Activity of the Chimeric Lysin ClyR against Common Gram-Positive Oral Microbes and Its Anticaries Efficacy in Rat Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Cloning, Expression, and Purification of ClyR
2.3. Bactericidal Assay
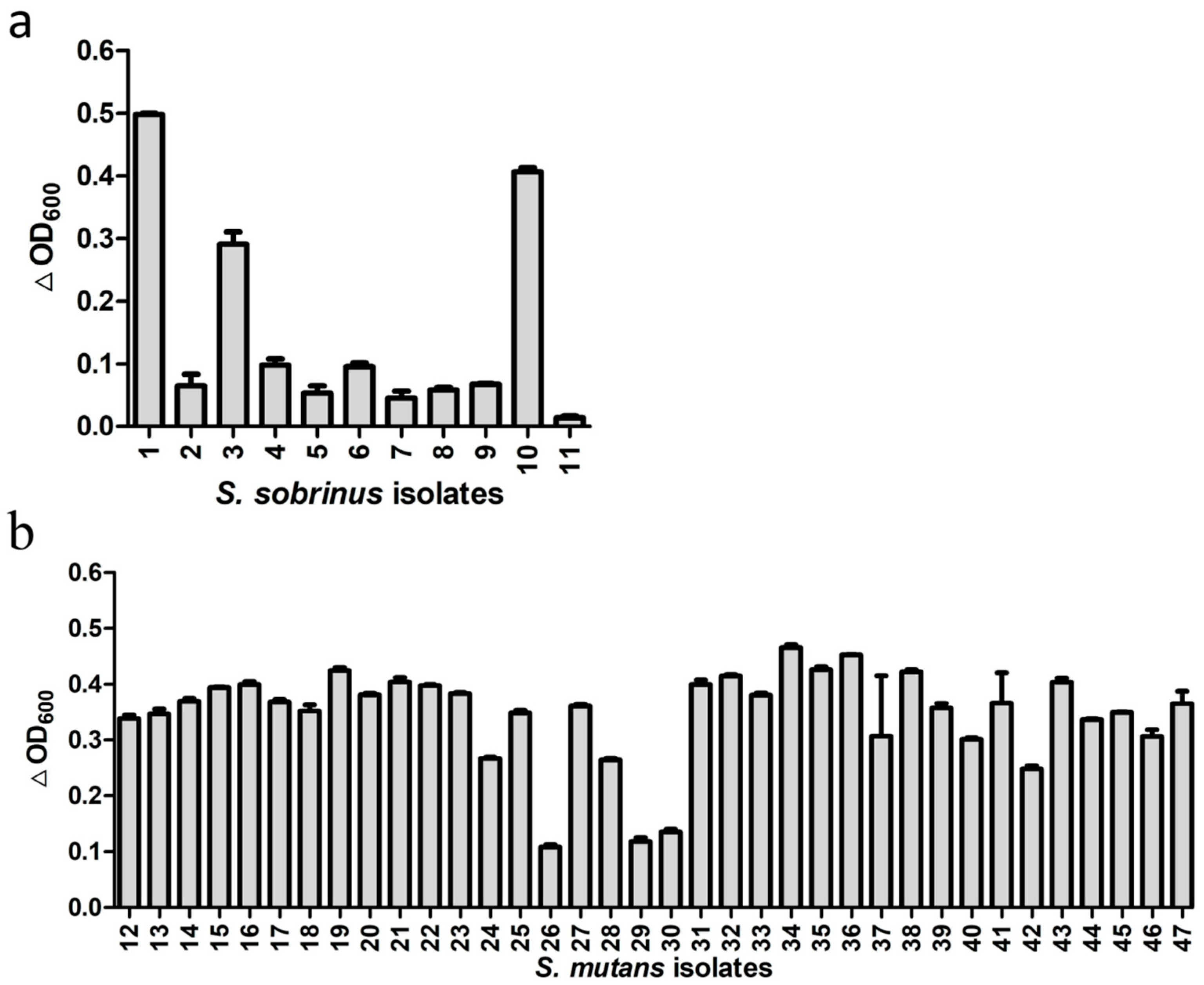
2.4. ClyR Activity against Clinical S. mutans and S. sobrinus Isolates
2.5. Transmission Electron Microscopy (TEM)
2.6. Scanning Electron Microscopy (SEM)
2.7. Confocal Laser Scanning Microscopy (CLSM)
2.8. Quantify Recovered Biofilm Bacteria in Vitro
2.9. Animal Study
2.10. Statistical Analysis
3. Results
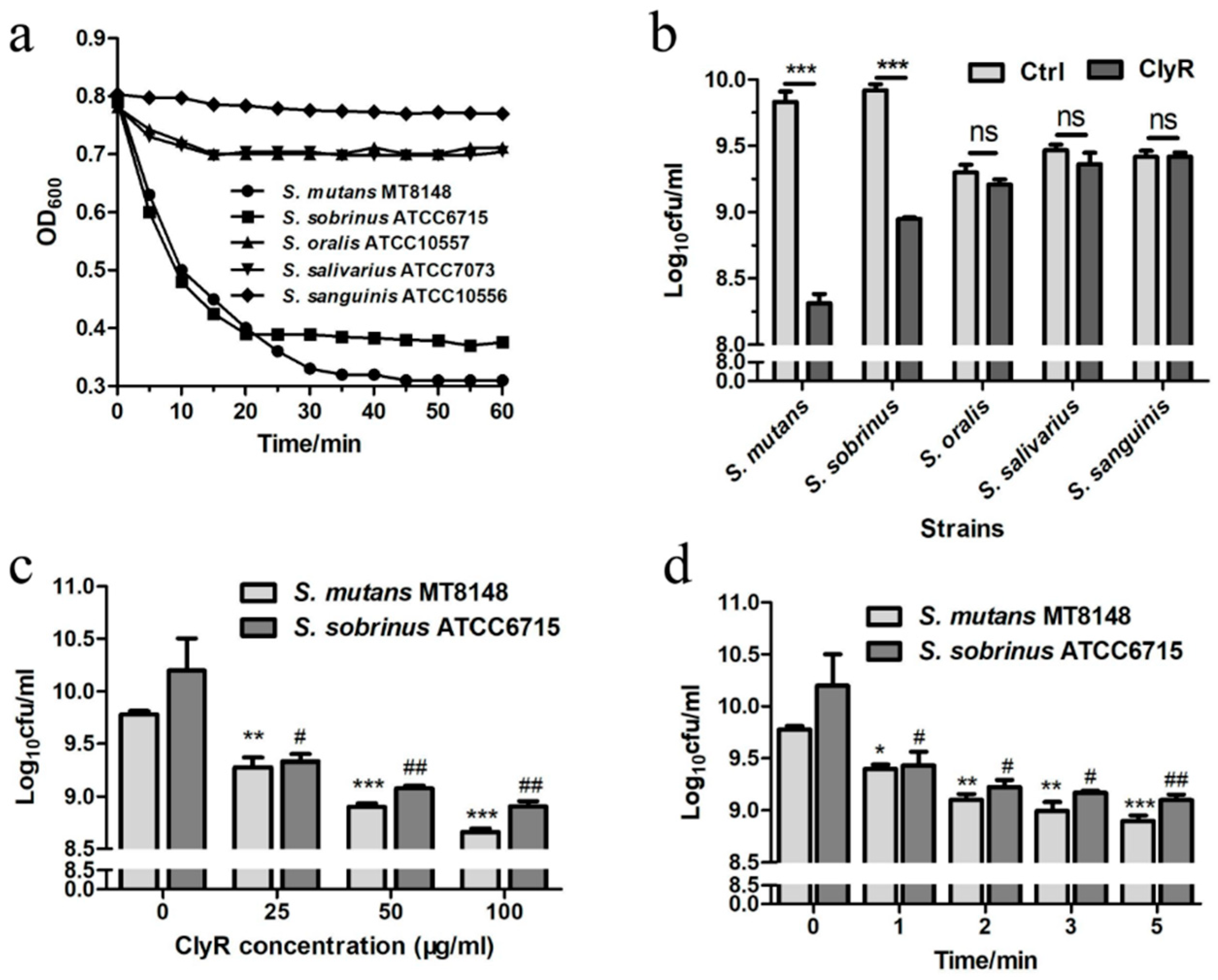
3.1. High Bactericidal Activity of ClyR against Planktonic S. mutans and S. sobrinus
3.2. Broad Lytic Activity
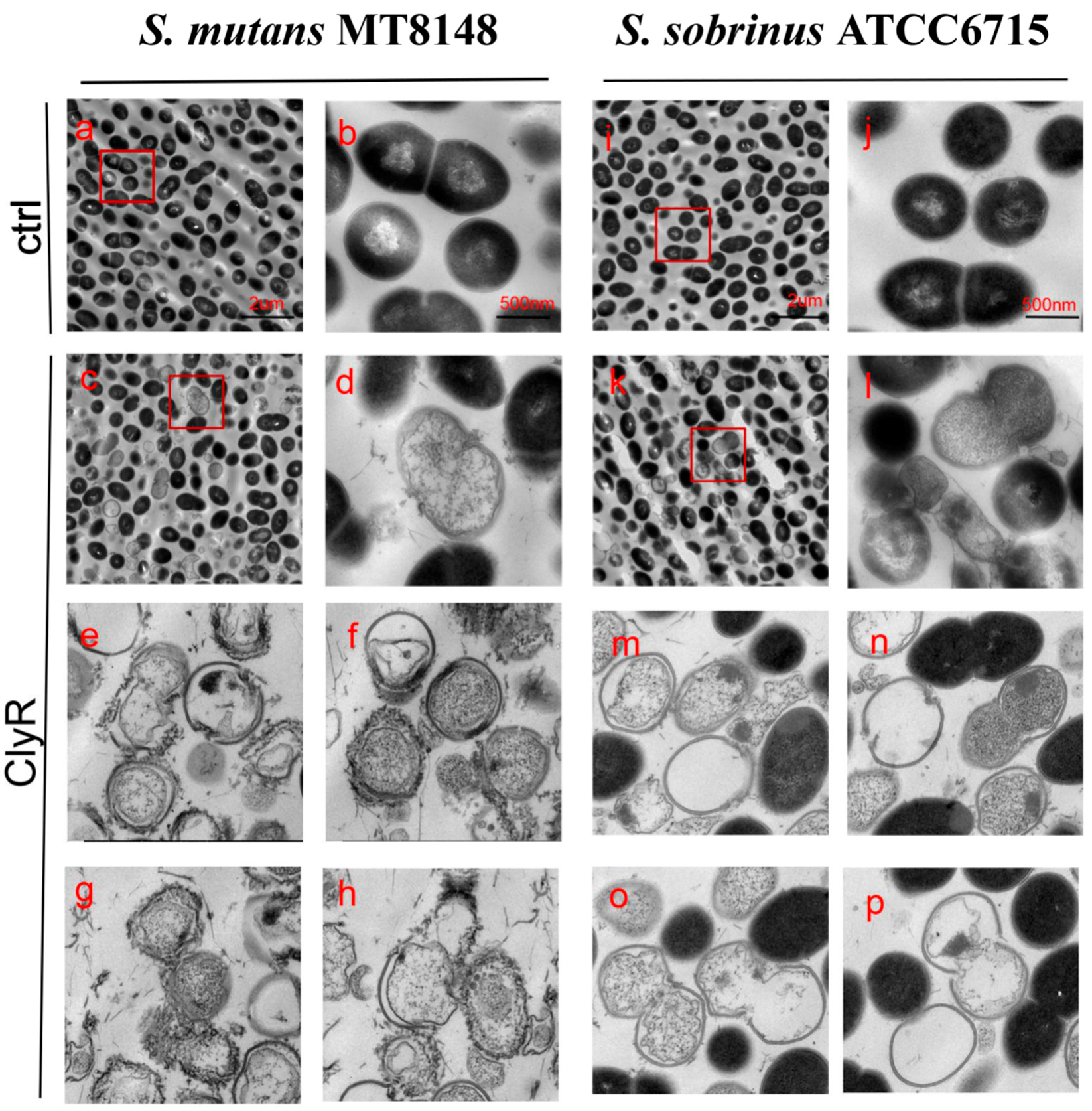
3.3. TEM Analysis of S. mutans and S. sobrinus Exposed to ClyR
3.4. Activity of ClyR against S. mutans and S. sobrinus Biofilms
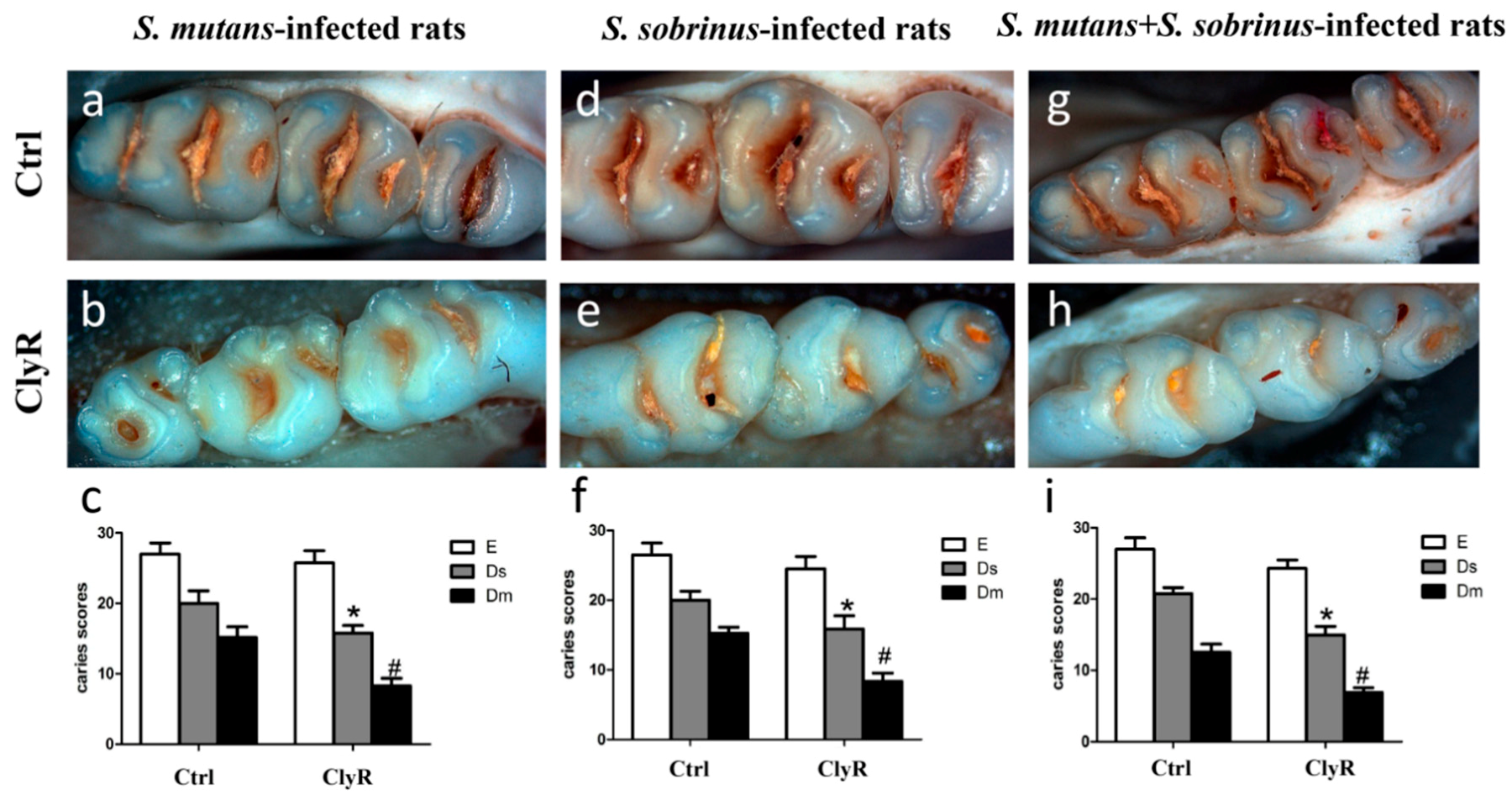
3.5. Anticaries Efficacy of ClyR in Rat Models
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Petersen, P.E.; Kwan, S. World Health Organization global oral health strategies for oral health promotion and disease prevention in the twenty-first century. Prvention Und Gesundheitsfrderung 2009, 4, 100–104. [Google Scholar] [CrossRef]
- Miller, W.D. The Microorganisms of Human Mouth. Am. J. Med. Sci. 1970, 98, 275–276. [Google Scholar] [CrossRef]
- Tanzer, J.M.; Livingston, J.; Thompson, A.M. The microbiology of primary dental caries in humans. J. Dent. Educ. 2001, 65, 1028–1037. [Google Scholar] [PubMed]
