Antiviral Activity of Fermented Ginseng Extracts against a Broad Range of Influenza Viruses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells, Viruses, and Ginseng Samples
2.2. Mice and Treatment
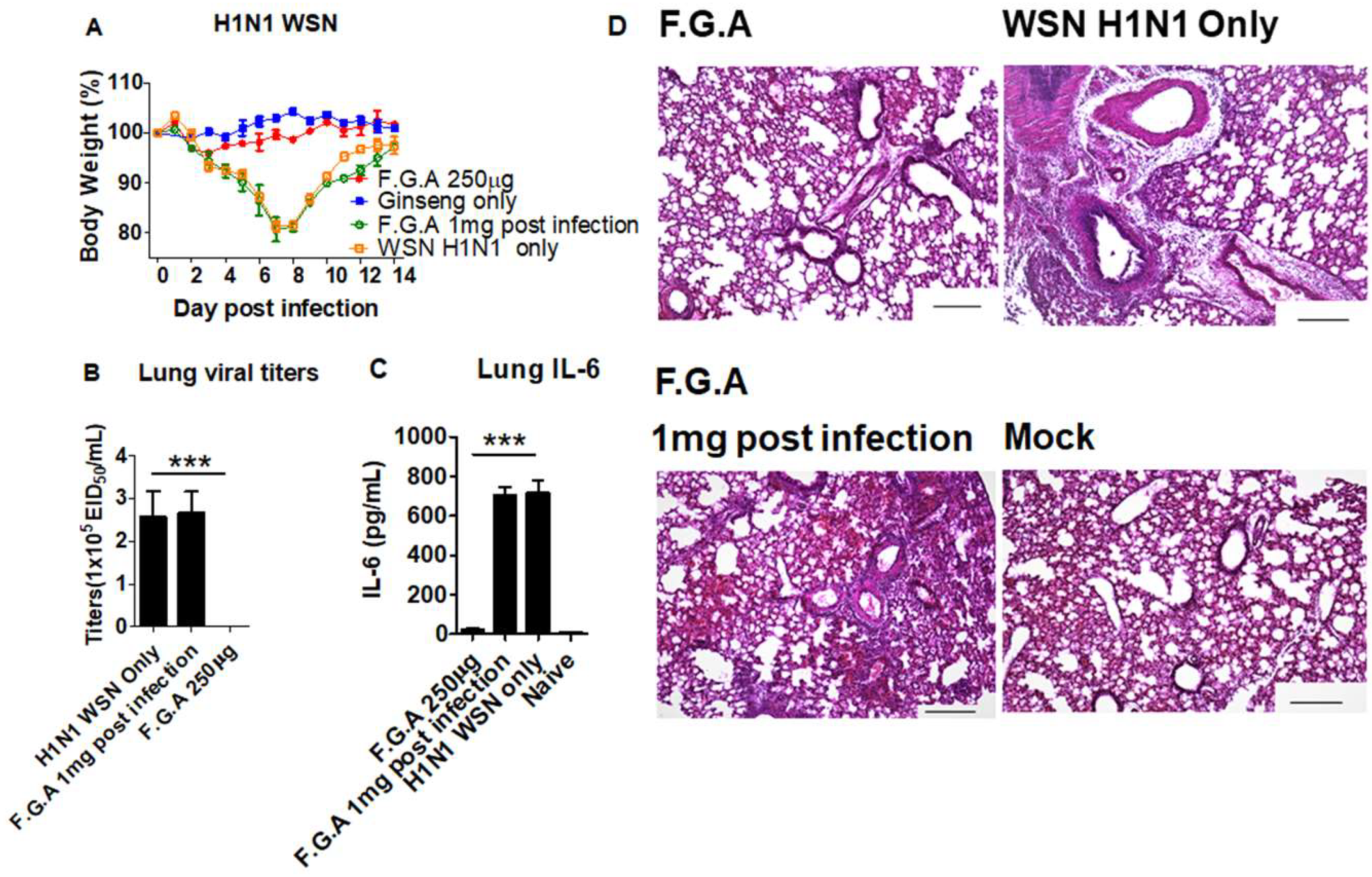
2.3. Lung Viral Titers, Histology and Cytokine ELISA
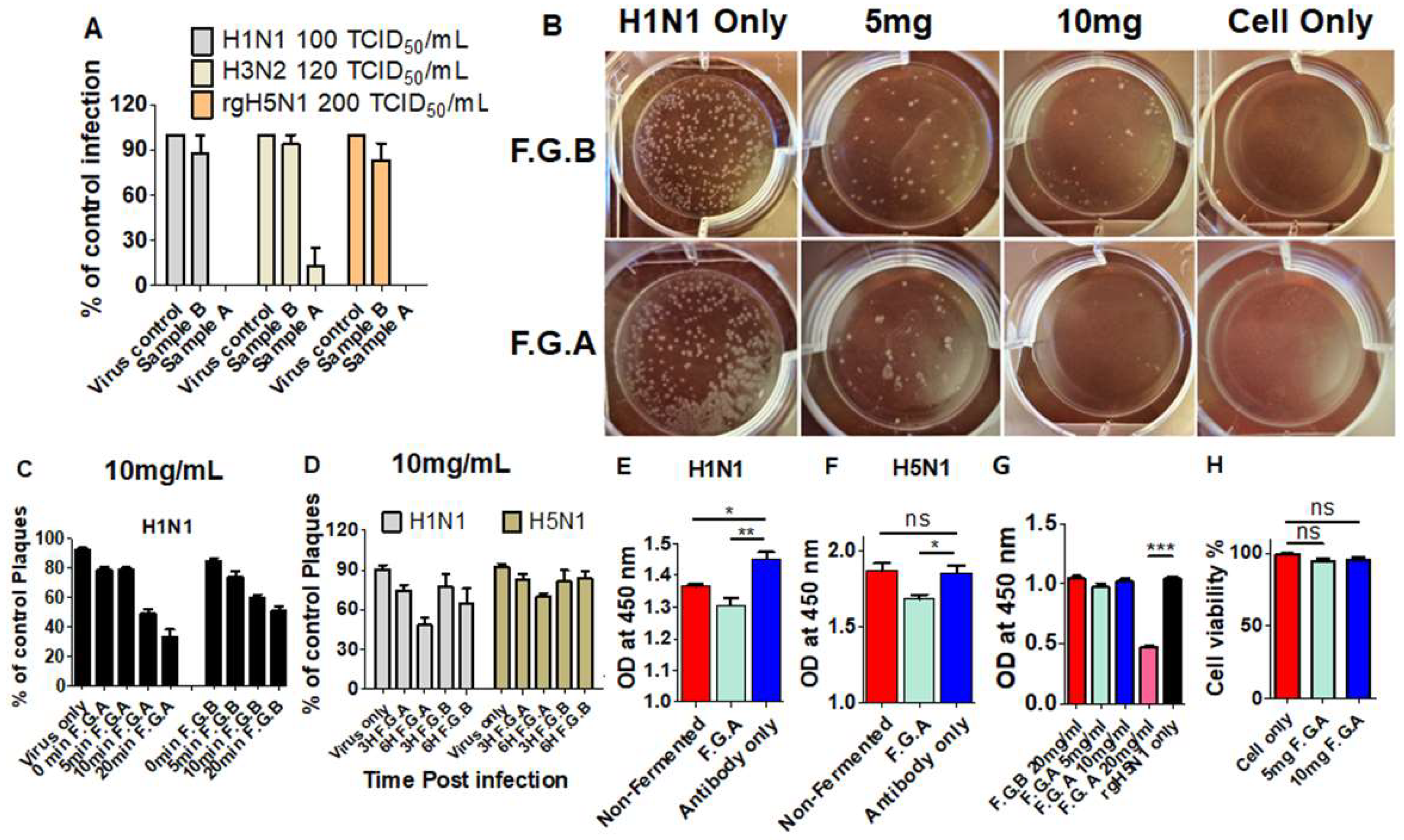
2.4. Virus Microneutralization and Plaque Assays and Cell Viability Tests in MDCK Cells
2.5. Hemagglutinin Assay
2.6. Neuraminidase Assay
2.7. Statistical Analysis Methods
3. Results
3.1. Fermented Ginseng Samples Exhibit Higher Antiviral Protection against Influenza Virus than Nonfermented Ginseng
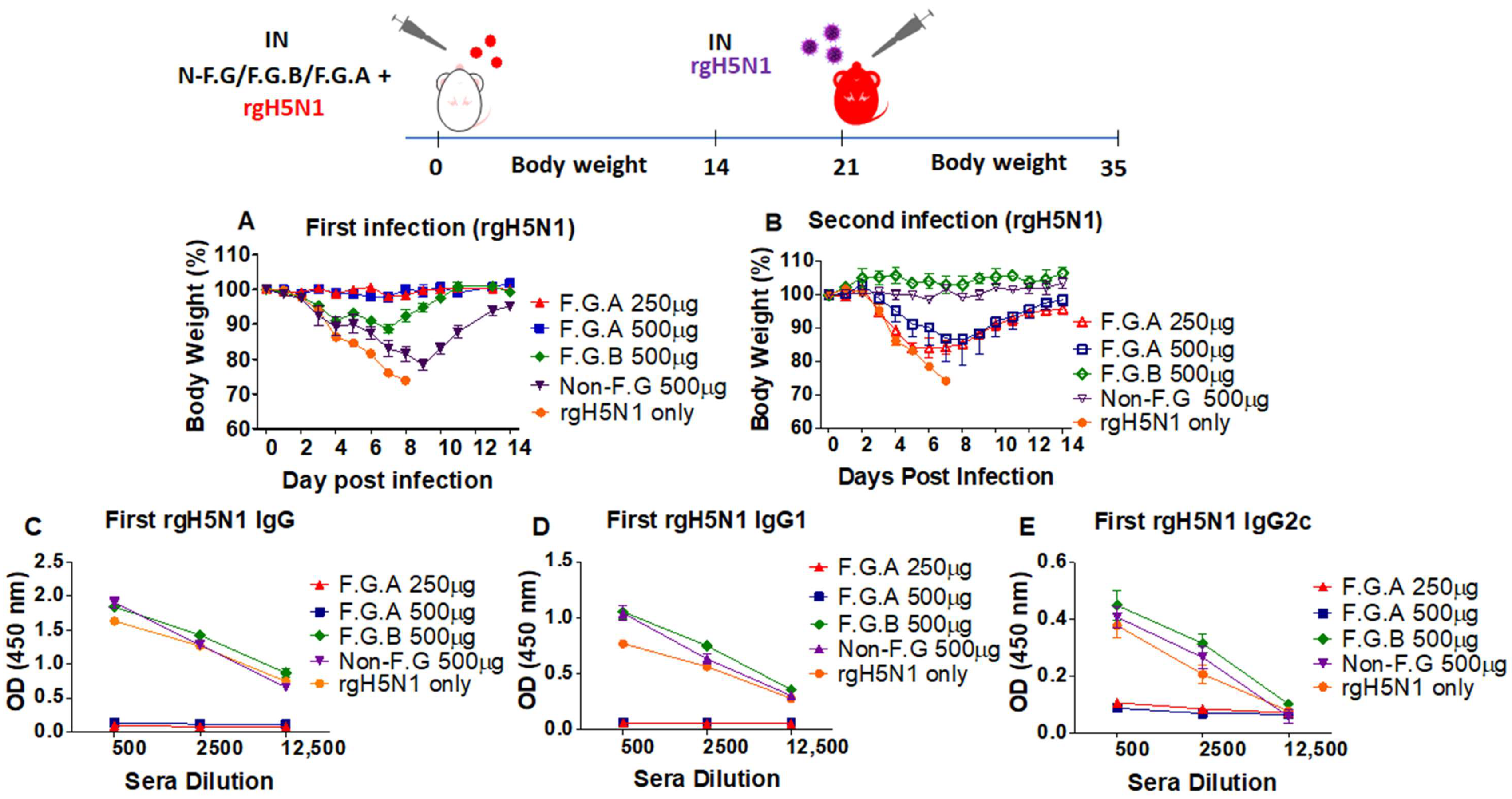
3.2. Protected Mice with Fermented Ginseng and rgH5N1 Influenza Virus Inoculation Develops Immunity against Virus Infection in Future
3.3. Fermented Ginseng Sample Has Antiviral Protective Effects against H3N2 Influenza Virus in CD8-Deficient Mice
3.4. Antiviral Protection by Fermented Ginseng Extract Is Observed Regardless of Influenza Strains and in Severe Immune-Deficient Condition
3.5. Inoculation of Mice with Fermented Ginseng Extract Samples and Virus Together Inhibits Viral Replication and Lung Inflammation
3.6. The Mice That Survive Primary Virus Inoculation with Fermented Ginseng Extract Samples Develop Immunity against Heterosubtypic Secondary Virus
3.7. Fermented Ginseng Extract Shows In Vitro Antiviral Activity against Influenza Virus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- CDC. Disease Burden of Influenza. Available online: www.cdc.gov/flu/about/disease/burden.htm (accessed on 1 August 2018).
- Centers for Disease Control and Prevention. Estimates of deaths associated with seasonal influenza—United States, 1976–2007. Morb. Mortal. Wkly. Rep. 2010, 59, 1057–1062. [Google Scholar]
