1. Introduction
The porcine epidemic diarrhea virus (PEDV), a member of the family
Coronaviridae, is an enveloped, single-stranded, positive-sense RNA virus that causes severe enteric diseases with a high mortality rate in suckling piglets [
1]. For decades, pathogenic strains of PEDV have caused recurrent outbreaks with enormous economic losses worldwide. The 28-kb PEDV genome has a gene organization typical of an
Alphacoronavirus, i.e.,
ORF 1a/b, spike (
S), open reading frame 3 (
ORF3), envelope (
E), membrane (
M) and the nucleocapsid (
N) genes [
2]. Although not entirely elucidated, it is assumed that PEDV replication is initiated by the interaction of the S protein to cellular receptors, followed by endocytosis-mediated viral internalization [
3]. After the release of viral genomic RNA into the cytoplasm, replicase proteins undergo extensive intracellular membranes modification to promote viral RNA synthesis. Shortly after synthesis of structural proteins, nascent virions are assembled in the endoplasmic reticulum-Golgi intermediate compartment (ERGIC), before being released, via smooth-walled vesicles, by exocytosis. While the roles of structural proteins in coronavirus (CoV) replication have been extensively studied, the contribution of the accessory ORF3 protein, particularly in PEDV replication, remains quite elusive. Mounting evidence has suggested that ORF3 is one of the key determinants of PEDV virulence [
4]. Nevertheless, the mechanisms by which it modulates viral pathogenicity are as yet clearly defined.
The ORF3 protein exhibits a genetic variation among PEDVs. The full-length ORF3 (675 nt; 224 aa) is found in wild-type PEDVs, while the truncated ORF3 (624 nt; 207 aa; 82–98 amino acid deletion) is common in cell-adapted or attenuated PEDVs [
4]. Structurally, ORF3 has been predicted to harbor multiple transmembrane domains, existing as a tetrameric ion channel [
5]. However, results from assays using cells stably expressing ORF3 revealed that the protein was predominantly expressed in the cytoplasm [
6]. Cells expressing ORF3 also displayed a prolonged S phase of the cell cycle and augmented vesicle formation [
