A VP26-mNeonGreen Capsid Fusion HSV-2 Mutant Reactivates from Viral Latency in the Guinea Pig Genital Model with Normal Kinetics
Abstract
:1. Introduction
2. Materials and Methods
In Vivo
3. Results
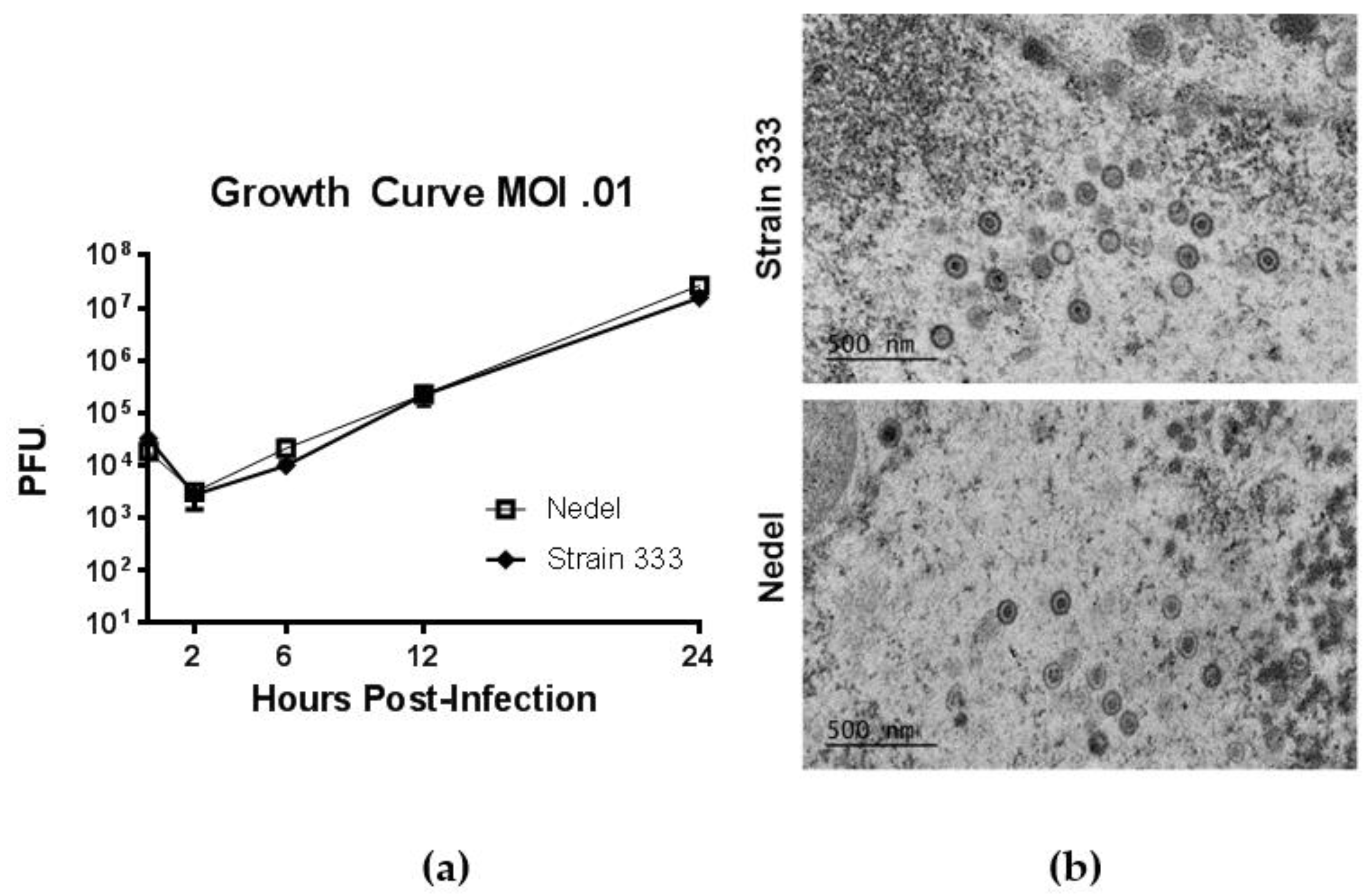
3.1. In Vitro Characterization
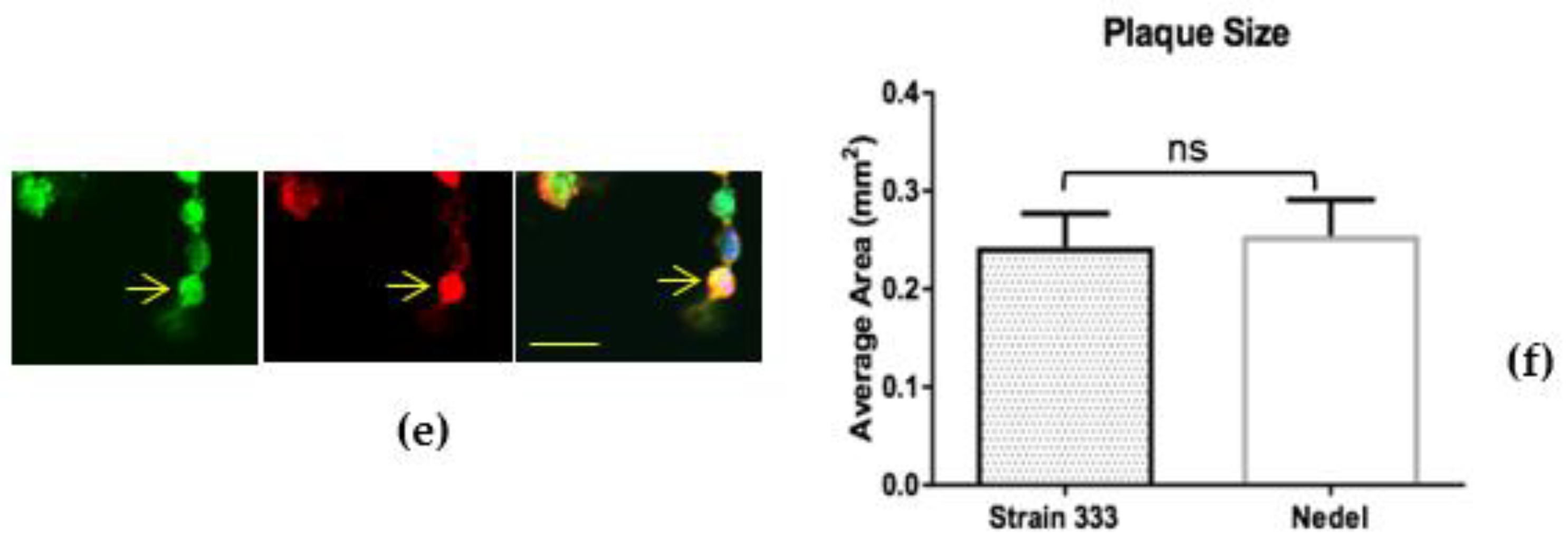
3.2. In Vivo Characterization
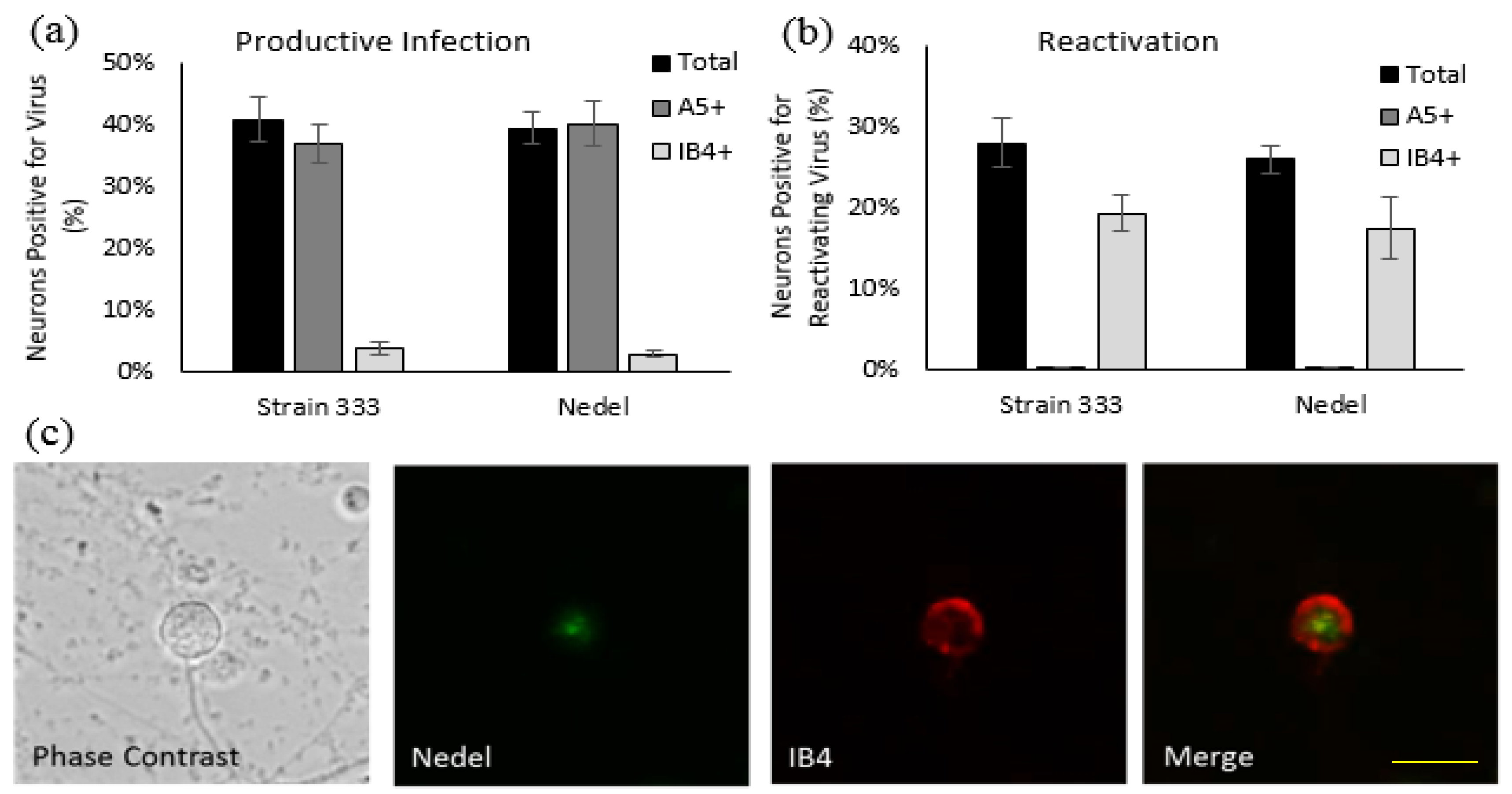
3.3. Ex Vivo Characterization
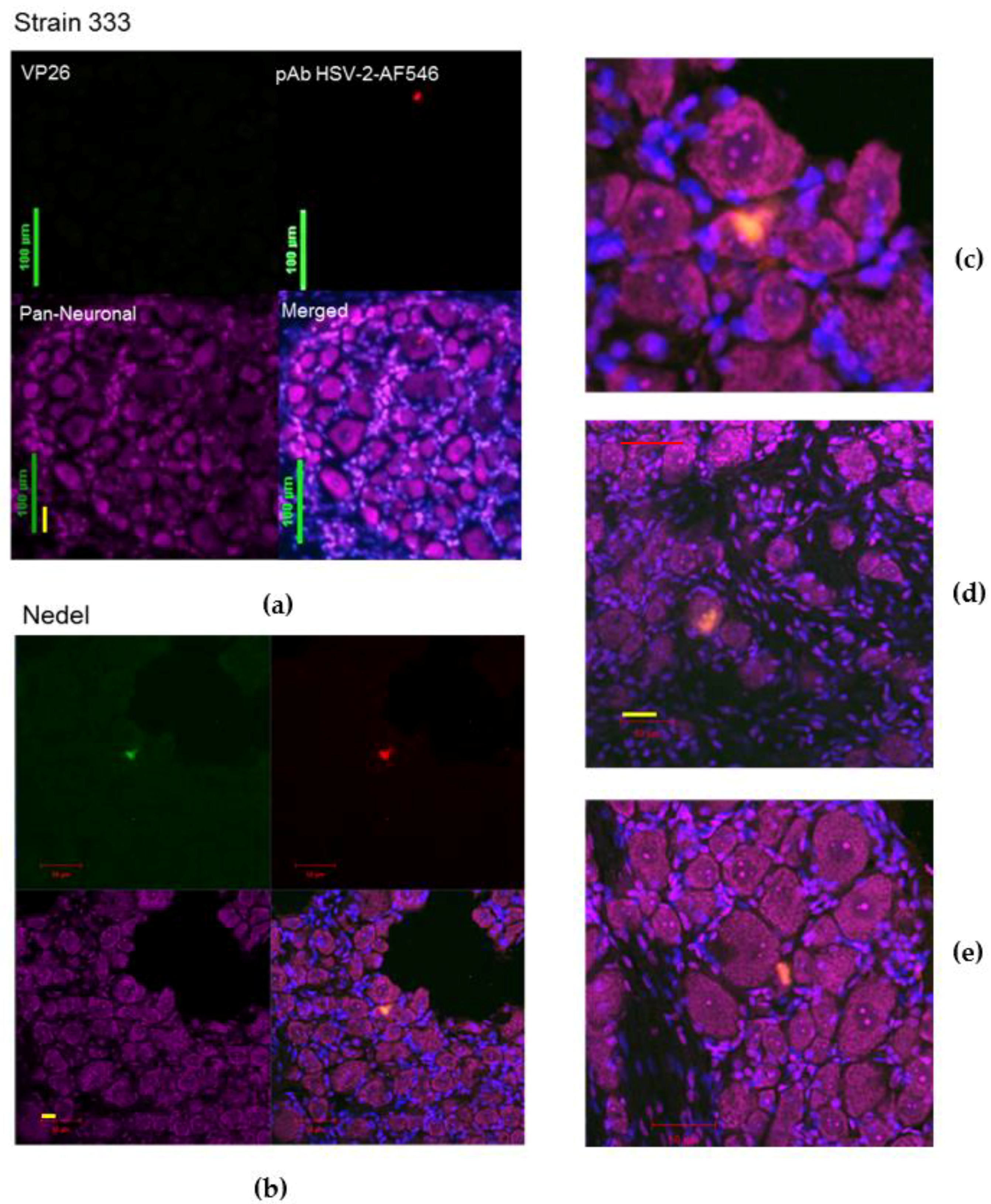
3.4. In Vivo Cryosections
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Booy, F.P.; Trus, B.L.; Newcomb, W.W.; Brown, J.C.; Conway, J.F.; Steven, A.C. Finding a needle in a haystack: Detection of a small protein (the 12-kDa VP26) in a large complex (the 200-MDa capsid of herpes simplex virus). Proc. Natl. Acad. Sci. USA 1994, 91, 5652–5656. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.; Person, S. Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J. Virol. 1998, 72, 7563–7568. [Google Scholar] [PubMed]
- Snyder, A.; Wisner, T.W.; Johnson, D.C. Herpes simplex virus capsids are transported in neuronal axons without an envelope containing the viral glycoproteins. J. Virol. 