- Xu, J.; Davis, C.T.; Christman, M.C.; Rivailler, P.; Zhong, H.; Donis, R.O.; Lu, G. Evolutionary history and phylodynamics of influenza A and B neuraminidase (NA) genes inferred from large-scale sequence analyses. PLoS ONE 2012, 7, e38665. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, J.M.; Chan, R.W.; Russell, R.J.; Air, G.M.; Peiris, J.S. Evolving complexities of influenza virus and its receptors. Trends Microbiol. 2008, 16, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Zhu, X.; Li, Y.; Shi, M.; Zhang, J.; Bourgeois, M.; Yang, H.; Chen, X.; Recuenco, S.; Gomez, J.; et al. New world bats harbor diverse influenza a viruses. PLoS Pathog. 2013, 9, e1003657. [Google Scholar] [CrossRef] [PubMed]
- Maines, T.R.; Lu, X.H.; Erb, S.M.; Edwards, L.; Guarner, J.; Greer, P.W.; Nguyen, D.C.; Szretter, K.J.; Chen, L.M.; Thawatsupha, P.; et al. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. J. Virol. 2005, 79, 11788–11800. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Pitol, A.K.; Bachmann, V.; Hacker, D.L.; Baldi, L.; Wurm, F.M. A simple plasmid-based transient gene expression method using High Five cells. J. Biotechnol. 2015, 216, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Huang, B.; Wang, A.; Deng, L.; Wu, D.; Lu, X.; Zhao, Q.; Xu, S.; Havers, F.; Wang, Y.; et al. Human influenza a (H7N9) virus infection associated with poultry farm, Northeastern China. Emerg. Infect. Dis. 2014, 20, 1902–1905. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.W. Rates of spontaneous mutation among RNA viruses. Proc. Natl. Acad. Sci. USA 1993, 90, 4171–4175. [Google Scholar] [CrossRef] [PubMed]
- Gultyaev, A.P.; Tsyganov-Bodounov, A.; Spronken, M.I.; van der Kooij, S.; Fouchier, R.A.; Olsthoorn, R.C. RNA structural constraints in the evolution of the influenza A virus genome NP segment. RNA Biol. 2014, 11, 942–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.S.; Hwang, H.S.; Ko, E.J.; Lee, Y.N.; Kwon, Y.M.; Kim, M.C.; Kang, S.M. Immunomodulatory activity of red ginseng