Role of T-Helper 9 Cells in Chronic Hepatitis C-Infected Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Subjects
2.3. Sample Processing
2.4. Cell Preparation
2.5. Flow Cytometry Analysis
2.6. Real-Time PCR
2.7. Statistical Analysis
3. Results
3.1. Alteration of the Frequency of Th9 Cells in Infected Patients
3.2. Correlation of Th9 Cells with Clinical Parameters
3.3. Th9 Levels in Relation to Liver Disease Severity
3.4. Th9 Levels in Relation to Response to Treatment
3.5. Predictive Accuracy and Determination of the Best Cut-Off Value of Th9 Cells for Predicting HCV-Related Chronic Liver Disease
3.6. Expression of IL9 Receptor (IL9R) in HCV-Infected Patients
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boulant, S.; Douglas, M.W.; Moody, L.; Budkowska, A. Hepatitis c virus core protein induces lipid droplet redistribution in a microtubule-and dynein-dependent manner. Traffic 2008, 9, 1268–1282. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, A.; Allam, N.; Elsharkawy, A.; El Kassas, M.; Waked, I. Hepatitis c infection in egypt: Prevalence, impact and management strategies. Hepat. Med. 2017, 9, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Kouyoumjian, S.P.; Chemaitelly, H.; Abu-Raddad, L.J. Characterizing hepatitis C virus epidemiology in egypt: Systematic reviews, meta-analyses, and meta-regressions. Sci. Rep. 2018, 8, 1661. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Hetta, H.F.; Abdel-Hameed, E.A.; Rouster, S.D.; Hossain, M.; Mekky, M.A.; Khalil, N.K.; Mohamed, W.A.; El-Feky, M.A.; Ahmed, S.H.; et al. Association between il28b rs12979860 single nucleotide polymorphism and the frequency of colonic treg in chronically HCV-infected patients. Arch. Virol. 2016, 161, 3161–3169. [Google Scholar] [CrossRef] [PubMed]
- Alberti, A.; Chemello, L.; Benvegnu, L. Natural history of hepatitis C. J. Hepatol. 1999, 31 (Suppl. 1), 17–24. [Google Scholar] [CrossRef]
