Differential Role of Anti-Viral Sensing Pathway for the Production of Type I Interferon β in Dendritic Cells and Macrophages Against Respiratory Syncytial Virus A2 Strain Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Mice Genotyping
2.3. RSV Propagation
2.4. RSV Infection In Vitro and Cytokine Measurements
2.5. Flow Cytometry
2.6. Statistics
3. Results
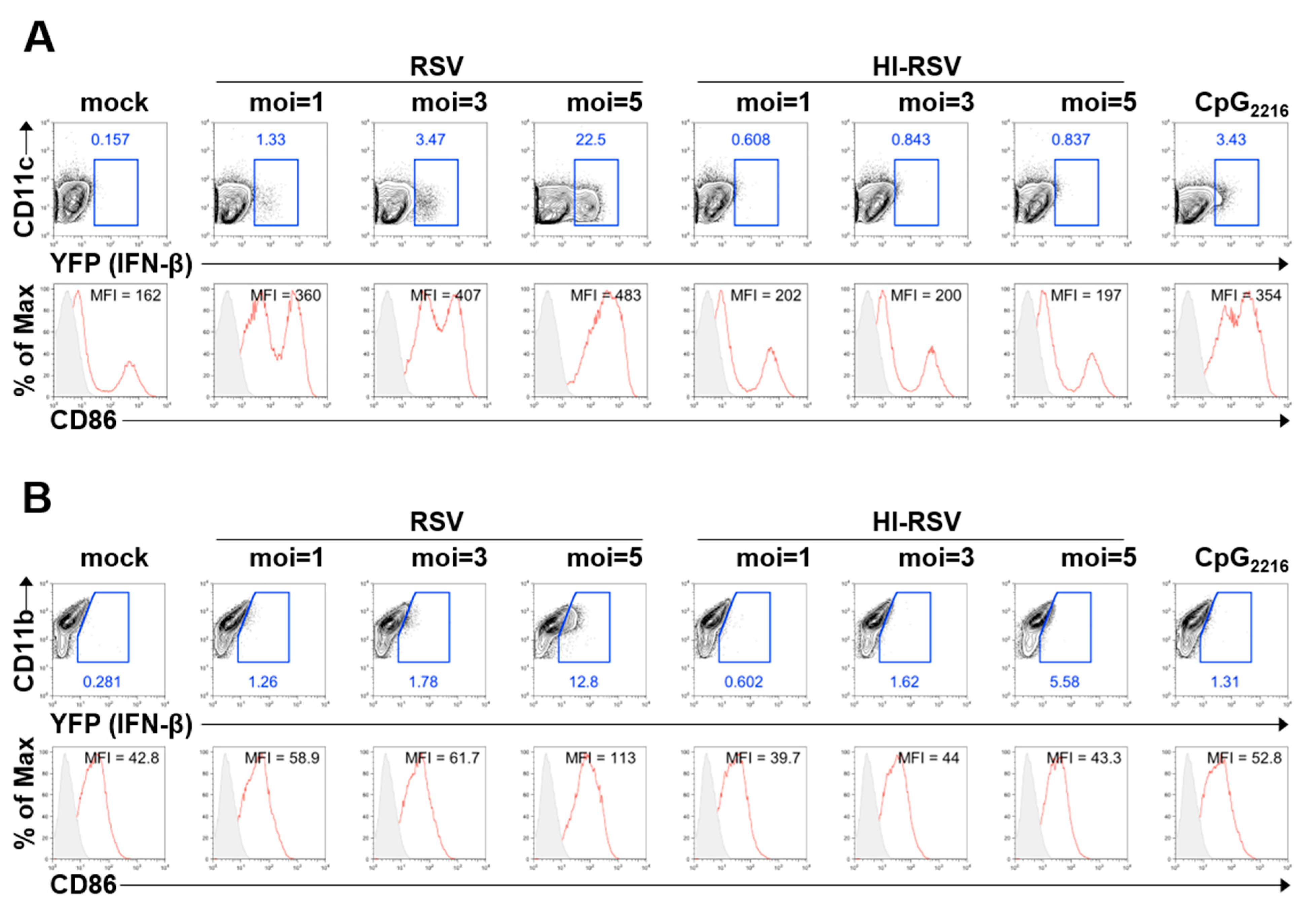
3.1. BM-DCs and BM-DMs Produce IFN-β in Response to RSV A2 Strain Infection
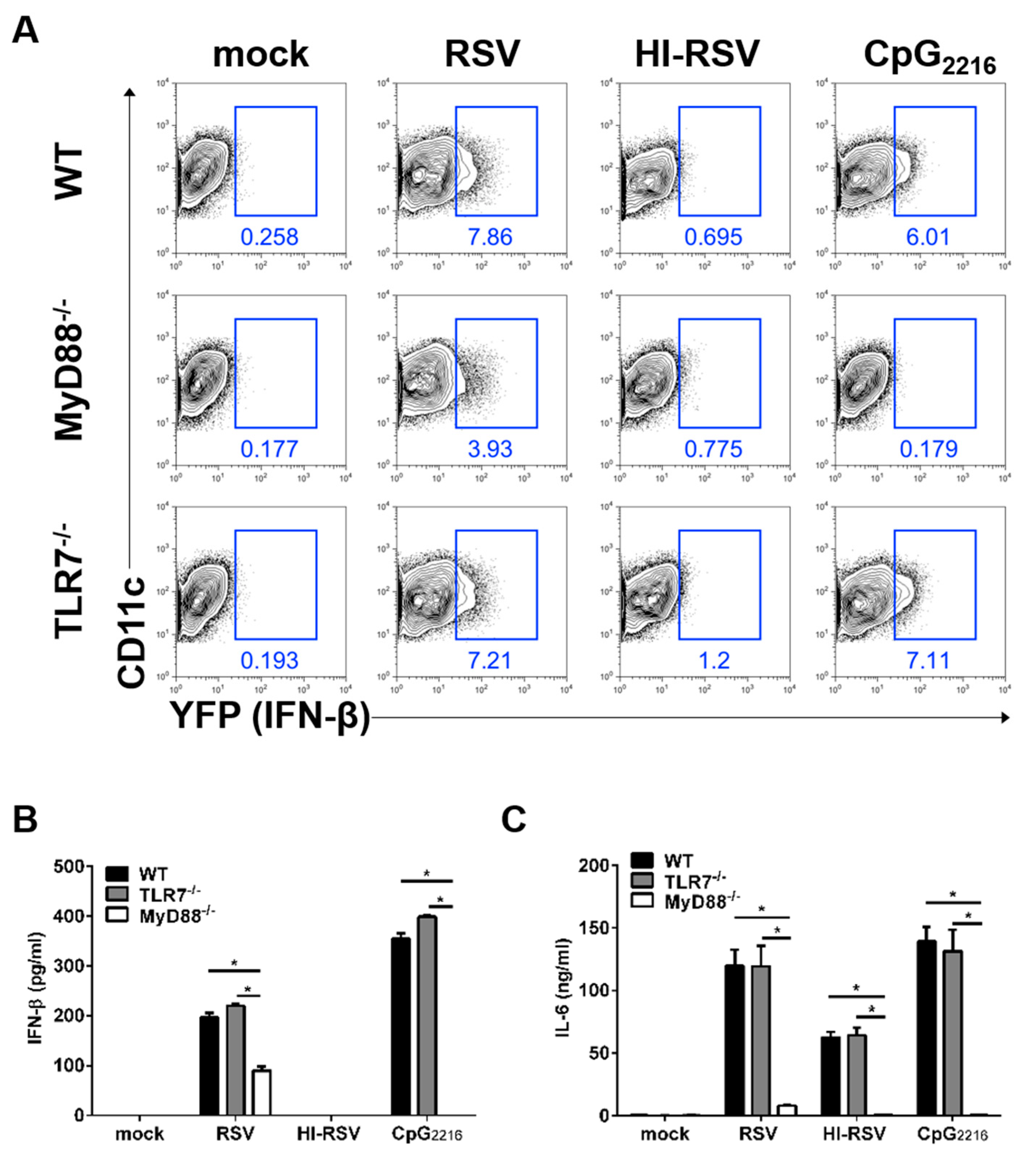
3.2. The MyD88- and TLR7-Mediated Pathways are Required to Induce IFN-β in BM-DCs
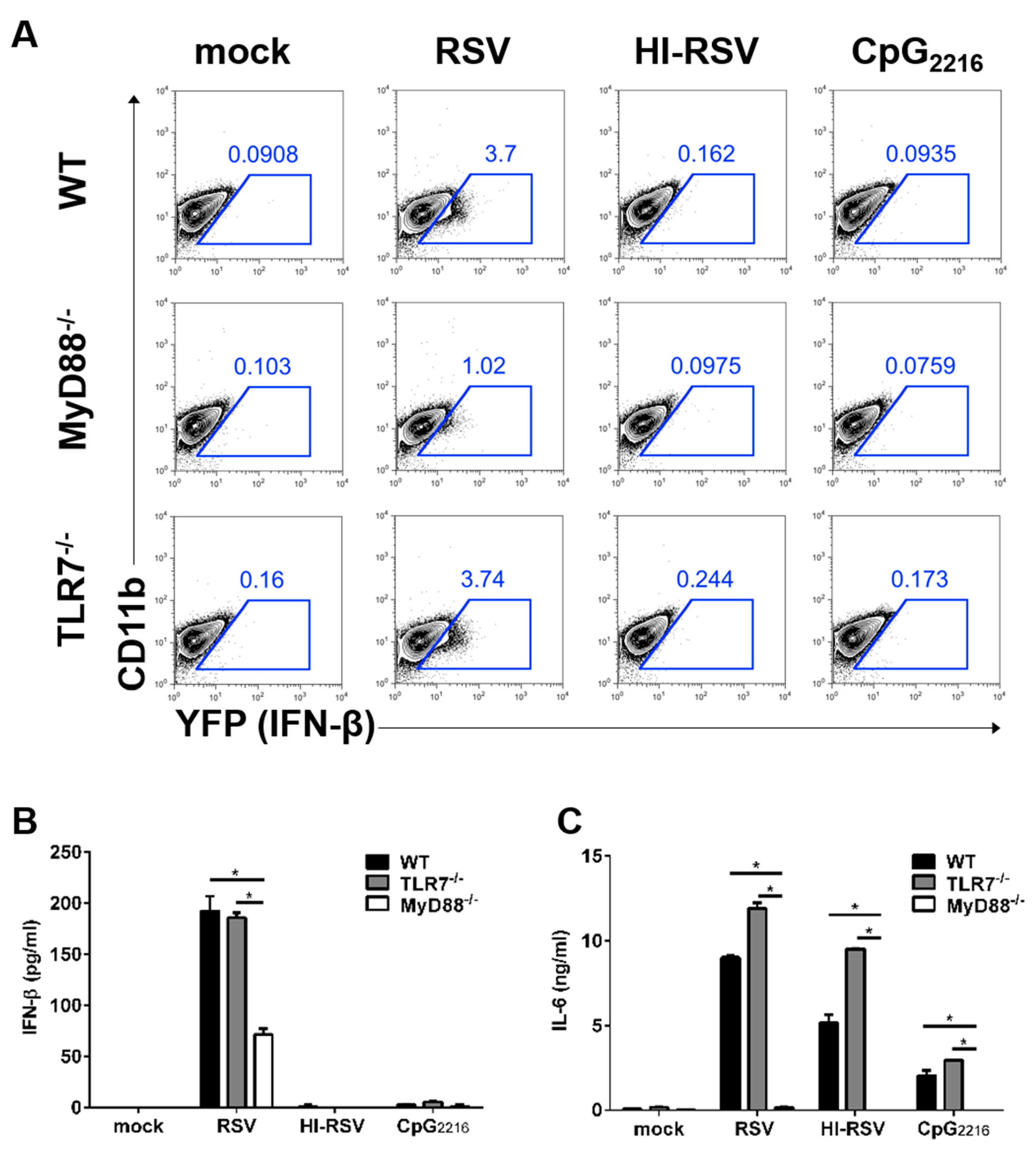
3.3. The MyD88-Mediated Pathways are Required to Induce IFN-β in BM-DMs
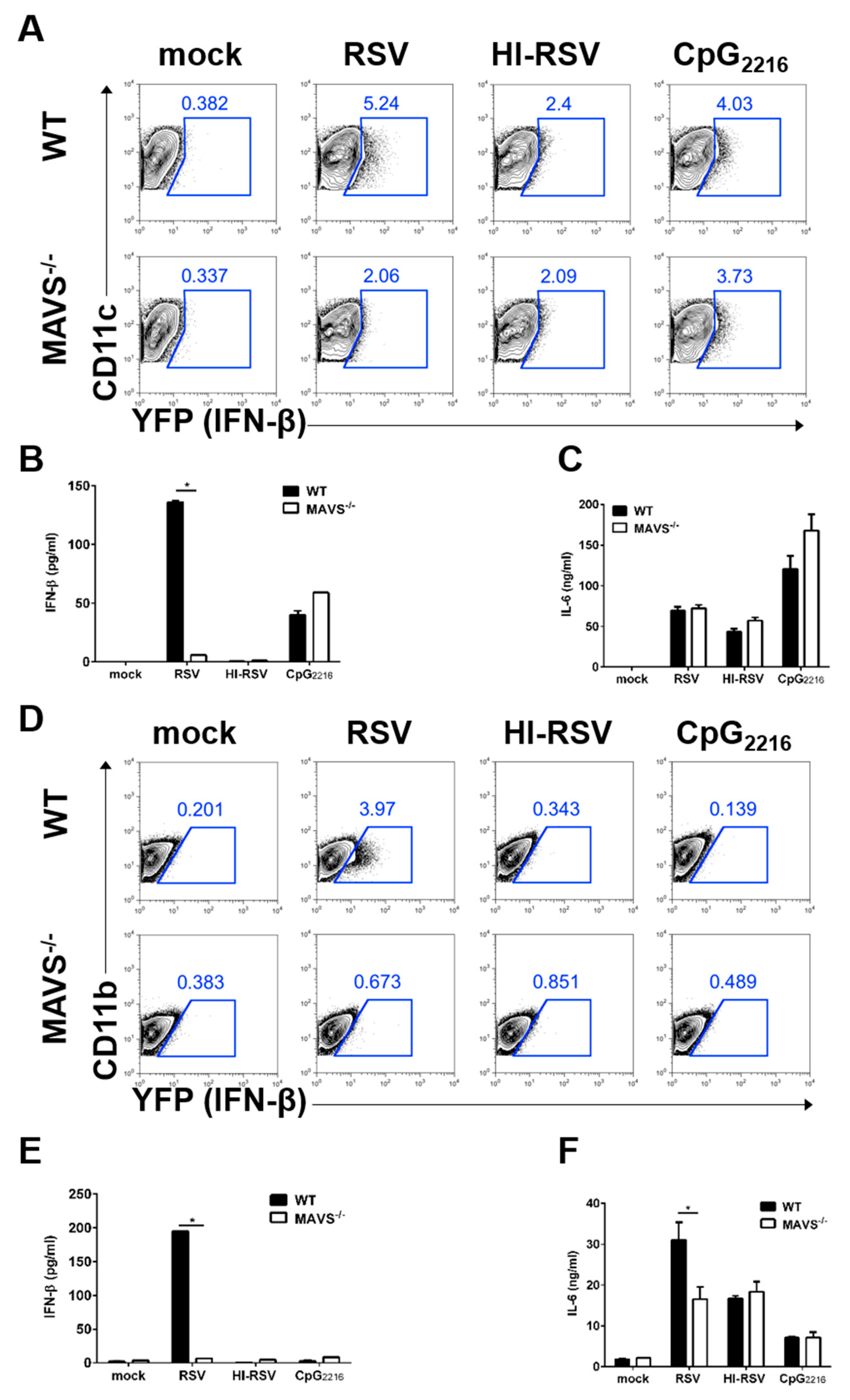
3.4. MAVS-Mediated Pathways are Required to Induce IFN-β in BM-DCs and BM-DMs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Collins, P.L.; Graham, B.S. Viral and host factors in human respiratory syncytial virus pathogenesis. J. Virol. 2008, 82, 2040–2055. [Google Scholar] [CrossRef] [PubMed]
- Borchers, A.T.; Chang, C.; Gershwin, M.E.; Gershwin, L.J. Respiratory syncytial virus--a comprehensive review. Clin. Rev. Allergy Immunol. 2013, 45, 331–379. [Google Scholar] [CrossRef] [PubMed]
- Luoto, R.; Jartti, T.; Ruuskanen, O.; Waris, M.; Lehtonen, L.; Heikkinen, T. Review of the clinical significance of respiratory virus infections in newborn infants. Acta Paediatr. 2016, 105, 1132–1139. [Google Scholar] [CrossRef]
- Anderson, L.J.; Dormitzer, P.R.; Nokes, D.J.; Rappuoli, R.; Roca, A.; Graham, B.S. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine 2013, 31 (Suppl. 2), B209–B215. [Google Scholar] [CrossRef] [Green Version]