against influenza A virus infection. Nutrients 2014, 6, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.G.; Kim, M.C.; Park, M.K.; Park, K.M.; Quan, F.S.; Song, J.M.; Wee, J.J.; Wang, B.Z.; Cho, Y.K.; Compans, R.W.; et al. Protective effect of ginseng polysaccharides on influenza viral infection. PLoS ONE 2012, 7, e33678. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.M.; Wu, H.; Li, H.; Chen, J.; Chen, J.Y.; Hu, S.L.; Shen, C. Effects and mechanisms of Geniposide on rats with adjuvant arthritis. Int. Immunopharmacol. 2014, 20, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.J.; Hwang, J.E.; Jung, J.; Jee, H.S.; Kim, K.T.; Paik, H.D. Short communication: Antioxidative and antibacterial activities on Staphylococcus aureus and Escherichia coli O157:H4 in milk with added ginseng marc extract fermented by Lactobacillus plantarum KCCM 11613P. J. Dairy Sci. 2017, 100, 7788–7792. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Park, J.; Kim, S.H.; Choi, K.M.; Ko, E.S.; Cha, J.D.; Lee, Y.R.; Jang, H.; Jang, Y.S. Oral administration of red ginseng powder fermented with probiotic alleviates the severity of dextran-sulfate sodium-induced colitis in a mouse model. Chin. J. Nat. Med. 2017, 15, 192–201. [Google Scholar] [CrossRef]
- Jang, S.H.; Park, J.; Kim, S.H.; Choi, K.M.; Ko, E.S.; Cha, J.D.; Lee, Y.R.; Jang, H.; Jang, Y.S. Red ginseng powder fermented with probiotics exerts antidiabetic effects in the streptozotocin-induced mouse diabetes model. Pharm. Boil. 2017, 55, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Song, J.M.; Van Rooijen, N.; Bozja, J.; Compans, R.W.; Kang, S.M. Vaccination inducing broad and improved cross protection against multiple subtypes of influenza A virus. Proc. Natl. Acad. Sci. USA 2011, 108, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Chen, J.; Sakwiwatkul, K.; Li, R.; Hu, S. Enhancement of immune responses to influenza vaccine (H3N2) by ginsenoside Re. Int. Immunopharmacol. 