2006, 80, 11165–11177. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, A.P.; Glauser, D.L.; Laimbacher, A.S.; Strasser, R.; Schraner, E.M.; Wild, P.; Ziegler, U.; Breakefield, X.O.; Ackermann, M.; Fraefel, C. Live visualization of herpes simplex virus type 1 compartment dynamics. J. Virol. 2008, 82, 4974–4990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antinone, S.E.; Shubeita, G.T.; Coller, K.E.; Lee, J.I.; Haverlock-Moyns, S.; Gross, S.P.; Smith, G.A. The herpesvirus capsid surface protein, VP26, and the majority of the tegument proteins are dispensable for capsid transport toward the nucleus. J. Virol. 2006, 80, 5494–5498. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Uema, M.; Sagara, H.; Tanaka, M.; Sata, T.; Hashimoto, Y.; Kawaguchi, Y. Simultaneous tracking of capsid, tegument, and envelope protein localization in living cells infected with triply fluorescent herpes simplex virus 1. J. Virol. 2008, 82, 5198–5211. [Google Scholar] [CrossRef] [PubMed]
- Nagel, C.-H.; Döhner, K.; Fathollahy, M.; Strive, T.; Borst, E.M.; Messerle, M.; Sodeik, B. Nuclear egress and envelopment of herpes simplex virus capsids analyzed with dual-color fluorescence HSV1(17+). J. Virol. 2008, 82, 3109–3124. [Google Scholar] [CrossRef] [PubMed]