- Alazawi, W.; Cunningham, M.; Dearden, J.; Foster, G.R. Systematic review: Outcome of compensated cirrhosis due to chronic hepatitis C infection. Aliment. Pharmacol. Ther. 2010, 32, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.P.; Gow, P.; Fink, M.; Dev, A.; Roberts, S.; Nicoll, A.; Lubel, J.; Kronborg, I.; Arachchi, N.; Ryan, M. Novel population based study finding higher than reported hepatocellular carcinoma incidence suggests an updated approach is needed. Hepatology 2016, 63, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Hassany, S.M.; Moustafa, E.F.; Taher, M.E.; Abdeltwab, A.A.; Blum, H.E. Screening for hepatocellular carcinoma by egyptian physicians. World J. Gastrointest. Oncol. 2015, 7, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Pawlotsky, J.M. Hepatitis C virus resistance to direct-acting antiviral drugs in interferon-free regimens. Gastroenterology 2016, 151, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Gower, E.; Estes, C.; Blach, S.; Razavi-Shearer, K.; Razavi, H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J. Hepatol. 2014, 61, S45–S57. [Google Scholar] [CrossRef] [PubMed]
- Geginat, J.; Paroni, M.; Facciotti, F.; Gruarin, P.; Kastirr, I.; Caprioli, F.; Pagani, M.; Abrignani, S. The CD4-centered universe of human T cell subsets. Semin. Immunol. 2013, 25, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.; Jabeen, R.; Yagi, R.; Pham, D.; Zhu, J.; Goenka, S.; Kaplan, M.H. STAT6-dependent regulation of Th9 development. J. Immunol. 2012, 188, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Stassen, M.; Steinborn, A.; Lohoff, M.; Schild, H. Interferon-regulatory factor 4 is essential for the developmental program of t helper 9 cells. Immunity 2010, 33, 192–202. [Google Scholar]
- Kaplan, M.H. Th9 cells: Differentiation and disease. Immunol. Rev. 2013, 252, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Grencis, R.; Hültner, L.; Else, K. Host protective immunity to trichinella spiralis in mice: Activation of th cell subsets and lymphokine secretion in mice expressing different response phenotypes. Immunology 1991, 74, 329–332. [Google Scholar] [PubMed]