- Goritzka, M.; Durant, L.R.; Pereira, C.; Salek-Ardakani, S.; Openshaw, P.J.; Johansson, C. Alpha/beta interferon receptor signaling amplifies early proinflammatory cytokine production in the lung during respiratory syncytial virus infection. J. Virol. 2014, 88, 6128–6136. [Google Scholar] [CrossRef] [PubMed]
- Makris, S.; Paulsen, M.; Johansson, C. Type I interferons as regulators of lung inflammation. Front. Immunol. 2017, 8, 259. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, H.K. Innate immune recognition of respiratory syncytial virus infection. BMB Rep. 2014, 47, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Lambert, L.; Sagfors, A.M.; Openshaw, P.J.; Culley, F.J. Immunity to RSV in Early-Life. Front. Immunol. 2014, 5, 466. [Google Scholar] [CrossRef]
- Sun, Y.; Lopez, C.B. The innate immune response to RSV: Advances in our understanding of critical viral and host factors. Vaccine 2017, 35, 481–488. [Google Scholar] [CrossRef]
- Lukacs, N.W.; Smit, J.J.; Mukherjee, S.; Morris, S.B.; Nunez, G.; Lindell, D.M. Respiratory virus-induced TLR7 activation controls IL-17-associated increased mucus via IL-23 regulation. J. Immunol. 2010, 185, 2231–2239. [Google Scholar] [CrossRef]
- Goritzka, M.; Makris, S.; Kausar, F.; Durant, L.R.; Pereira, C.; Kumagai, Y.; Culley, F.J.; Mack, M.; Akira, S.; Johansson, C. Alveolar macrophage-derived type I interferons orchestrate innate immunity to RSV through recruitment of antiviral monocytes. J. Exp. Med. 2015, 212, 