6]. These results altogether point to the possibility that ORF3 might not be a typical ion channel, but rather a multifunctional protein that is involved in many cellular processes. Notably, since cell culture-adapted variants of PEDV usually possess abortive ORF3, be it absent or truncated [
4], it is also likely that ORF3 might interfere with PEDV replication in cultured cells [
7].
To date, the investigation of ORF3 is hampered by technical challenges associated with obtaining productively-growing PEDV with intact ORF3. It is also difficult to assess ORF3 expression in virus-infected cells as specific antibodies are either not accessible or ineffective. In this study, we constructed recombinant PEDV bearing ORF3 with specific epitope tags and evaluated the ORF3 expression in infected cells in comparison to those transfected by the expression plasmid. We demonstrated that the expression of ORF3 in PEDV-infected cells is distinct from that transiently expressed in individual cells. Furthermore, we found that ORF3 and S are co-localized in several cellular compartments in infected cells and the ORF3-S interaction was also confirmed. Collectively, our results suggest that ORF3 may interact with S during PEDV assembly and consequently exert its activity at this step of virus replication.
2. Materials and Methods
2.1. Cell Line and Culture Condition
Human embryonic kidney (HEK) 293T and African green monkey (VeroE6) cells stably expressing porcine aminopeptidase N (VeroE6-APN) were grown and maintained in Opti-MEM medium supplemented with 10% heat-inactivated fetal bovine serum (FBS). Cells were cultured in humidified air containing 5% CO2 at 37 °C.
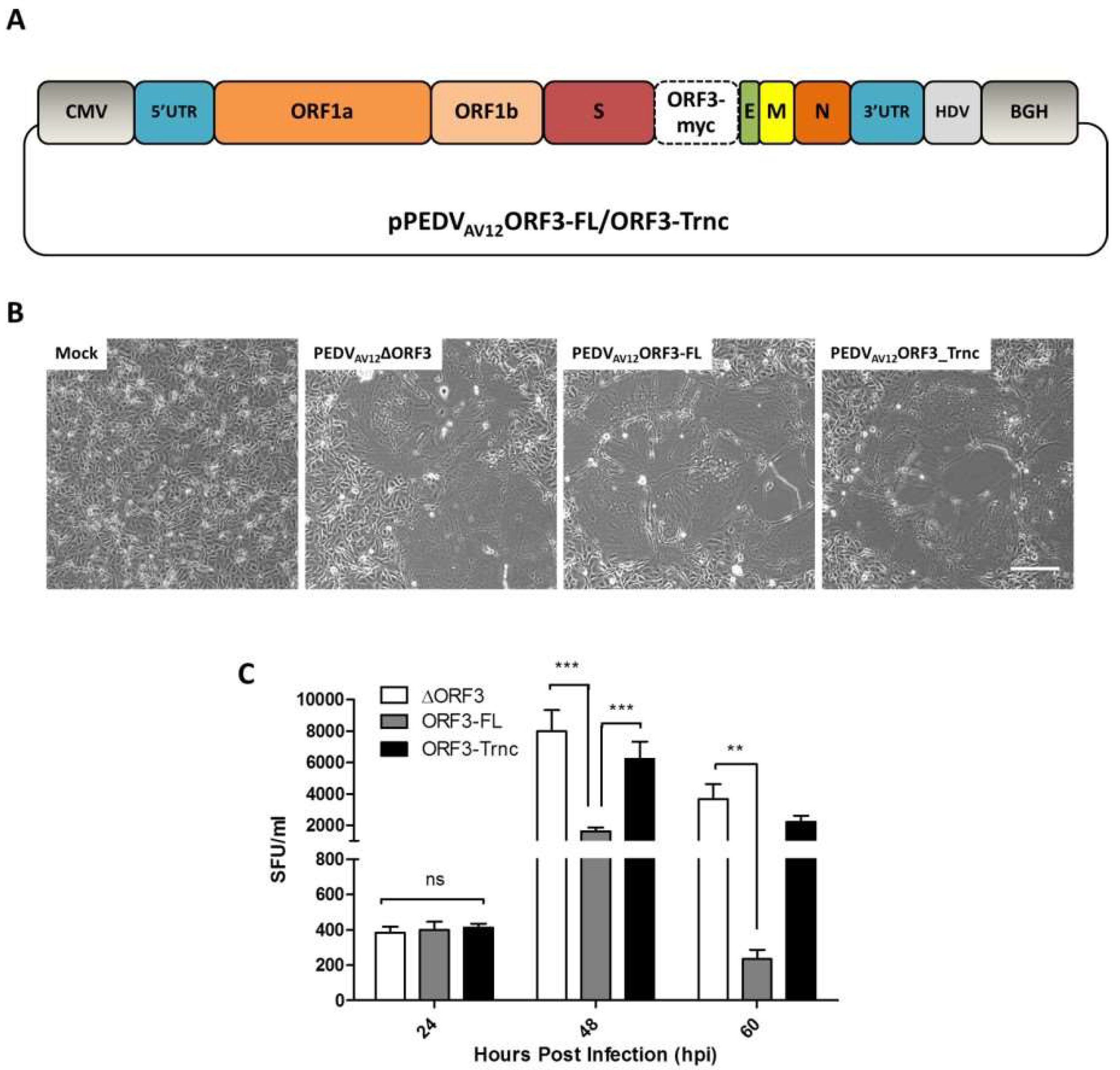
2.2. Construction of Expression Plasmids and PEDV Infectious Clones Carrying Tagged-ORF3s
The ORF3 sequence was synthesized for expression in mammalian cells and codon-optimized as shown in
Table S1. The full-length (ORF3-FL) and truncated ORF3 (ORF3-Trnc) (deletion of the amino acid at positions 82–98) with myc-tag at the C-terminus were cloned into the pCAGGS expression plasmid, designated pCAGGS_ORF3-FL and-Trnc. The pCAGGS_FlagORF3-FL and-Trnc were also constructed by insertion of Flag-tag at the N terminus of ORF3s.
To generate PEDV infectious clones, the plasmids pCAGGS_ORF3-FL and-Trnc were used as templates for amplification of ORF3-myc flanked with
BsiWI and