- Purwar, R.; Schlapbach, C.; Xiao, S.; Kang, H.S.; Elyaman, W.; Jiang, X.; Jetten, A.M.; Khoury, S.J.; Fuhlbrigge, R.C.; Kuchroo, V.K. Robust tumor immunity to melanoma mediated by interleukin-9-producing t cells. Nat. Med. 2012, 18, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.-Y.; Lu, D.-H.; Guo, X.-Y.; Luo, W.; Hu, B.-L.; Huang, X.-L.; Chen, M.; Wang, J.-X.; Ma, S.-J.; Yang, X.-W. A deleterious role for Th9/Il-9 in hepatic fibrogenesis. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, R.; Edwards, E.; Freeman, R.; Harper, A.; Kim, R.; Kamath, P.; Kremers, W.; Lake, J.; Howard, T.; Merion, R.M.; et al. Model for end-stage liver disease (meld) and allocation of donor livers. Gastroenterology 2003, 124, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Feng, L.; Fang, X.; Jiang, Y.; Wang, X. Overexpression of Il-9 receptor in diffuse large b-cell lymphoma. Int. J. Clin. Exp. Pathol. 2013, 6, 911–916. [Google Scholar] [PubMed]
- Li, H.-J.; Sun, Q.-M.; Liu, L.-Z.; Zhang, J.; Huang, J.; Wang, C.-H.; Ding, R.; Song, K.; Tong, Z. High expression of Il-9r promotes the progression of human hepatocellular carcinoma and indicates a poor clinical outcome. Oncol. Rep. 2015, 34, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Hoppenot, D.; Malakauskas, K.; Lavinskiene, S.; Bajoriuniene, I.; Kalinauskaite, V.; Sakalauskas, R. Peripheral blood Th9 cells and eosinophil apoptosis in allergic asthma patients. Clin. Transl. Allergy 2014, 4, O11. [Google Scholar] [CrossRef]
- Ouyang, H.; Shi, Y.; Liu, Z.; Feng, S.; Li, L.; Su, N.; Lu, Y.; Kong, S. Increased interleukin-9 and CD4+ Il-9+ T cells in patients with systemic lupus erythematosus. Mol. Med. Rep. 2013, 7, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Liu, W.; Li, C.; Wang, Z.; Luo, L.; Tan, D.; Hu, G.; Zhang, Z. Circulating Th1 and Th2 cytokines in patients with hepatitis C virus infection. Mediat. Inflamm. 1998, 7, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Zignego, A.L. Enhanced Th1 cytokine production in hepatitis C virus-infected patients with mixed cryoglobulinemia: Understanding the pathological issues. J. Hepatol. 