699–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demoor, T.; Petersen, B.C.; Morris, S.; Mukherjee, S.; Ptaschinski, C.; De Almeida Nagata, D.E.; Kawai, T.; Ito, T.; Akira, S.; Kunkel, S.L.; et al. IPS-1 signaling has a nonredundant role in mediating antiviral responses and the clearance of respiratory syncytial virus. J. Immunol. 2012, 189, 5942–5953. [Google Scholar] [CrossRef] [PubMed]
- Scheu, S.; Dresing, P.; Locksley, R.M. Visualization of IFNbeta production by plasmacytoid versus conventional dendritic cells under specific stimulation conditions in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 20416–20421. [Google Scholar] [CrossRef] [PubMed]
- Adachi, O.; Kawai, T.; Takeda, K.; Matsumoto, M.; Tsutsui, H.; Sakagami, M.; Nakanishi, K.; Akira, S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 1998, 9, 143–150. [Google Scholar] [CrossRef]
- Lund, J.M.; Alexopoulou, L.; Sato, A.; Karow, M.; Adams, N.C.; Gale, N.W.; Iwasaki, A.; Flavell, R.A. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 2004, 101, 5598–5603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Sun, L.; Liu, H.H.; Chen, X.; Seth, R.B.; Forman, J.; Chen, Z.J. The specific and essential role of MAVS in antiviral innate immune responses. Immunity 2006, 24, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.R.; Lee, H.K. Caspase-1 independent viral clearance and adaptive immunity against mucosal respiratory syncytial virus infection. Immune Netw. 2015, 15, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Joo, D.H.; Lee, J.B.; Shim, B.S.; Cheon, I.S.; Jang, J.E.; Song, H.H.; Kim, K.H.; Song, M.K.; Chang, J. Dual Role of Respiratory Syncytial Virus Glycoprotein Fragment as a Mucosal Immunogen and Chemotactic Adjuvant. