights on Adenovirus as Vaccine Vectors. Mol. Ther. 2009, 17, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Thacker, E.E.; Timares, L.; Matthews, Q.L. Strategies to overcome host immunity to adenovirus vectors in vaccine development. Expert Rev. Vaccines 2009, 8, 761–777. [Google Scholar] [CrossRef]
- Hartman, Z.C.; Appledorn, D.M.; Amalfitano, A. Adenovirus vector induced innate immune responses: Impact upon efficacy and toxicity in gene therapy and vaccine applications. Virus Res. 2008, 132, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nwanegbo, E.; Vardas, E.; Gao, W. T.; Whittle, H.; Sun, H. J.; Rowe, D.; Robbins, P.D.; Gambotto, A. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clin. Diagn. Labo. Immunol. 2004, 11, 351–357. [Google Scholar]
- Zaiss, A.K.; Machado, H.B.; Herschman, H.R. The influence of innate and pre-existing immunity on adenovirus therapy. J. Cell Biochem. 2009, 108, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Y.; Figueredo, J.; Kobinger, G.P.; Hagan, H.; Calcedo, R.; Miller, J.R.; Gao, G.; Wilson, J.M. Efficacy of severe acute respiratory syndrome vaccine based on a nonhuman primate adenovirus in the presence of immunity against human adenovirus. Hum. Gene Ther. 2006, 17, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Kobinger, G.P.; Feldmann, H.; Zhi, Y.; Schumer, G.; Gao, G.; Feldmann, F.; Jones, S.; Wilson, J.M. Chimpanzee adenovirus vaccine protects against Zaire Ebola virus. Virology 2006, 346, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Vogels, R.; Zuijdgeest, D.; van Rijnsoever, R.; Hartkoorn, E.; Damen, I.; de Bethune, M.P.; Kostense, S.; Penders, G.; Helmus, N.; Koudstaal, W.; Cecchini, M.; Wetterwald, A.; Sprangers, M.; Lemckert, A.; Ophorst, O.; Koel, B.; van Meerendonk, M.; Quax, P.; Panitti, L.; Grimbergen, J.; Bout, A.; Goudsmit, J.; Havenga, M. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J. Virol. 2003, 77, 8263–8271. [Google Scholar] [CrossRef] [PubMed]
- Barouch, D.H.; Pau, M.G.; Custers, J.H.; Koudstaal, W.; Kostense, S.; Havenga, M.J.; Truitt, D.M.; Sumida, S.M.; Kishko, M.G.; Arthur, J.C.; Korioth-Schmitz, B.; Newberg, M.H.; Gorgone, D.A.; Lifton, M.A.; Panicali, D.L.; Nabel, G.J.; Letvin, N.L.; Goudsmit, J. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 2004, 172, 6290–6297. [Google Scholar] [PubMed]
- Lemckert, A.A.; Sumida, S.M.; Holterman, L.; Vogels, R.; Truitt, D.M.; Lynch, D.M.; Nanda, A.; Ewald, B.A.; Gorgone, D.A.; Lifton, M.A.; Goudsmit, J.; Havenga, M.J.; Barouch, D.H. Immunogenicity of heterologous prime-boost regimens involving recombinant adenovirus serotype 11 (Ad11) and Ad35 vaccine vectors in the presence of anti-ad5 immunity. J. Virol. 2005, 79, 9694–9701. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Zhi, Y.; Kobinger, G.P.; Figueredo, J.; Calcedo, R.; Miller, J.R.; Feldmann, H.; Wilson, J.M. Generation of an adenoviral vaccine vector based on simian adenovirus 21. J. Gen. Virol. 2006, 87, 2477–2485. [Google Scholar] [CrossRef] [PubMed]
- Croyle, M.A.; Patel, A.; Tran, K.N.; Gray, M.; Zhang, Y.; Strong, J.E.; Feldmann, H.; Kobinger, G.P. Nasal delivery of an adenovirus-based vaccine bypasses pre-existing immunity to the vaccine carrier and improves the immune response in mice . PLoS One 2008, 3, e3548. [Google Scholar] [CrossRef] [PubMed]
- Weaver, E.A.; Barry, M.A. Effects of Shielding Adenoviral Vectors with Polyethylene Glycol (PEG) on Vector-specific and Vaccine-mediated Immune Responses. Hum. Gene Ther. 2008, 19, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Share and Cite
Wonganan, P.; Croyle, M.A. PEGylated Adenoviruses: From Mice to Monkeys. Viruses 2010, 2, 468-502. https://doi.org/10.3390/v2020468
Wonganan P, Croyle MA. PEGylated Adenoviruses: From Mice to Monkeys. Viruses. 2010; 2(2):468-502. https://doi.org/10.3390/v2020468
Chicago/Turabian StyleWonganan, Piyanuch, and Maria A. Croyle. 2010. "PEGylated Adenoviruses: From Mice to Monkeys" Viruses 2, no. 2: 468-502. https://doi.org/10.3390/v2020468