2004, 41, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Barjon, C.; Dahlqvist, G.; Calmus, Y.; Conti, F. Role of regulatory T-cells during hepatitis C infection: From the acute phase to post-transplantation recurrence. Dig. Liver Dis. 2015, 47, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Bălănescu, P.; Lădaru, A.; Voiosu, T.; Nicolau, A.; Ene, M.; Bălănescu, E. Th17 and Il-17 immunity in chronic hepatitis C infection. Rom. J. Intern. Med. 2012, 50, 13–18. [Google Scholar] [PubMed]
- Saiz, J.C.; Vazquez-Calvo, A.; Blazquez, A.B.; Merino-Ramos, T.; Escribano-Romero, E.; Martin-Acebes, M.A. Zika virus: The latest newcomer. Front. Microbiol. 2016, 7, 496. [Google Scholar] [CrossRef] [PubMed]
- McNamara, P.S.; Flanagan, B.F.; Baldwin, L.M.; Newland, P.; Hart, C.A.; Smyth, R.L. Interleukin 9 production in the lungs of infants with severe respiratory syncytial virus bronchiolitis. Lancet 2004, 363, 1031–1037. [Google Scholar] [CrossRef]
- Jimenez-Sousa, M.A.; Almansa, R.; De la Fuente, C.; Caro-Paton, A.; Ruiz, L.; Sanchez-Antolín, G.; Gonzalez, J.M.; Aller, R.; Alcaide, N.; Largo, P. Increased Th1, Th17 and pro-fibrotic responses in hepatitis C-infected patients are down-regulated after 12 weeks of treatment with pegylated interferon plus ribavirin. Eur. Cytokine Netw. 2010, 21, 84–91. [Google Scholar] [PubMed]
- Guzmán-Fulgencio, M.; Jiménez, J.L.; Berenguer, J.; Fernández-Rodríguez, A.; López, J.C.; Cosín, J.; Miralles, P.; Micheloud, D.; Muñoz-Fernández, M.Á.; Resino, S. Plasma Il-6 and Il-9 predict the failure of interferon-α plus ribavirin therapy in HIV/HCV-coinfected patients. J. Antimicrob. Chemother. 2012, 67, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Nowak, E.C.; Noelle, R.J. Interleukin-9 as a t helper type 17 cytokine. Immunology 2010, 131, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Stephens, G.L.; Swerdlow, B.; Benjamin, E.; Coyle, A.J.; Humbles, A.; Kolbeck, R.; Fung, M. Il-9 is a Th17-derived cytokine that limits pathogenic activity in organ-specific autoimmune disease. Eur. J. Immunol. 