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- McKimm-Breschkin, J.L. A simplified plaque assay for respiratory syncytial virus--direct visualization of plaques without immunostaining. J. Virol. Methods 2004, 120, 113–117. [Google Scholar] [CrossRef]
- Ichinohe, T.; Lee, H.K.; Ogura, Y.; Flavell, R.; Iwasaki, A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J. Exp. Med. 2009, 206, 79–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillyer, P.; Mane, V.P.; Chen, A.; Dos Santos, M.B.; Schramm, L.M.; Shepard, R.E.; Luongo, C.; Le Nouen, C.; Huang, L.; Yan, L.; et al. Respiratory syncytial virus infection induces a subset of types I and III interferons in human dendritic cells. Virology 2017, 504, 63–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhoj, V.G.; Sun, Q.; Bhoj, E.J.; Somers, C.; Chen, X.; Torres, J.P.; Mejias, A.; Gomez, A.M.; Jafri, H.; Ramilo, O.; et al. MAVS and MyD88 are essential for innate immunity but not cytotoxic T lymphocyte response against respiratory syncytial virus. Proc. Natl. Acad. Sci. USA 2008, 105, 14046–14051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.H.; Lee, H.K. Differential roles of lung dendritic cell subsets against respiratory virus infection. Immune Netw. 2014, 14, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; Le Nouen, C.; Luongo, C.; Buchholz, U.J.; Collins, P.L.; Bukreyev, A. Nonstructural proteins 1 and 2 of respiratory syncytial virus suppress maturation of human dendritic cells. J. Virol. 2008, 82, 8780–8796. [Google Scholar] [CrossRef] [PubMed]