- Hamilton, I.R.; Buckley, N.D. Adaptation by Streptococcus mutans to acid tolerance. Oral Microbiol. Immunol. 1991, 6, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Monchois, V.; Willemot, R.; Monsan, P. Glucansucrases: Mechanism of action and structure–function relationships. FEMS Microbiol. Rev. 1999, 23, 131–151. [Google Scholar] [CrossRef] [PubMed]
- De Soet, J.J.; Van, L.C.; Lammens, A.J.; Pavicić, M.J.; Homburg, C.H.; ten Cate, J.M.; de, G.J. Differences in cariogenicity between fresh isolates of Streptococcus sobrinus and Streptococcus mutans. Caries Res. 1991, 25, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Sánchezacedo, M.; Montielcompany, J.M.; Dasífernández, F.; Almerichsilla, J.M. Streptococcus mutans and Streptococcus sobrinus detection by Polymerase Chain Reaction and their relation to dental caries in 12 and 15 year-old schoolchildren in Valencia (Spain). Med. Oral Patol. Oral Cir. Bucal 2013, 18, E839–E845. [Google Scholar] [CrossRef]
- Okada, M.; Kawamura, M.; Oda, Y.; Yasuda, R.; Kojima, T.; Kurihara, H. Caries prevalence associated with Streptococcus mutans and Streptococcus sobrinus in Japanese schoolchildren. Int. J. Paediatr. Dent. 2012, 22, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Kishi, M.; Abe, A.; Kishi, K.; Ohara-Nemoto, Y.; Kimura, S.; Yonemitsu, M. Relationship of quantitative salivary levels of Streptococcus mutans and S. sobrinus in mothers to caries status and colonization of mutans streptococci in plaque in their 2.5-year-old children. Commun. Dent. Oral Epidemiol. 2009, 37, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.D. Prevention and reversal of dental caries: Role of low level fluoride. Commun. Dent. Oral Epidemiol. 1999, 27, 31–40. [Google Scholar] [CrossRef]