2011, 41, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Sousa, G.M.; Oliveira, I.S.; Andrade, L.J.; Sousa-Atta, M.L.B.; Paraná, R.; Atta, A.M. Serum levels of th17 associated cytokines in chronic hepatitis C virus infection. Cytokine 2012, 60, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Sung, S.S.J.; Krueger, P.D.; Jo, Y.A.; Rosen, H.R.; Ziegler, S.F.; Hahn, Y.S. Hepatitis C virus promotes T-helper (th) 17 responses through thymic stromal lymphopoietin production by infected hepatocytes. Hepatology 2013, 57, 1314–1324. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Kang, H.S.; Kim, B.S. Th17 cells enhance viral persistence and inhibit T cell cytotoxicity in a model of chronic virus infection. J. Exp. Med. 2009, 206, 313–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, P.M.J.; Neuman, M.G. Immunopathogenesis of hepatitis C viral infection: Th1/Th2 responses and the role of cytokines. Clin. Biochem. 2001, 34, 167–171. [Google Scholar] [CrossRef]
- Flynn, J.K.; Dore, G.J.; Hellard, M.; Yeung, B.; Rawlinson, W.D.; White, P.A.; Kaldor, J.M.; Lloyd, A.R.; Ffrench, R.A. Maintenance of Th1 hepatitis C virus (HCV)-specific responses in individuals with acute HCV who achieve sustained virological clearance after treatment. J. Gastroenterol. Hepatol. 2013, 28, 1770–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak, E.C.; Noelle, R.J. Interleukin-9 and T cell subsets. Cell Cycle 2009, 8, 3798–3799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, H.; Wang, S.; Zhao, L. A tumor-promoting role of Th9 cells in hepatocellular carcinoma through CCL20 and STAT3 pathways. Clin. Exp. Pharmacol. Physiol. 2017, 44, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-W.; Jiang, H.-X.; Huang, X.-L.; Ma, S.-J.; Qin, S.-Y. Role of Th9 cells and Th17 cells in the pathogenesis of malignant ascites. Asian Pac. J. Trop. Biomed. 2015, 5, 806–811. [Google Scholar] [CrossRef]