- Ravi, L.I.; Li, L.; Sutejo, R.; Chen, H.; Wong, P.S.; Tan, B.H.; Sugrue, R.J. A systems-based approach to analyse the host response in murine lung macrophages challenged with respiratory syncytial virus. BMC Genom. 2013, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Mufson, M.A.; Orvell, C.; Rafnar, B.; Norrby, E. Two distinct subtypes of human respiratory syncytial virus. J. Gen. Virol. 1985, 66, 2111–2124. [Google Scholar] [CrossRef]
- Walsh, E.E.; Hall, C.B.; Schlesinger, J.J.; Brandriss, M.W.; Hildreth, S.; Paradiso, P. Comparison of Antigenic Sites of Subtype-Specific Respiratory Syncytial Virus Attachment Proteins. J. Gen. Virol. 1989, 70, 2953–2961. [Google Scholar] [CrossRef]
- Johnson, T.R.; Graham, B.S. Contribution of respiratory syncytial virus G antigenicity to vaccine-enhanced illness and the implications for severe disease during primary respiratory syncytial virus infection. Pediatr. Infect. Dis. J. 2004, 23, S46–S57. [Google Scholar] [CrossRef] [Green Version]
- Bohmwald, K.; Espinoza, J.A.; Rey-Jurado, E.; Gomez, R.S.; Gonzalez, P.A.; Bueno, S.M.; Riedel, C.A.; Kalergis, A.M. Human Respiratory Syncytial Virus: Infection and Pathology. Semin. Respir. Crit. Care Med. 2016, 37, 522–537. [Google Scholar] [CrossRef]
- Fonseca, A.; Scott, N.; Strickland, D.; Everard, M. Persistence of respiratory syncytial virus replication in lung dendritic cells. Eur. Respir. Soc. 2015. [Google Scholar] [CrossRef]
- Rivera-Toledo, E.; Gomez, B. Respiratory syncytial virus persistence in macrophages alters the profile of cellular gene expression. Viruses 2012, 4, 3270–3280. [Google Scholar] [CrossRef] [PubMed]