XhoI restriction sites. The amplified ORF3 variants were cloned into pTZ5R/T carrying
S,
E,
M and
N genesof PEDV
AVCT12 at
BsiWI and
XhoI sites to incorporate ORF3 variants into the PEDV
AVCT12 genome as previously described [
8], designated pTZ_ORF3-FL and-Trnc. The pPEDV
AVCT12 infectious cDNA clone was subsequently digested with
PacI and
MluI restriction enzymes to allow the insertion of the ORF3s-myc fragment. PEDV
AVCT12 genes including S, ORF3-myc, E, M, and the N terminus of the N gene regions in pTZ_ORF3 plasmids were then inserted into the linearized pPEDV
AVCT12 infectious cDNA clone. The ORF3-myc in positive clones, designated pPEDV
AV12_ORF3-FL and-Trnc, were verified by direct nucleotide sequencing.
2.3. Western Blot Assay
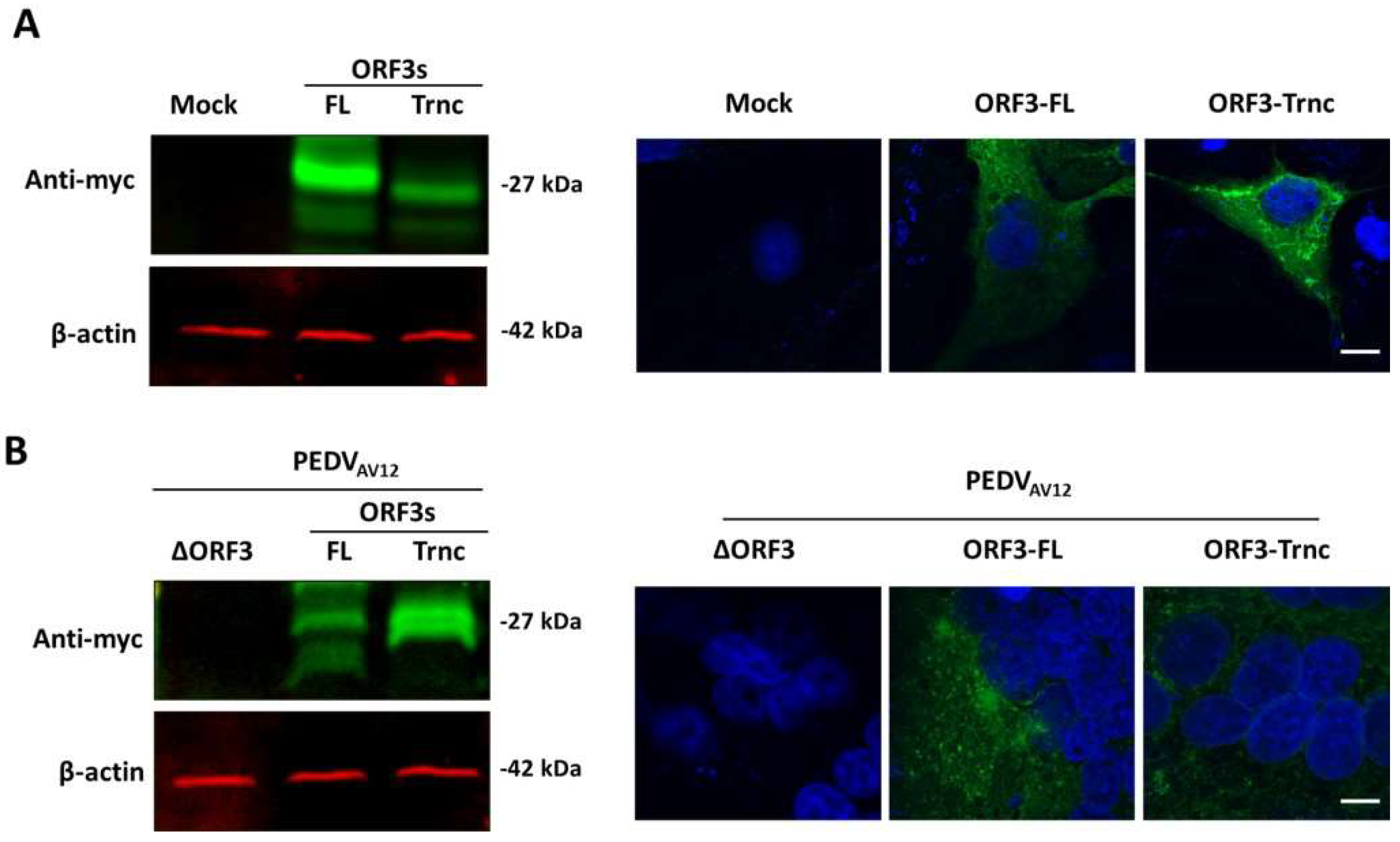
HEK293T or VeroE6-APN cells were grown in 6-well plates and transfected with the pCAGGS_ORF3-FL, pCAGGS_ORF3-Trnc or pCAGGS_SAV12 using Fugene HD transfection reagent (Promega, Madison, WI, USA) following the manufacturer’s instructions. At 24 h post transfection (hpt), transfected cells were collected and re-suspended in mammalian cell lysis buffer. Fifty micrograms of protein samples were incubated with protein loading buffer at 37 °C for 1 h, loaded on to 10% polyacrylamide gel, and transferred to nitrocellulose membranes. Membranes were then probed with rabbit anti-myc antibodies (Abcam, Cambridge, MA, USA) and followed by IRDye® 800CW goat anti-rabbit IgG (Li-COR bioscience, Lincoln, NE, USA) antibodies. Protein bands were visualized using a Li-COR Odyssey CLx imager (Li-COR bioscience).
2.4. Generation of Recombinant Viruses Expressing Tagged-ORF3
VeroE6-APN cells grown in a 6-well plate were transfected with 2 µg of pPEDVAV12_ORF3-FL or-Trnc and treated with 0.1% recombinant trypsin (Thermo Scientific, Waltham, MA, USA) at 24 hpt. At 60 hpt, cell supernatants were collected and adsorbed onto fresh VeroE6-APN cells to further propagate the virus.
Viral RNA was isolated using an RNA isolation kit (Geneaid, New Taipei, Taiwan). The purified viral RNA was then subject to RT-PCR using PrimeScript™ One-Step RT-PCR Kit (Takara, Tokyo, Japan) using primers specific for the inserted ORF3 gene. PCR products were cloned into pTZ5R/T vector, and five independent clones were sequence verified.