- Saito, T.; Nishikawa, H.; Wada, H.; Nagano, Y.; Sugiyama, D.; Atarashi, K.; Maeda, Y.; Hamaguchi, M.; Ohkura, N.; Sato, E. Two FOXP3+ CD4+ T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat. Med. 2016, 22, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.-L.L.; Zhu, S.-X.X.; Zhang, Y.-Y.Y.; Wu, Y.-F.F.; Wang, X.-J.J. [changes in peripheral blood t helper 9 cells and interleukin-9 in children in the acute stage of kawasaki disease]. Zhongguo Dang Dai er Ke Za Zhi Chin. J. Contemp. Pediatr. 2016, 18, 721–725. [Google Scholar]
- Yu, X.; Zheng, Y.; Deng, Y.; Li, J.; Guo, R.; Su, M.; Ming, D.; Lin, Z.; Zhang, J.; Su, Z. Serum interleukin (Il)-9 and Il-10, but not T-helper 9 (Th9) cells, are associated with survival of patients with acute-on-chronic hepatitis b liver failure. Medicine 2016, 95, e3405. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Lv, Y.; Lu, J.; Zhang, W.; Duan, Y.; Huang, Y.; Yang, L.; Li, M.; Liu, W.; Liu, D. Decreased frequency of circulating Th9 cells in patients with chronic hepatitis b infection. J. Clin. Lab. Anal. 2018, 32. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.P.; Schön, M.P.; Wallbrecht, K.; Gruber-Wackernagel, A.; Wang, X.-J.; Wolf, P. Involvement of Il-9 in Th17-associated inflammation and angiogenesis of psoriasis. PLoS ONE 2013, 8, e51752. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Kakinuma, S.; Nishimura, M.; Kobayashi, Y.; Nagata, K.; Shimada, Y. Interleukin-9 receptor gene is transcriptionally regulated by nucleolin in T-cell lymphoma cells. Mol. Carcinog. 2012, 51, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Wang, X. Role of Il-9 and stats in hematological malignancies. Oncol. Lett. 2014, 7, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Barata, J.T.; Keenan, T.D.; Silva, A.; Nadler, L.M.; Boussiotis, V.A.; Cardoso, A.A. Common gamma chain-signaling cytokines promote proliferation of t-cell acute lymphoblastic leukemia. Haematologica 2004, 89, 1459–1467. [Google Scholar] [PubMed]
- Wagner, E.F.; Nebreda, Á.R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 2009, 9, 537–549. [Google Scholar] [CrossRef] [PubMed]
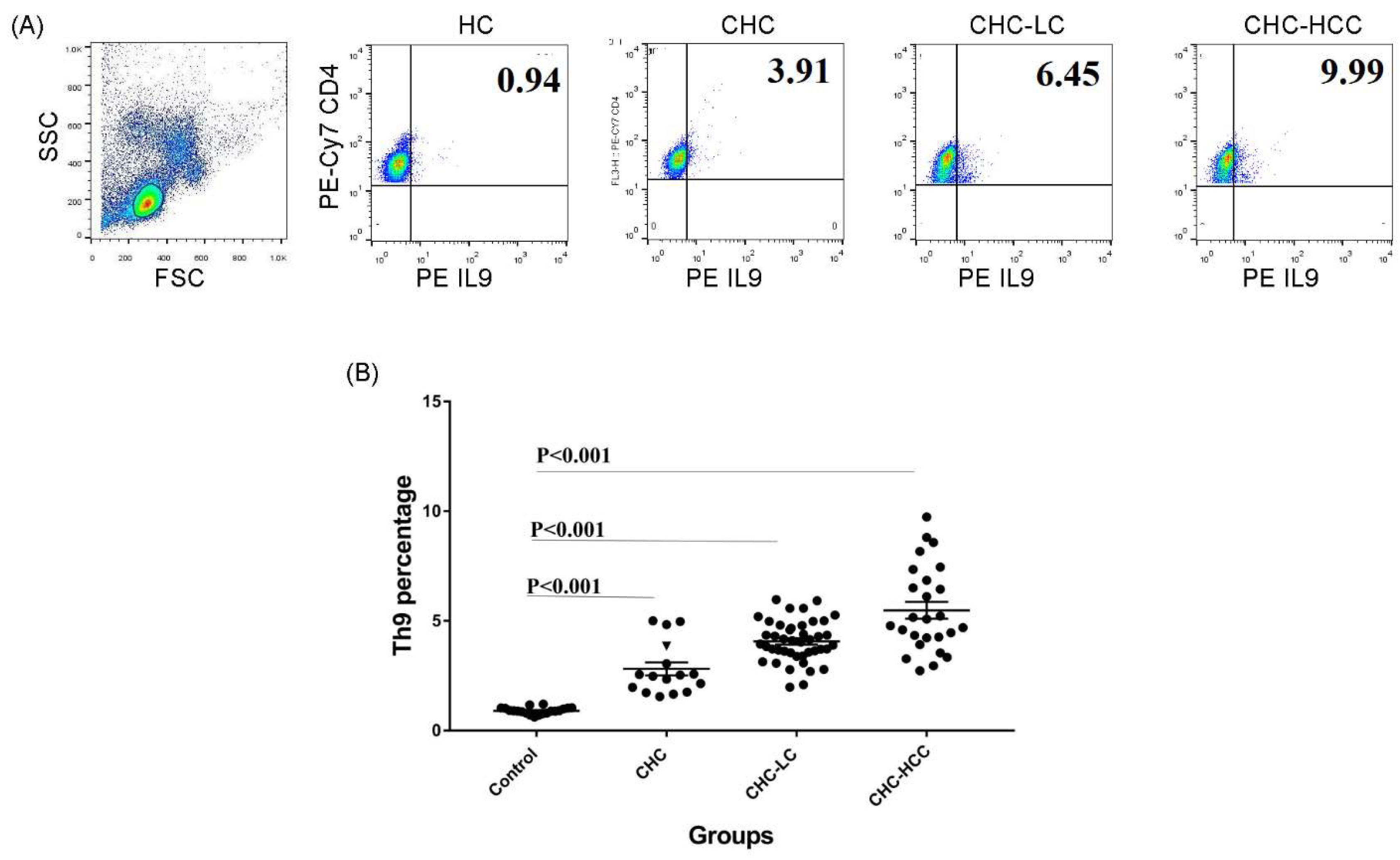
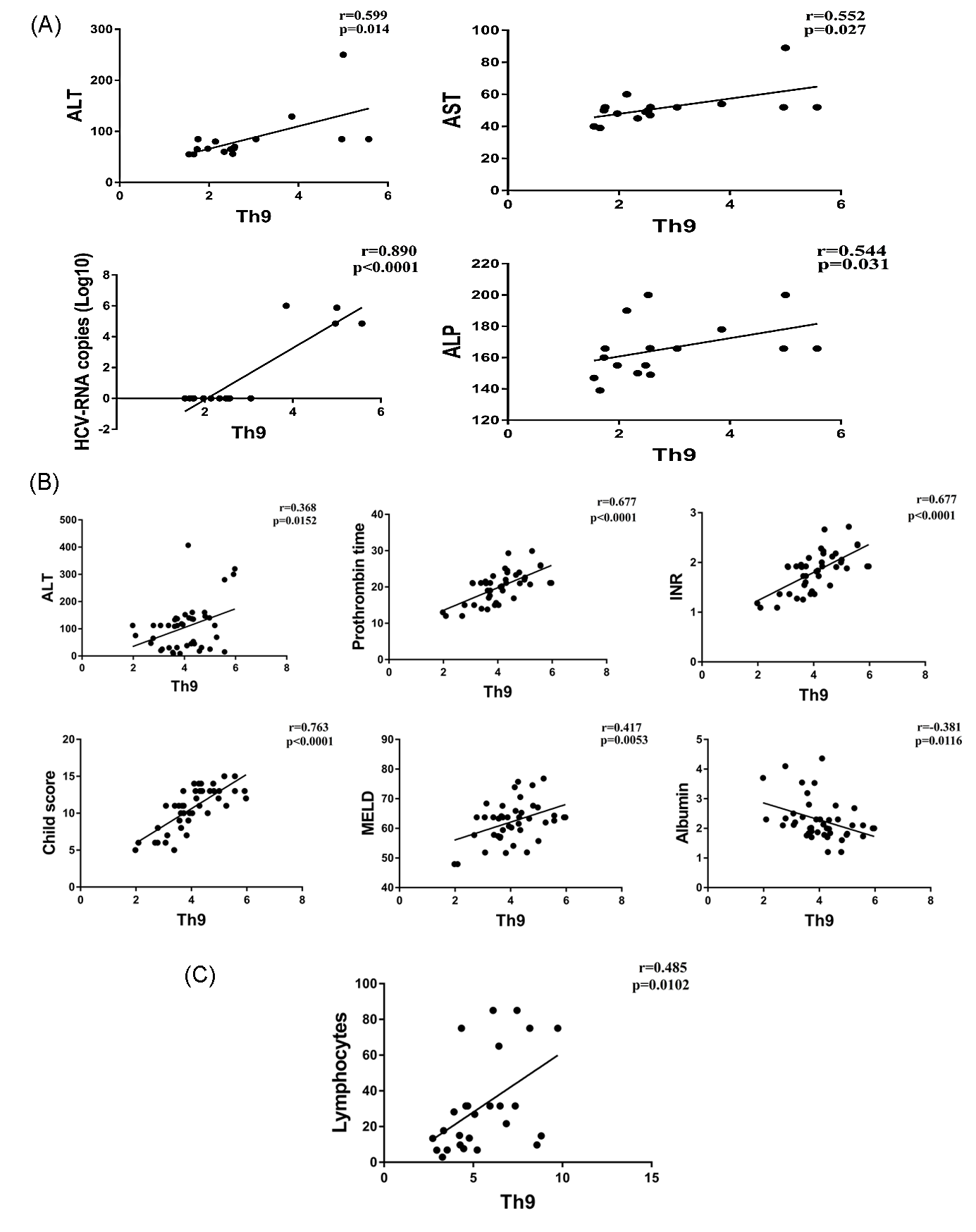
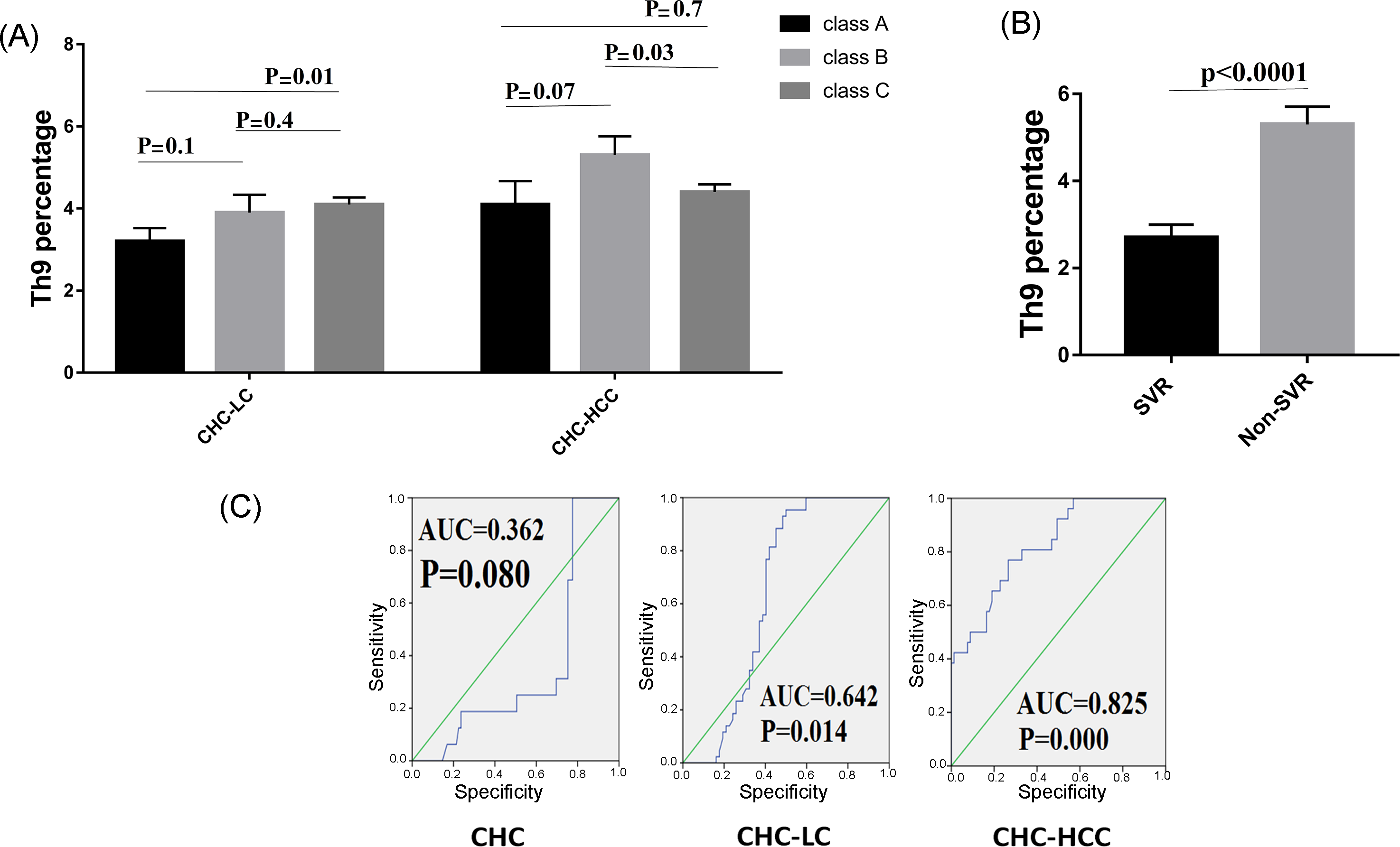