2010, 10, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Quan, F.S.; Compans, R.W.; Nguyen, H.H.; Kang, S.M. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J. Virol. 2008, 82, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Lee, Y.T.; Ngo, V.L.; Cho, Y.H.; Ko, E.J.; Hong, S.M.; Kim, K.H.; Jang, J.H.; Oh, J.S.; Park, M.K.; et al. Heat-killed Lactobacillus casei confers broad protection against influenza A virus primary infection and develops heterosubtypic immunity against future secondary infection. Sci. Rep. 2017, 7, 17360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.N.; Kim, M.C.; Lee, Y.T.; Hwang, H.S.; Lee, J.; Kim, C.; Kang, S.M. Cross Protection against Influenza A Virus by Yeast-Expressed Heterologous Tandem Repeat M2 Extracellular Proteins. PLoS ONE 2015, 10, e0137822. [Google Scholar] [CrossRef] [PubMed]
- Sha, Z.; Kang, S.M.; Compans, R.W. Mucosal immunization of CD4(+) T cell-deficient mice with an inactivated virus induces IgG and IgA responses in serum and mucosal secretions. Virology 2005, 331, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, L.; Clarke, M.J.; Schonberger, L.B.; Arden, N.H.; Cox, N.J.; Fukuda, K. Pandemic versus epidemic influenza mortality: A pattern of changing age distribution. J. Infect. Dis. 1998, 178, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Doyle, T.M.; Hashem, A.M.; Li, C.; Van Domselaar, G.; Larocque, L.; Wang, J.; Smith, D.; Cyr, T.; Farnsworth, A.; He, R.; et al. Universal anti-neuraminidase antibody inhibiting all influenza A subtypes. Antivir. Res. 2013, 100, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Doyle, T.M.; Jaentschke, B.; Van Domselaar, G.; Hashem, A.M.; Farnsworth, A.; Forbes, N.E.; Li, C.; Wang, J.; He, R.; Brown, E.G.; et al. The universal epitope of influenza A viral neuraminidase fundamentally contributes to enzyme activity and viral replication. J. Biol. Chem. 2013, 288, 18283–18289. [Google Scholar] [CrossRef] [PubMed]