- Rudd, B.D.; Schaller, M.A.; Smit, J.J.; Kunkel, S.L.; Neupane, R.; Kelley, L.; Berlin, A.A.; Lukacs, N.W. MyD88-mediated instructive signals in dendritic cells regulate pulmonary immune responses during respiratory virus infection. J. Immunol. 2007, 178, 5820–5827. [Google Scholar] [CrossRef] [PubMed]
- Cyr, S.L.; Angers, I.; Guillot, L.; Stoica-Popescu, I.; Lussier, M.; Qureshi, S.; Burt, D.S.; Ward, B.J. TLR4 and MyD88 control protection and pulmonary granulocytic recruitment in a murine intranasal RSV immunization and challenge model. Vaccine 2009, 27, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Openshaw, P.J.M.; Chiu, C.; Culley, F.J.; Johansson, C. Protective and Harmful Immunity to RSV Infection. Annu. Rev. Immunol. 2017, 35, 501–532. [Google Scholar] [CrossRef] [PubMed]
- Sparrer, K.M.; Gack, M.U. Intracellular detection of viral nucleic acids. Curr. Opin. Microbiol. 2015, 26, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, D.S.; Oh, J.E.; Jung, H.E.; Lee, H.K. Transient Depletion of CD169(+) Cells Contributes to Impaired Early Protection and Effector CD8(+) T Cell Recruitment against Mucosal Respiratory Syncytial Virus Infection. Front. Immunol. 2017, 8, 819. [Google Scholar] [CrossRef]
- Morris, S.; Swanson, M.S.; Lieberman, A.; Reed, M.; Yue, Z.; Lindell, D.M.; Lukacs, N.W. Autophagy-mediated dendritic cell activation is essential for innate cytokine production and APC function with respiratory syncytial virus responses. J. Immunol. 2011, 187, 3953–3961. [Google Scholar] [CrossRef]