2.5. Confocal Microscopy
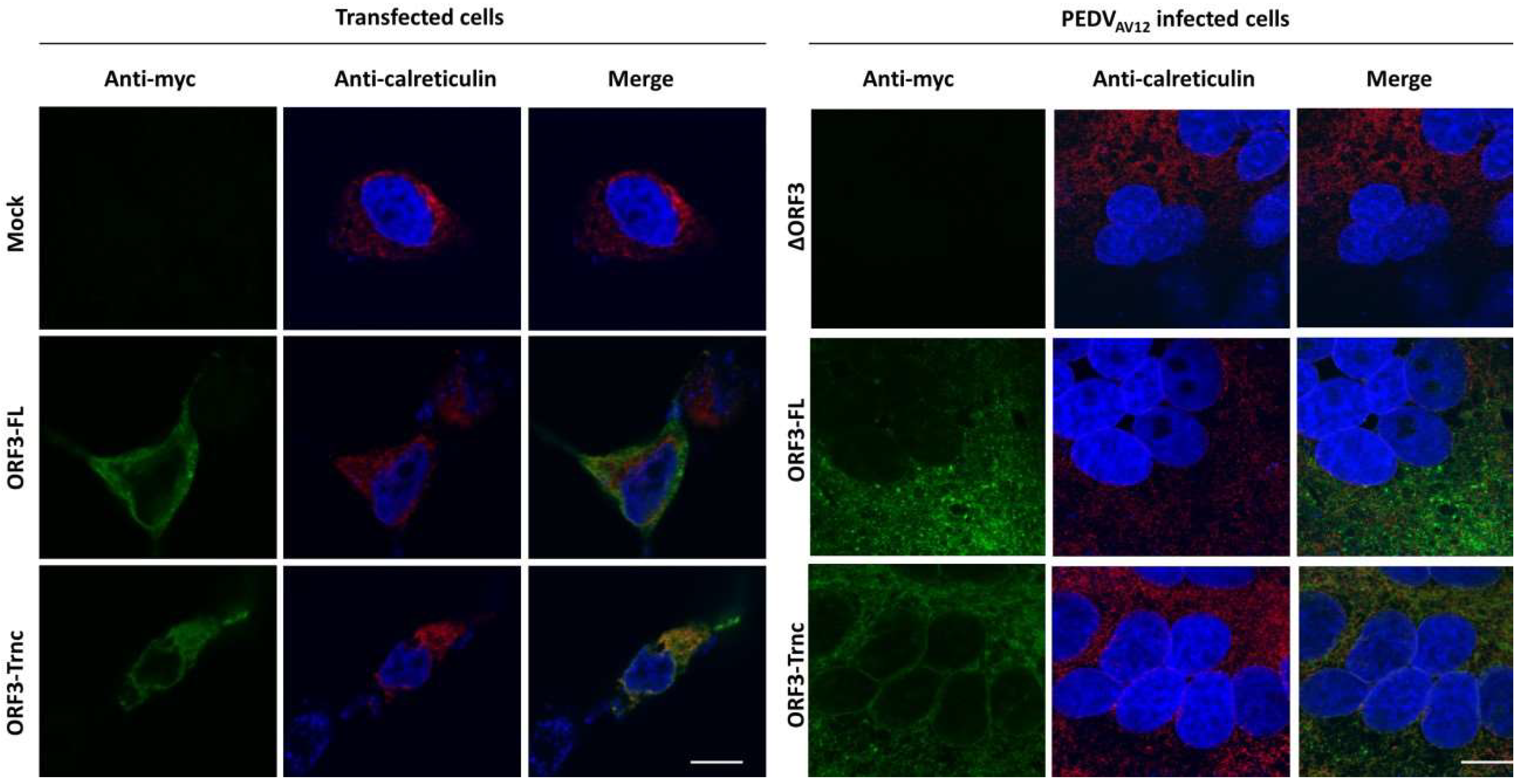
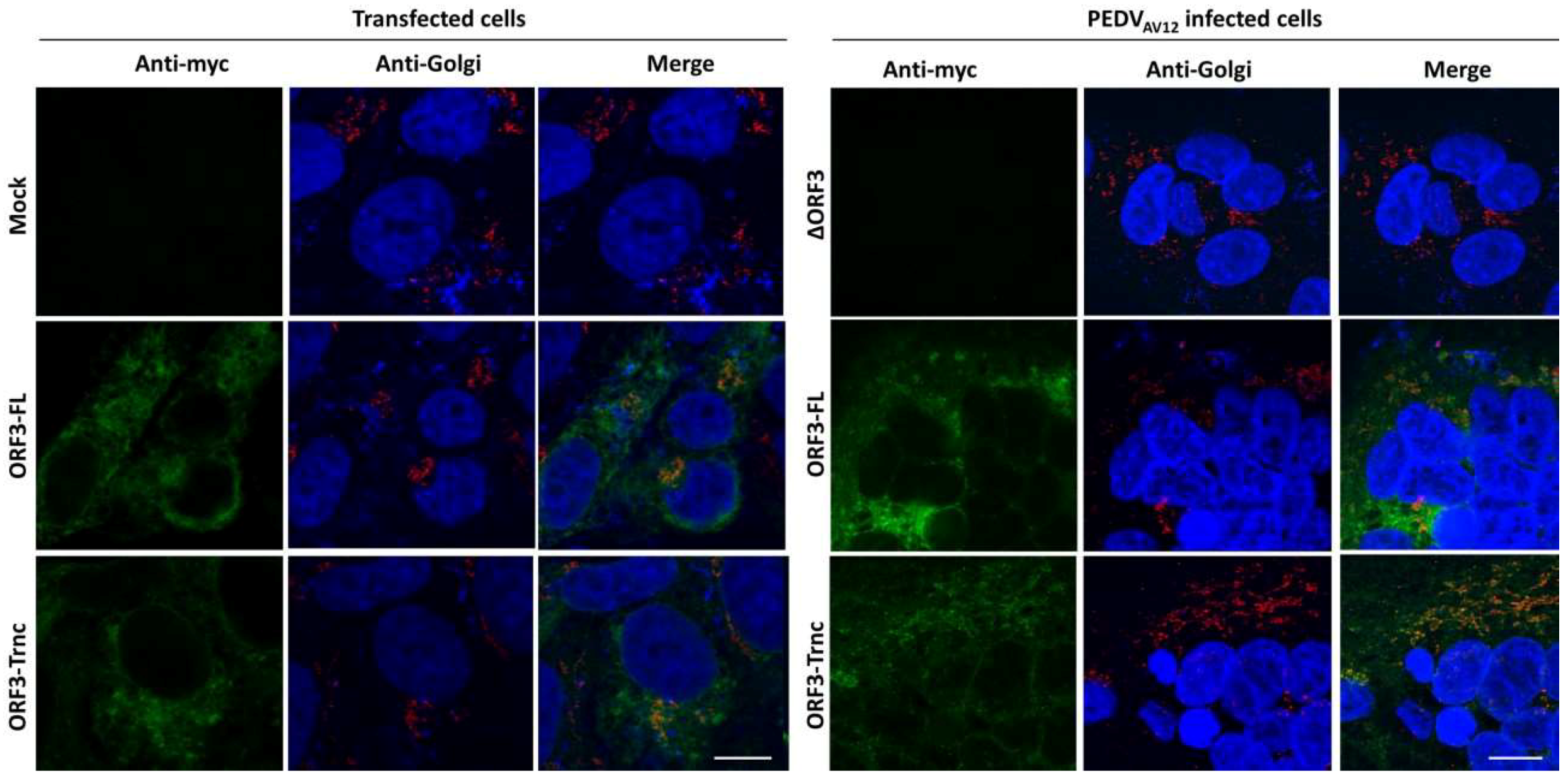
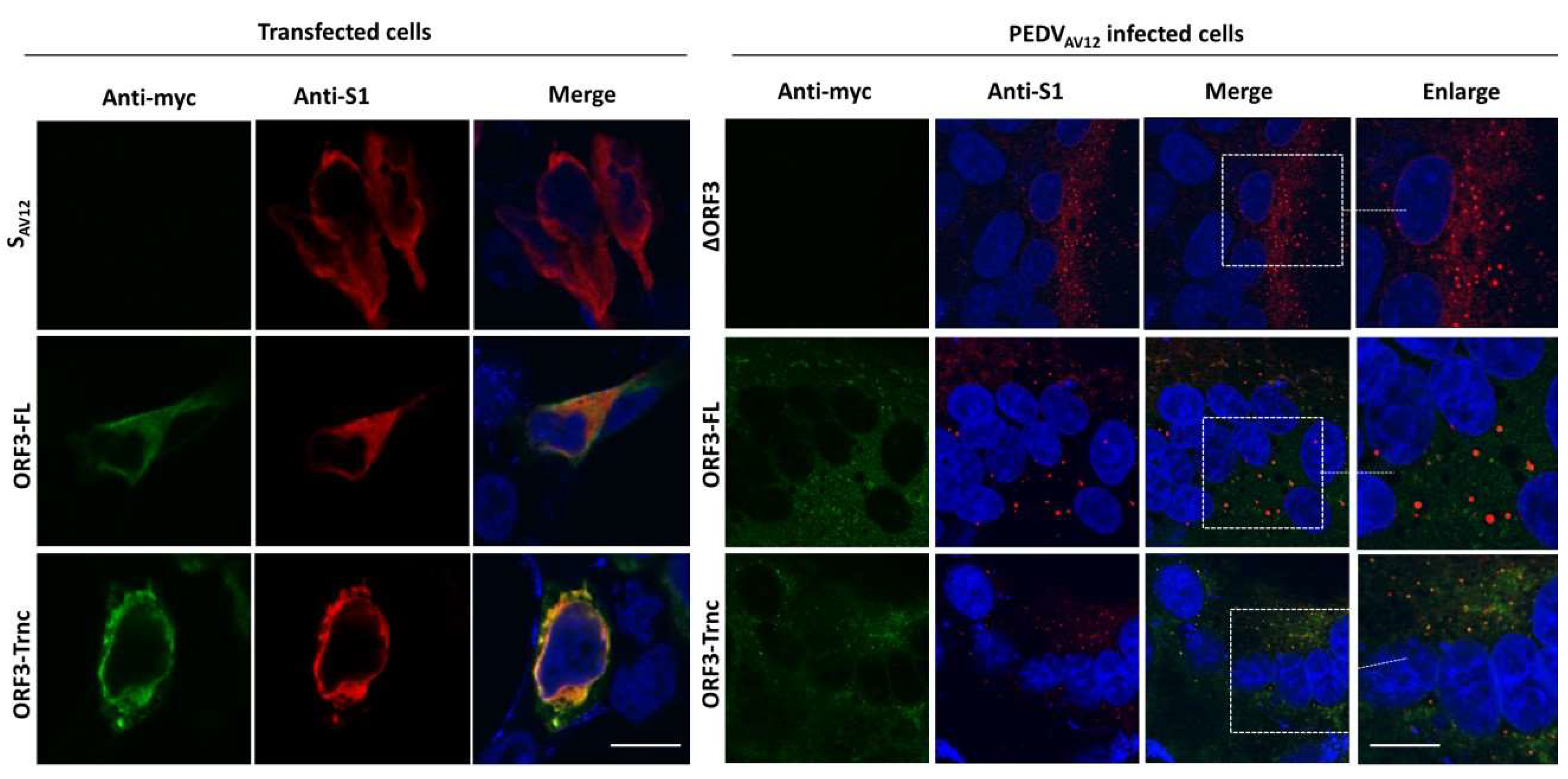
HEK293T or VeroE6-APN cells were grown on coverslips in 6-well plates. At 24 hpt (or h post infection; hpi), cells were washed with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde for 20 min at 4 °C. After incubation, cells were washed three times with PBS and blocked with PBS containing 10% FBS, 1% bovine serum albumin (BSA) and 0.2% TritonX-100 for 1 h. Cells were subsequently incubated for 1 h with rabbit anti-myc antibodies (Abcam) and mouse anti-calreticulin ER marker (Abcam) or mouse anti-58K Golgi protein antibodies (Abcam) in 10% FBS at a dilution 1:500 and 1:250, respectively. After washing thrice with PBST, goat anti-rabbit IgG Alexa flour 488 (Abcam) and goat anti-mouse IgG Alexa flour 647 antibodies (Abcam) in 10% FBS at a dilution 1:1000 was added and further incubated for 1 h. The glass slips were mounted on slides with Prolong Gold Antifade Mountant with DAPI (Invitrogen, Carlsbad, CA, USA). The samples were analyzed by FluoviewTM FV1000 confocal microscopy (Olympus, Tokyo, Japan). The Pearson’s correlation coefficients (PCC, Pasadena, CA, USA) were employed to determine co-localization using the ImageJ analysis program [
9] and the PSC co-localization plug-in as described elsewhere [
10,
11,
12]. Mean Pearson’s coefficient values were calculated from at least ten independent acquired images.