- Osuji, O.O.; Leake, J.L.; Chipman, M.L.; Nikiforuk, G.; Locker, D.; Levine, N. Risk factors for dental fluorosis in a fluoridated community. J. Dent. Res. 1988, 67, 1488–1492. [Google Scholar] [CrossRef] [PubMed]
- Gessner, B.D.; Beller, M.; Middaugh, J.P.; Whitford, G.M. Acute fluoride poisoning from a public water system. New Engl. J. Med. 1994, 330, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Stauder, M.; Papetti, A.; Daglia, M.; Vezzulli, L.; Gazzani, G.; Varaldo, P.E.; Pruzzo, C. Inhibitory activity by barley coffee components towards Streptococcus mutans biofilm. Curr. Microbiol. 2010, 61, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Duarte, S.; Murata, R.M.; Scottanne, K.; Gregoire, S.; Watson, G.E.; Singh, A.P.; Vorsa, N. Influence of Cranberry Proanthocyanidins on Formation of Biofilms by Streptococcus mutans on Saliva-Coated Apatitic Surface and on Dental Caries Development in vivo. Caries Res. 2010, 44, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Nittayananta, W.; Limsuwan, S.; Srichana, T.; Sae-Wong, C.; Amnuaikit, T. Oral spray containing plant-derived compounds is effective against common oral pathogens. Arch. Oral Biol. 2018, 90, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Fan, M.; Wu, H.; Melander, C.; Liu, C. A new small molecule inhibits Streptococcus mutans biofilms in vitro and in vivo. J. Appl. Microbiol. 2015, 119, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Hayacibara, M.F.; Schobel, B.D.; Cury, J.A.; Rosalen, P.L.; Park, Y.K.; Vacca-Smith, A.M.; Bowen, W.H. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J. Antimicrob. Chemother. 