- Boyapalle, S.; Wong, T.; Garay, J.; Teng, M.; San Juan-Vergara, H.; Mohapatra, S.; Mohapatra, S. Respiratory syncytial virus NS1 protein colocalizes with mitochondrial antiviral signaling protein MAVS following infection. PLoS ONE 2012, 7, e29386. [Google Scholar] [CrossRef]
- Spann, K.M.; Tran, K.C.; Collins, P.L. Effects of nonstructural proteins NS1 and NS2 of human respiratory syncytial virus on interferon regulatory factor 3, NF-kappaB, and proinflammatory cytokines. J. Virol. 2005, 79, 5353–5362. [Google Scholar] [CrossRef]
- Runkel, L.; Pfeffer, L.; Lewerenz, M.; Monneron, D.; Yang, C.H.; Murti, A.; Pellegrini, S.; Goelz, S.; Uze, G.; Mogensen, K. Differences in activity between alpha and beta type I interferons explored by mutational analysis. J. Biol. Chem. 1998, 273, 8003–8008. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, D.S.; Kim, T.H.; Lee, H.K. Differential Role of Anti-Viral Sensing Pathway for the Production of Type I Interferon β in Dendritic Cells and Macrophages Against Respiratory Syncytial Virus A2 Strain Infection. Viruses 2019, 11, 62. https://doi.org/10.3390/v11010062
Oh DS, Kim TH, Lee HK. Differential Role of Anti-Viral Sensing Pathway for the Production of Type I Interferon β in Dendritic Cells and Macrophages Against Respiratory Syncytial Virus A2 Strain Infection. Viruses. 2019; 11(1):62. https://doi.org/10.3390/v11010062
Chicago/Turabian StyleOh, Dong Sun, Tae Hoon Kim, and Heung Kyu Lee. 2019. "Differential Role of Anti-Viral Sensing Pathway for the Production of Type I Interferon β in Dendritic Cells and Macrophages Against Respiratory Syncytial Virus A2 Strain Infection" Viruses 11, no. 1: 62. https://doi.org/10.3390/v11010062