- Doyle, T.M.; Li, C.; Bucher, D.J.; Hashem, A.M.; Van Domselaar, G.; Wang, J.; Farnsworth, A.; She, Y.M.; Cyr, T.; He, R.; et al. A monoclonal antibody targeting a highly conserved epitope in influenza B neuraminidase provides protection against drug resistant strains. Biochem. Biophys. Res. Commun. 2013, 441, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Wohlbold, T.J.; Nachbagauer, R.; Xu, H.; Tan, G.S.; Hirsh, A.; Brokstad, K.A.; Cox, R.J.; Palese, P.; Krammer, F. Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous, but not heterosubtypic, cross-protection against influenza virus infection in mice. MBio 2015, 6, e02556. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.G.; Keating, R.; Hulse-Post, D.J.; Doherty, P.C. Cell-mediated protection in influenza infection. Emerg. Infect. Dis. 2006, 12, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Pleschka, S.; Stein, M.; Schoop, R.; Hudson, J.B. Anti-viral properties and mode of action of standardized Echinacea purpurea extract against highly pathogenic avian influenza virus (H5N1, H7N7) and swine-origin H1N1 (S-OIV). Virol. J. 2009, 6, 197. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Farooqui, A.; Leon, A.J.; Kelvin, D.J. Inhibition of influenza A virus infection by ginsenosides. PLoS ONE 2017, 12, e0171936. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Moki, T.; Takizawa, T.; Shiratsuchi, A.; Nakanishi, Y. Evidence for phagocytosis of influenza virus-infected, apoptotic cells by neutrophils and macrophages in mice. J. Immunol. 2007, 178, 2448–2457. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Santiago, F.; Lambert, K.; Takimoto, T.; Topham, D.J. T cell-mediated protection against lethal 2009 pandemic H1N1 influenza virus infection in a mouse model. J. Virol. 