2003, 52, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guo, L.; Lux, R.; Eckert, R.; Yarbrough, D.; He, J.; Anderson, M.; Shi, W. Targeted Antimicrobial Therapy Against Streptococcus mutans Establishes Protective Non-cariogenic Oral Biofilms and Reduces Subsequent Infection. Int. J. Oral Sci. 2010, 2, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, C.W.; Sim, J.H.; Shah, K.R.; Kolesnikovakaplan, A.; Shi, W.; Eckert, R. Selective Membrane Disruption: Mode of Action of C16G2, a Specifically Targeted Antimicrobial Peptide. Antimicrob. Agents Chemother. 2011, 55, 3446–3452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Worthington, R.J.; Melander, C.; Wu, H. A new small molecule specifically inhibits the cariogenic bacterium Streptococcus mutans in multispecies biofilms. Antimicrob. Agents Chemother. 2011, 55, 2679–2687. [Google Scholar] [CrossRef] [PubMed]
- Roach, D.R.; Donovan, D.M. Antimicrobial bacteriophage-derived proteins and therapeutic applications. Bacteriophage 2015, 5, e1062590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmelcher, M.; Shen, Y.; Nelson, D.C.; Eugster, M.R.; Eichenseher, F.; Hanke, D.C.; Loessner, M.J.; Dong, S.; Pritchard, D.G.; Lee, J.C. Evolutionarily distinct bacteriophage endolysins featuring conserved peptidoglycan cleavage sites protect mice from MRSA infection. J. Antimicrob. Chemother. 2015, 70, 1453–1465. [Google Scholar] [CrossRef] [PubMed]
- Gilmer, D.B.; Schmitz, J.E.; Euler, C.W.; Fischetti, V.A. Novel bacteriophage lysin with broad lytic activity protects against mixed infection by Streptococcus pyogenes and methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2013, 57, 2743–2750. [Google Scholar] [CrossRef] [PubMed]