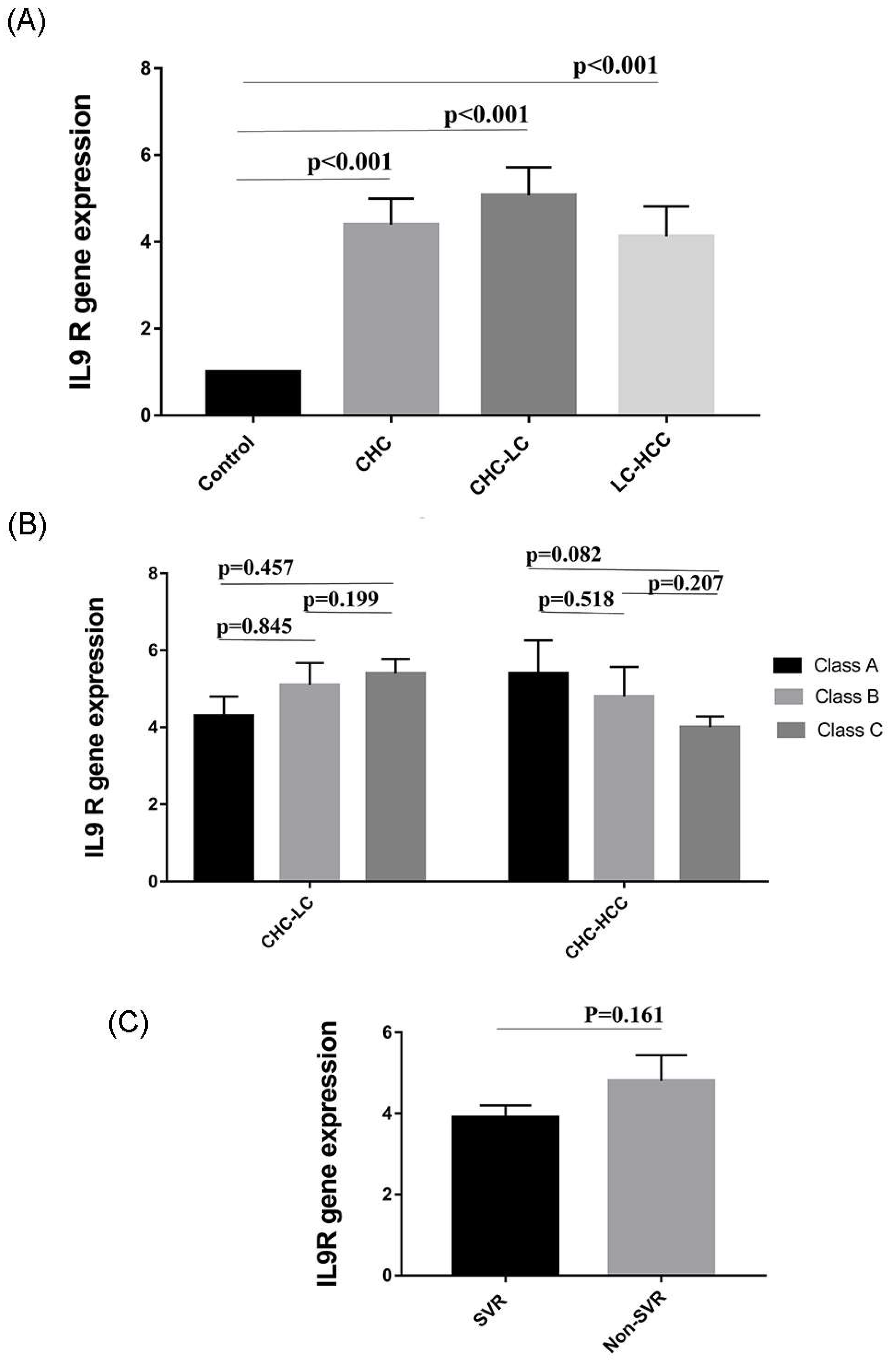
| Variable | CHC (n = 16) | LC without HCC (n = 43) | LC with HCC (n = 26) | Total (n = 85) |
|---|---|---|---|---|
| Age (years) a | 47 ± 13.4 (25–65) | 59.9 ± 8.4 (45–78) | 58.3 ± 9.4 (41–80) | 57 ± 10.7 (25–80) |
| Sex b | ||||
| Male | 8 (50%) | 24 (55.8%) | 14 (53.8%) | 46 (54.1%) |
| Female | 8 (50%) | 19 (44.2%) | 12 (46.2%) | 39 (45.9%) |
| Co-morbid diseases b | 2 (13%) | 11 (26%) | 12 (46%) | 25 (29.4%) |
| Abdominal pain b | 5 (31%) | 6 (14%) | 13 (50%) | 24 (28.2%) |
| Ascites b | 0 | 32 (74%) | 15 (58%) | 47 (81%) |
| GIT bleeding b | 0 | 8 (19%) | 8 (31%) | 16 (18.8%) |
| Hepatic Encephalopathy b | 0 | 25 (58%) | 10 (38%) | 35 (41%) |
| Serum albumin a | 4.1 ± 0.7 | 2.3 ± 0.7 | 2.6 ± 0.7 | 2.7 ± 0.9 |
| Serum total bilirubin (gm/dL) c | 1.8 (1.2–4) | 3.1 (0.4–7.2) | 1.9 (0.8–24.1) | 2 (0.4–24.1) |
| Alanine transaminase (IU/L) c | 68.5 (55–250) | 112.2 (8–407) | 100.1 (22–310) | 84.8 (8–407) |
| Aspartate transaminase (IU/L) c | 51 (39–89) | 71 (14–222) | 92 (32–476.3) | 66 (14–476.3) |
| Alkaline phosphatase (IU/L) c | 165.8 (139–200) | 168 (55–420) | 179 (57–340) | 166 (55–420) |
| Prothrombin time (seconds) a | 14 ± 1.5 | 19.5 ± 4.4 | 20.5 ± 10.5 | 19.8 ± 7.7 |
| INR a | 1.1 ± 0.3 | 1.7 ± 0.4 | 1.9 ± 0.9 | 1.7 ± 0.7 |
| WBC(x106/uL) c | 4.3 (2.4–5.2) | 6.1 (2.4–33.5) | 8.5 (1.5–31.8) | 7.5 (1.5–33.5) |
| Lymphocytes c | 15.2 (11.3–76) | 19.6 (6.1–75) | 15 (2.9–85) | 17.7 (2.9–85) |
| Neutrophils c | 70.1 (55–78) | 71.2 (52.2–83.2) | 76 (53.4–89.5) | 73.6 (52.2–89.5) |
| Hematocrit value c | 31 (22–35) | 30 (11.7–43.5) | 28.9 (13.5–49.1) | 29.6 ± 7.7 |
| Serum creatinine (mg/dL) c | 1.1 (0.8–1.3) | 1.4 (0.5–4) | 1.2 (0.5–5.9) | 1.2 (0.5–5.9) |
| Alfa Fetoprotein (IU/L) c | NA | NA | 357 (61.6–2526.6) | 357 (61.6–2526.6) |
| Child–Pugh score a | 0 | 10.7 ± 2.7 | 10.2 ± 2.8 | 10.5 ± 2.7 |
| Child–Pugh classification b | ||||
| Child–Pugh A | NA | 6 (14%) | 3 (12%) | 9 (8.9%) |
| Child–Pugh B | NA | 9 (21%) | 10 (38%) | 19 (18.1%) |
| Child–Pugh C | NA | 28 (65%) | 13 (50%) | 41 (39%) |