2011, 85, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Moldoveanu, Z.; Novak, M.J.; van Ginkel, F.W.; Ban, E.; Kiyono, H.; McGhee, J.R.; Mestecky, J. Heterosubtypic immunity to lethal influenza A virus infection is associated with virus-specific CD8(+) cytotoxic T lymphocyte responses induced in mucosa-associated tissues. Virology 1999, 254, 50–60. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.; Krauss, S.L.; Riberdy, J.M.; Webster, R.G.; Woodland, D.L. Heterologous protection against lethal A/HongKong/156/97 (H5N1) influenza virus infection in C57BL/6 mice. J. Gen. Virol. 2000, 81, 2689–2696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.H.; van Ginkel, F.W.; Vu, H.L.; McGhee, J.R.; Mestecky, J. Heterosubtypic immunity to influenza A virus infection requires B cells but not CD8+ cytotoxic T lymphocytes. J. Infect. Dis. 2001, 183, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Zemlin, M.; Ivanov, I.I.; Andrasi, J.; Zemlin, C.; Vu, H.L.; Schelonka, R.; Schroeder, H.W., Jr.; Mestecky, J. Heterosubtypic immunity to influenza A virus infection requires a properly diversified antibody repertoire. J. Virol. 2007, 81, 9331–9338. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.C.; Lee, Y.N.; Kim, Y.J.; Choi, H.J.; Kim, K.H.; Lee, Y.J.; Kang, S.M. Immunogenicity and efficacy of replication-competent recombinant influenza virus carrying multimeric M2 extracellular domains in a chimeric hemagglutinin conjugate. Antivir. Res. 2017, 148, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Cho, M.K.; Hwang, H.S.; Ko, E.J.; Lee, Y.N.; Kwon, Y.M.; Kim, M.C.; Kim, K.H.; Lee, Y.T.; Jung, Y.J.; et al. Ginseng diminishes lung disease in mice immunized with formalin-inactivated respiratory syncytial virus after challenge by modulating host immune responses. J. Interferon Cytokine Res. 2014, 34, 902–914. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Lee, Y.N.; Lee, Y.T.; Hwang, H.S.; Kim, K.H.; Ko, E.J.; Kim, M.C.; Kang, S.M. Ginseng protects against respiratory syncytial virus by modulating multiple immune cells and inhibiting viral replication. Nutrients 2015, 7, 1021–1036. [Google Scholar] [CrossRef] [PubMed]