2.6. Flow Cytometry
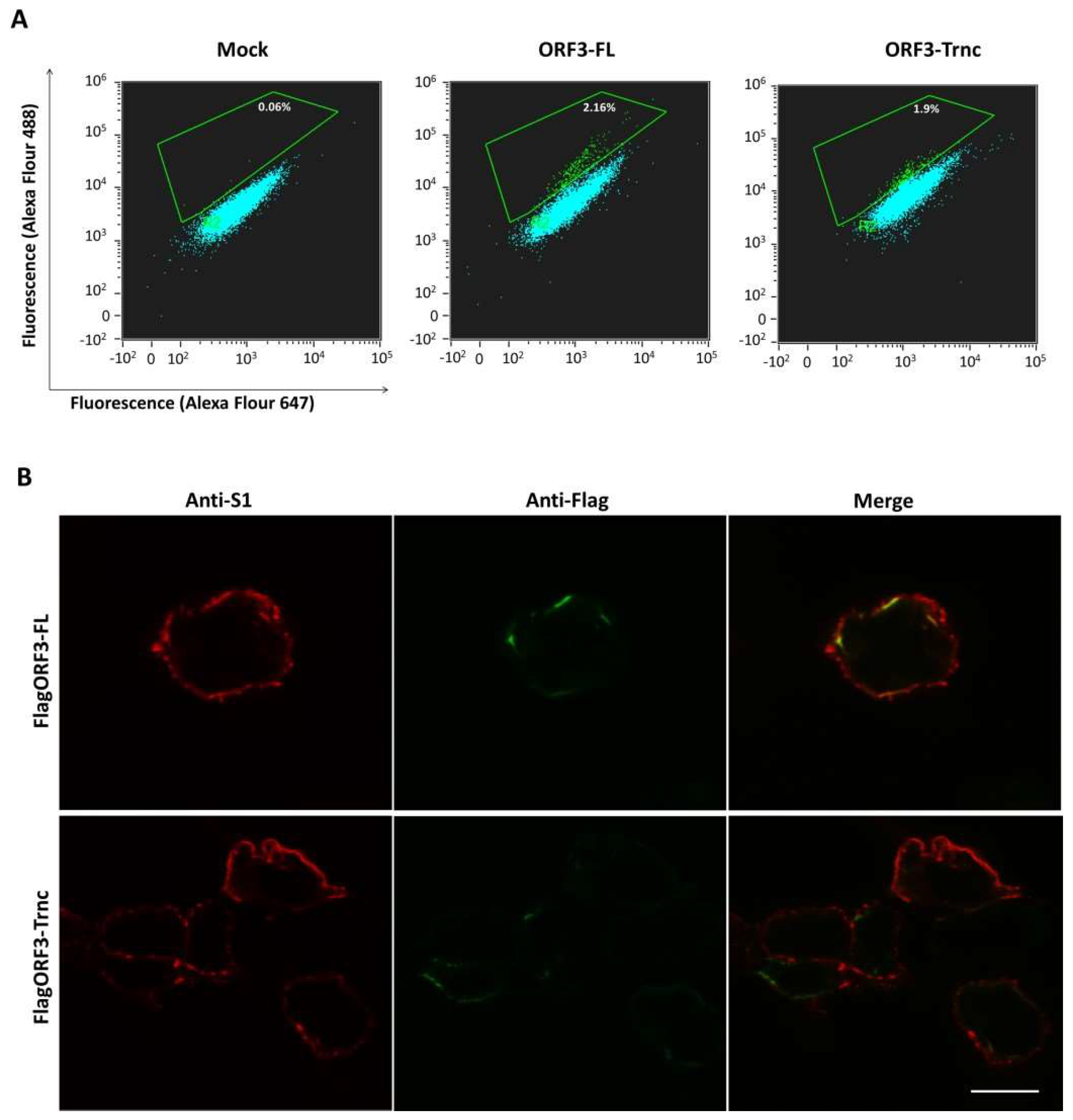
HEK293T cells were transfected with pCAGGS_FlagORF3-FL and-Trnc for 24 h before being trypsinized and fixed with 4% paraformaldehyde. Cells were blocked in blocking buffer (2% FBS and 0.5% BSA in PBS) for 1 h and then incubated with rabbit anti-Flag tag antibodies (Abcam) or mouse anti-S1 monoclonal antibodies for 1 h. A mouse anti-calreticulin ER marker was used as a non-permeabilized condition control. Cells were washed with PBS and incubated with a goat anti-rabbit Alexa flour 488 (IgG H + L) or anti-mouse Alexa flour 647 (IgG H + L) antibodies (Abcam) for 1 h. Cells were washed and analyzed on a FlowSight® Imaging Flow Cytometer (Merck, Kennborough, NJ, USA).