| MELD score a | 0 | 17.7 ± 7.1 | 19.5 ± 8.9 | 18.4 ± 7.8 |
| Death b | 0 | 1 (2.3%) | 6 (23%) | 7 (8.2%) |
| Variable | CHC | CHC-LC | CHC-HCC | |||
|---|---|---|---|---|---|---|
| r/rho | p Value | r/rho | p Value | r/rho | p Value | |
| Age | 0.242 | 0.241 | 0.187 | 0.405 | 0.181 | 0.387 |
| Serum albumin | −0.436 | 0.091 | 0.091 | 0.560 | −0.370 | 0.063 |
| Serum total bilirubin | 0.392 | 0.134 | 0.141 | 0368 | 0.036 | 0.861 |
| ALT | 0.133 | 0.804 | 0.186 | 0.231 | 0.186 | 0.676 |
| AST | 0.141 | 0.879 | 0.175 | 0.261 | 0.157 | 0.782 |
| ALP | 0.198 | 0.719 | −0.064 | 0.686 | 0.192 | 0.346 |
| Prothrombin time | 0.324 | 0.221 | 0.202 | 0.194 | 0.098 | 0.634 |
| INR | 0.111 | 0.420 | 0.177 | 0.261 | 0.166 | 0.417 |
| WBC | 0.084 | 0.784 | 0.085 | 0.694 | 0.102 | 0.697 |
| Lymphocyte | - | - | 0.091 | 0.688 | 0.224 | 0.329 |
| Neutrophil | - | - | 0.164 | 0.503 | 0.175 | 0.533 |
| Hb | 0.587 | 0.298 | −0.156 | 0.410 | −0.025 | 0.915 |
| HCT | 0.571 | 0.315 | −0.089 | 0.635 | −0.176 | 0.445 |
| Serum creatinine | 0.121 | 0.811 | 0.068 | 0.706 | 0.135 | 0.630 |
| HCV-RNA | 0.311 | 0.280 | 0.054 | 0.791 | 0.341 | 0.233 |
| Child-Pugh score | - | - | 0.127 | 0.423 | 0.308 | 0.126 |
| MELD | - | - | 0.197 | 0.281 | 0.081 | 0.786 |
| AFP | - | - | - | - | 0.632 | 0.368 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, M.E.; El-Badawy, O.; Afifi, N.A.; Eldin, A.S.; Hassan, E.A.; Halby, H.M.; El-Mokhtar, M.A. Role of T-Helper 9 Cells in Chronic Hepatitis C-Infected Patients. Viruses 2018, 10, 341. https://doi.org/10.3390/v10070341
Ali ME, El-Badawy O, Afifi NA, Eldin AS, Hassan EA, Halby HM, El-Mokhtar MA. Role of T-Helper 9 Cells in Chronic Hepatitis C-Infected Patients. Viruses. 2018; 10(7):341. https://doi.org/10.3390/v10070341
Chicago/Turabian StyleAli, Mohamed E., Omnia El-Badawy, Noha A. Afifi, Abeer Sharaf Eldin, Elham Ahmed Hassan, Hamada M. Halby, and Mohamed Ahmed El-Mokhtar. 2018. "Role of T-Helper 9 Cells in Chronic Hepatitis C-Infected Patients" Viruses 10, no. 7: 341. https://doi.org/10.3390/v10070341