- Matrosovich, M.N.; Matrosovich, T.Y.; Gray, T.; Roberts, N.A.; Klenk, H.D. Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. J. Virol. 2004, 78, 12665–12667. [Google Scholar] [CrossRef] [PubMed]
| Ginsenosides | Red Ginseng Extracts | Fermented Ginseng A | Fermented Ginseng B |
|---|---|---|---|
| Rg1 | 1.36 | 0.60 | 1.27 |
| Re | 1.43 | 0.49 | 0.87 |
| Rf | 1.02 | 0.29 | 0.13 |
| Rb1 | 6.86 | 0.95 | 2.74 |
| Rc | 2.76 | 0.04 | 0.05 |
| Rg2 | 1.03 | 0.65 | 0.25 |
| Rh1 | 1.02 | 0.58 | 0.19 |
| Rb2 | 2.46 | 0.02 | 0.26 |
| Rb3 | 0.57 | 0.01 | 0.02 |
| F1 | - | 0.28 | 0.17 |
| Rd | 0.82 | 0.62 | 0.75 |
| F2 | - | 0.55 | 0.47 |
| Rg3 | 1.51 | 0.62 | 0.54 |
| PPT | - | 0.88 | 0.33 |
| CK | - | 4.19 | 1.69 |
| Rh2 | - | 1.49 | 0.43 |
| PPD | - | 2.13 | 0.93 |
| Total ginsenosides | 21.0 mg/g | 14.39 mg/g | 11.09 mg/g |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Jung, Y.-J.; Kim, K.-H.; Kwon, Y.; Kim, Y.-J.; Zhang, Z.; Kang, H.-S.; Wang, B.-Z.; Quan, F.-S.; Kang, S.-M. Antiviral Activity of Fermented Ginseng Extracts against a Broad Range of Influenza Viruses. Viruses 2018, 10, 471. https://doi.org/10.3390/v10090471
Wang Y, Jung Y-J, Kim K-H, Kwon Y, Kim Y-J, Zhang Z, Kang H-S, Wang B-Z, Quan F-S, Kang S-M. Antiviral Activity of Fermented Ginseng Extracts against a Broad Range of Influenza Viruses. Viruses. 2018; 10(9):471. https://doi.org/10.3390/v10090471
Chicago/Turabian StyleWang, Ye, Yu-Jin Jung, Ki-Hye Kim, Youngman Kwon, Yu-Jin Kim, Zhan Zhang, Heun-Soo Kang, Bao-Zhong Wang, Fu-Shi Quan, and Sang-Moo Kang. 2018. "Antiviral Activity of Fermented Ginseng Extracts against a Broad Range of Influenza Viruses" Viruses 10, no. 9: 471. https://doi.org/10.3390/v10090471