- Lo, P.; Yu, X.; Atanasov, I.; Chandran, B.; Zhou, Z.H. Three-dimensional localization of porf65 in kaposi's sarcoma-associated herpesvirus capsid. J. Virol. 2003, 77, 4291–4297. [Google Scholar] [CrossRef] [PubMed]
- Hogue, I.; Bosse, J.; Engel, E.; Scherer, J.; Hu, J.-R.; del Rio, T.; Enquist, L. Fluorescent protein approaches in alpha herpesvirus research. Viruses 2015, 7, 5933–5961. [Google Scholar] [CrossRef] [PubMed]
- Nagel, C.-H.; Döhner, K.; Binz, A.; Bauerfeind, R.; Sodeik, B. Improper tagging of the non-essential small capsid protein VP26 impairs nuclear capsid egress of herpes simplex virus. PLoS ONE 2012, 7, e44177. [Google Scholar] [CrossRef] [PubMed]
- Krautwald, M.; Maresch, C.; Klupp, B.G.; Fuchs, W.; Mettenleiter, T.C. Deletion or green fluorescent protein tagging of the pUL35 capsid component of pseudorabies virus impairs virus replication in cell culture and neuroinvasion in mice. J. Gen. Virol. 2008, 89, 1346–1351. [Google Scholar] [CrossRef] [PubMed]