- Poonacha, N.; Nair, S.; Desai, S.; Tuppad, D.; Hiremath, D.; Mohan, T.; Vipra, A.; Sharma, U. Efficient Killing of Planktonic and Biofilm-Embedded Coagulase-Negative Staphylococci by Bactericidal Protein P128. Antimicrob. Agents Chemother. 2017, 61, e00457-17. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Poonacha, N.; Desai, S.; Hiremath, D.; Tuppad, D.; Mohan, T.; Chikkamadaiah, R.; Durgaiah, M.; Kumar, S.; Channabasappa, S. Restoration of sensitivity of a diverse set of drug-resistant Staphylococcus clinical strains by bactericidal protein P128. J. Med. Microbiol. 2018, 67, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, R.; Domenech, M.; Iglesiasbexiga, M.; Menéndez, M.; García, P. Csl2, a novel chimeric bacteriophage lysin to fight infections caused by Streptococcus suis, an emerging zoonotic pathogen. Sci. Rep. 2017, 7, 16506. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.C.; Roach, D.R.; Chauhan, V.S.; Yang, S.; Juli, F.F.; Powell, A.M.; Gary, B.; Lease, R.A.; Homan, M.; Harty, W.J. Triple-acting Lytic Enzyme Treatment of Drug-Resistant and Intracellular Staphylococcus aureus. Sci. Rep. 2016, 6, 25063. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; González, S.; Campelo, A.B.; Martínez, B.; Rodríguez, A.; García, P. Downregulation of Autolysin-Encoding Genes by Phage-Derived Lytic Proteins Inhibits Biofilm Formation in Staphylococcus aureus. Antimicrob. Agents Chemother. 2017, 61, e02724-16. [Google Scholar]
- Hang, Y.; Linden, S.B.; Jing, W.; Yu, J.; Nelson, D.C.; Wei, H. A chimeolysin with extended-spectrum streptococcal host range found by an induced lysis-based rapid screening method. Sci. Rep. 2015, 5, 17257. [Google Scholar] [Green Version]