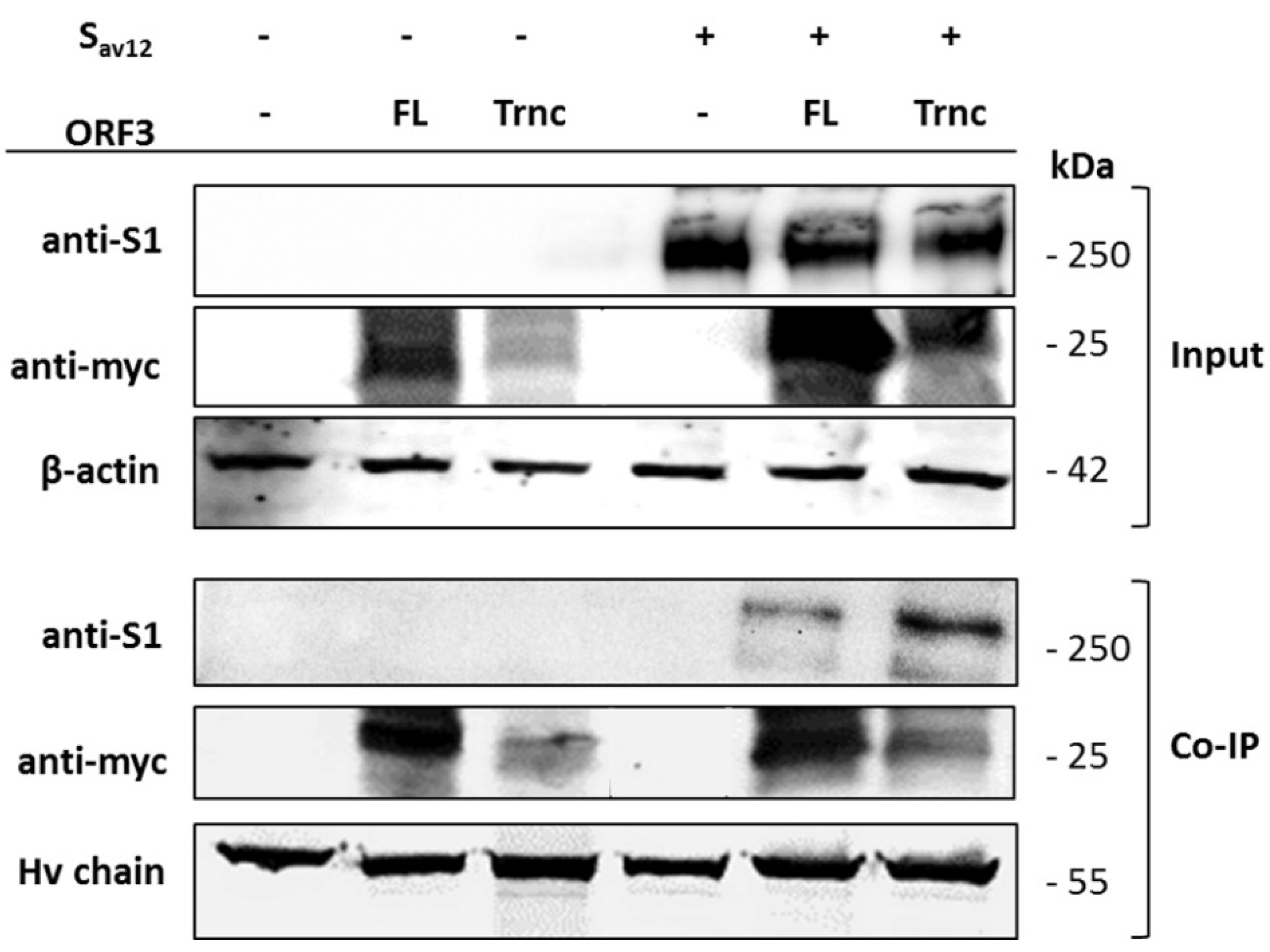
2.7. Co-Immunoprecipitation
HEK293T cells were co-transfected with pCAGGS_ORF3-FL or-Trnc and pCAGGS_SAV12. At 24 hpt, transfected cells were washed once with cold PBS. Cells were lysed with PierceTM lysis buffer (Thermo Scientific) supplemented with protease inhibitor cocktail (Thermo Scientific). Cell lysates were cleared by centrifugation at 14,000 times gravity (× g) for 5 min at 4 °C. Cleared lysates were then incubated with PierceTM anti-myc agarose (Thermo Scientific) with gentle rocking overnight at 4 °C. Immunoprecipitates were washed thrice with tris buffered saline and tween 20 (TBST;25 mM Tris-HCl, 0.15 M NaCl, 0.05% tween 20, pH 7.2) and eluted in sample buffer followed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot.
2.8. Virus Titration
VeroE6-APN cells were grown in 6-well plates and inoculated with 10-fold serial dilutions of the recombinant PEDVAV12. At 24 hpi, infected cells were fixed with 80% cold acetone for 10 min and washed twice with PBS. Blocking solution containing 10% FBS and 1% BSA was added and incubated at room temperature for 1 h with gentle agitation. After blocking, cells were incubated with mouse anti-PEDV N antibodies (Medgene, Brookings, SD, USA) and goat anti-mouse IgG alkaline phosphatase antibodies (Abcam). Syncytium forming unit was examined based on color formation after the addition of 1-Step™ NBT/BCIP Substrate Solution (Thermo Scientific).