- Yang, H.; Bi, Y.; Shang, X.; Wang, M.; Linden, S.B.; Li, Y.; Li, Y.; Nelson, D.C.; Wei, H. Antibiofilm Activities of a Novel Chimeolysin against Streptococcus mutans under Physiological and Cariogenic Conditions. Antimicrob. Agents Chemother. 2016, 60, 7436–7443. [Google Scholar] [PubMed]
- Li, Y.; Caufield, P.W. Arbitrarily primed polymerase chain reaction fingerprinting for the genotypic identification of mutans streptococci from humans. Mol. Oral Microbiol. 2010, 13, 17–22. [Google Scholar] [CrossRef]
- Keyes, P.H. Dental caries in the molar teeth of rats. 2. A method for diagnosing and scoring several types of lesions simultaneously. J. Dent. Res. 1958, 37, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, H.; Wang, J.; Yu, J.; Wei, H. A novel chimeric lysin with robust antibacterial activity against planktonic and biofilm methicillin-resistant Staphylococcus aureus. Sci. Rep. 2017, 7, 40182. [Google Scholar] [CrossRef] [PubMed]
- Gilmer, D.B.; Schmitz, J.E.; Thandar, M.; Euler, C.W.; Fischetti, V.A. The Phage Lysin PlySs2 Decolonizes Streptococcus suis from Murine Intranasal Mucosa. PLoS ONE 2017, 12, e0169180. [Google Scholar] [CrossRef] [PubMed]
- Schmelcher, M.; Loessner, M.J. Bacteriophage endolysins: Applications for food safety. Curr. Opin. Biotechnol. 2015, 37, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Czaplewski, L.; Bax, R.; Clokie, M.; Dawson, M.; Fairhead, H.; Fischetti, V.A.; Foster, S.; Gilmore, B.F.; Hancock, R.E.W.; Harper, D. Alternatives to antibiotics—A pipeline portfolio review. Lancet Infect. Dis. 2016, 16, 239–251. [Google Scholar] [CrossRef]
- Li, W.; Yang, H.; Gong, Y.; Wang, S.; Li, Y.; Wei, H. Effects of a Chimeric Lysin against Planktonic and Sessile Enterococcus faecalis Hint at Potential Application in Endodontic Therapy. Viruses 2018, 10, 290. [Google Scholar] [CrossRef] [PubMed]
- Burne, R.A.; Parsons, D.T.; Marquis, R.E. Cloning and expression in Escherichia coli of the genes of the arginine deiminase system of Streptococcus sanguis NCTC 10904. Infect. Immun. 1989, 57, 3540–3548. [Google Scholar] [PubMed]
- Li, Y.H.; Chen, Y.Y.; Burne, R.A. Regulation of urease gene expression by Streptococcus salivarius growing in biofilms. Environ. Microbiol. 2010, 2, 169–177. [Google Scholar] [CrossRef]
- Lee, S.H. Antagonistic effect of peptidoglycan of Streptococcus sanguinis on lipopolysaccharide of major periodontal pathogens. J. Microbiol. 2015, 53, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Kreth, J.; Zhang, Y.; Herzberg, M.C. Streptococcal antagonism in oral biofilms: Streptococcus sanguinis and Streptococcus gordonii interference with Streptococcus mutans. J. Bacteriol. 2008, 190, 4632–4640. [Google Scholar] [CrossRef] [PubMed]
- Hardie, J.M.; Bowden, G.H. Cell Wall and Serological Studies on Streptococcus mutans. Caries Res. 2009, 8, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Chałas, R.; Wójcikchęcińska, I.; Woźniak, M.J.; Grzonka, J.; Święszkowski, W.; Kurzydłowski, K.J. Dental plaque as a biofilm—A risk in oral cavity and methods to prevent. Postȩpy Higieny I Medycyny Doświadczalnej 2015, 69, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Costerton, W.; Veeh, R.; Shirtliff, M.; Pasmore, M.; Post, C.; Ehrlich, G. The application of biofilm science to the study and control of chronic bacterial infections. J. Clin. Investig. 