2.9. Statistical Analysis
GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA, USA) was used for statistical analyses. The differences in mean values of virus titer between groups were analyzed by two-way ANOVA. All results were represented as means ± standard error of means; p-values of <0.05 were considered statistically significant.
4. Discussion
We previously demonstrated that the ORF3 protein from different PEDV strains could have a distinct regulatory function on virus replication. Specifically, the naturally-occurring amino acid deletion could abolish the suppressive effect of some ORF3 variants on virus replication in vitro [
7]. In this study, we synthesized the full-length ORF3 gene carrying C-terminal myc-tag (ORF3-FL) as a representative of the wild-type ORF3. To evaluate the effect of the amino acid deletion, we constructed the truncated ORF3 (ORF3-Trnc). In addition, we employed reverse genetics to generate recombinant PEDV carrying ORF3-myc without disturbing the virus growth and were able to follow ORF3 subcellular localization in infected cells. It should be noted that, while the amino acid substitution or deletion on ORF3-FL gene were not detected upon multiple passages, random amino acid substitution could be detected in some selected clones of ORF3-Trnc.
The ORF3 protein of PEDV has been predicted to have transmembrane domains and ion-channel function [
5]. Similarly, accessory proteins from other coronaviruses such as U274 protein from Severe acute respiratory syndrome Coronavirus (SARS-CoV) [
15] and ORF3 from Human pathogenic coronavirus NL63 (hCoV-NL63) [
16] are also reported to have ion-channel activity. It is thus possible that these proteins may share common functions. Our results in this study, however, suggest that ORF3 may play multiple roles in addition to being ion channels during replication of PEDV in host cells. Consistent with this hypothesis, a recent study has demonstrated that ORF3 in cells stably expressing the protein was predominantly cytosolic [
6]. In addition, cells expressing ORF3 exhibited a prolonged S phase, suggesting that ORF3 may be associated with the host’s cell cycle machinery [
6]. Furthermore, our findings that the ORF3 protein expressed in transfected and infected cells displayed different patterns led us to speculate that the additional band in infected cells might be due to post-translational modifications that occurred during protein trafficking through the ER-Golgi compartments [
15]. Indeed, the in silico analysis of ORF3 by the WoLFPSORT program predicts its cellular localization in ER, Golgi apparatus, and vesicle of the secretory pathway, which is confirmed by our immunofluorescence data. These results are actually in line with what reported previously about ORF3 [
6,
7] and other coronaviruses [
16,
17].
It is noteworthy that the ORF3-Trnc has a deletion of amino acids sequences ‘YCPLLYYCGAFLDATII’, resulting in the disruption of the transmembrane domain [
5] and YxxΦ motif (x can be any residue, and Φ is a residue with a bulky hydrophobic side chain), a sorting signal for clathrin-mediated endocytosis [
18,
19]. The absence of YxxΦ motif within the SARS 3a protein was reported to increase retention of the ΔYxxΦ 3a protein in the Golgi apparatus, protein degradation and decreased protein expression on the cell surface [
20]. Unlike the SARS 3a protein, our results showed that both ORF3-FL and-Trnc partially localized in the ER and Golgi compartments and could be transported to the cells’ surface, suggesting that the lack of YxxΦ motif in the truncated ORF3 protein might not be crucial for its subcellular trafficking through the ER–Golgi compartment.
Our observation that ORF3 and S are both expressed on the cell surface points to the possibility that these two proteins might interact with each other. Indeed, we showed that both ORF3-FL and-Trnc proteins were co-localized with the S protein. Interestingly, ORF3-Trnc displayed a more apparent association with the S protein particularly at the perinuclear membrane of transfected cells and the vesicle-like compartments in infected cells. Ultrastructural characterization in PEDV infected cells has revealed that the PEDV virus replication site was mainly at the perinuclear space where maturation of the virus particles was formed and packaged into the vacuoles through the vesicular pathway in the ER and Golgi compartments [
21]. The fact that ORF3 were found co-localized with S protein at the perinuclear area and in the vesicular structures may suggest its contribution at this step of the replication cycle. It is also notable that other accessory proteins from other coronaviruses have been shown to interact with viral structural proteins such as S, M, and E proteins [
15,
16,
20]. In addition, recent evidence has suggested a possible association between S and ORF3 to increase virus replication and virulence in nursing piglets. Recombinant PEDV viruses lacking ORF3 were of very low virulence despite the expression of S derived from a highly pathogenic variant. Interestingly, when the functional ORF3 was restored, the recombinant viruses bearing S from the pathogenic strain and ORF3 displayed increased virulence [
22]. These data, therefore, suggest that S and ORF3 may work in concert to regulate PEDV replication in vivo.
Although the exact mechanism(s) by which the ORF3 protein takes part in virus replication has not been clarified in the present study, evidence of ORF3 characteristics described here uncovered its unprecedented functions and association with structural proteins that may play a role in virus assembly and growth. It is noteworthy that while some variants of full-length ORF3 were shown to inhibit PEDV replication [
8], some studies report that the protein could promote PEDV replication in vitro [
5,
6]. In this regard, we cannot definitively distinguish whether ORF3 functions in promoting or suppressing PEDV replication or what other viral proteins could contribute to the regulatory role of ORF3. Consequently, further work is required to determine precisely how ORF3 exerts its functions. Understanding these functions may, in turn, enable the development of new attenuated vaccines to prevent PED.