2003, 112, 1466–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbin, A.; Pitts, B.; Parker, A.; Stewart, P.S. Antimicrobial penetration and efficacy in an in vitro oral biofilm model. Antimicrob. Agents Chemother. 2011, 55, 3338–3344. [Google Scholar] [CrossRef] [PubMed]
- Fragkou, S.; Balasouli, C.; Tsuzukibashi, O.; Argyropoulou, A.; Menexes, G.; Kotsanos, N.; Kalfas, S. Streptococcus mutans, Streptococcus sobrinus and Candida albicans in oral samples from caries-free and caries-active children. Eur. Arch. Paediatr. Dent. 2016, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Cui, T.; Zeng, J.; Chen, L.; Zhang, W.; Xu, X.; Cheng, L.; Li, M.; Li, J.; Zhou, X.; et al. Molecule Targeting Glucosyltransferase Inhibits Streptococcus mutans Biofilm Formation and Virulence. Antimicrob. Agents Chemother. 2015, 60, 126–135. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Yang, H.; Bi, Y.; Li, W.; Wei, H.; Li, Y. Activity of the Chimeric Lysin ClyR against Common Gram-Positive Oral Microbes and Its Anticaries Efficacy in Rat Models. Viruses 2018, 10, 380. https://doi.org/10.3390/v10070380
Xu J, Yang H, Bi Y, Li W, Wei H, Li Y. Activity of the Chimeric Lysin ClyR against Common Gram-Positive Oral Microbes and Its Anticaries Efficacy in Rat Models. Viruses. 2018; 10(7):380. https://doi.org/10.3390/v10070380
Chicago/Turabian StyleXu, Jingjing, Hang Yang, Yongli Bi, Wuyou Li, Hongping Wei, and Yuhong Li. 2018. "Activity of the Chimeric Lysin ClyR against Common Gram-Positive Oral Microbes and Its Anticaries Efficacy in Rat Models" Viruses 10, no. 7: 380. https://doi.org/10.3390/v10070380