- Antinone, S.E.; Zaichick, S.V.; Smith, G.A. Resolving the assembly state of herpes simplex virus during axon transport by live-cell imaging. J. Virol. 2010, 84, 13019–13030. [Google Scholar] [CrossRef] [PubMed]
- Balliet, J.W.; Kushnir, A.S.; Schaffer, P.A. Construction and characterization of a herpes simplex virus type I recombinant expressing green fluorescent protein: Acute phase replication and reactivation in mice. Virology 2007, 361, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Bertke, A.S.; Swanson, S.M.; Chen, J.; Imai, Y.; Kinchington, P.R.; Margolis, T.P. A5-positive primary sensory neurons are nonpermissive for productive infection with herpes simplex virus 1 in vitro. J. Virol. 2011, 85, 6669–6677. [Google Scholar] [CrossRef] [PubMed]
- Bertke, A.S.; Apakupakul, K.; Ma, A.; Imai, Y.; Gussow, A.M.; Wang, K.; Cohen, J.I.; Bloom, D.C.; Margolis, T.P. Lat region factors mediating differential neuronal tropism of HSV-1 and HSV-2 do not act in trans. PLoS ONE 2012, 7, e53281. [Google Scholar] [CrossRef] [PubMed]
- Bertke, A.S.; Ma, A.; Margolis, M.S.; Margolis, T.P. Different mechanisms regulate productive herpes simplex virus 1 (HSV-1) and HSV-2 infections in adult trigeminal neurons. J. Virol. 2013, 87, 6512–6516. [Google Scholar] [CrossRef] [PubMed]
- Shaner, N.C.; Lambert, G.G.; Chammas, A.; Ni, Y.; Cranfill, P.J.; Baird, M.A.; Sell, B.R.; Allen, J.R.; Day, R.N.; Israelsson, M.; et al. A bright monomeric green fluorescent protein derived from branchiostoma lanceolatum. Nat. Methods 2013, 10, 407. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.R.; Stanberry, L.R.; Bourne, N.; Connelly, B.; Kurawadwala, J.F.; Patel, A.; Straus, S.E. Expression of the herpes simplex virus type 2 latency-associated transcript enhances spontaneous reactivation of genital herpes in latently infected guinea pigs. J. Exp. Med. 1995, 181, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Stanberry Lr Fau-Kern, E.R.; Kern Er Fau-Richards, J.T.; Richards Jt Fau-Abbott, T.M.; Abbott Tm Fau-Overall, J.C., Jr.; Overall, J.C., Jr. Genital herpes in guinea pigs: Pathogenesis of the primary infection and description of recurrent disease. J. Inf. Dis. 1982, 146, 397–404. [Google Scholar] [CrossRef]
- Stephen, A.S.; William, A.S.; Martin, W.W. Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J. Histochem. Cytochem. 1999, 47, 719–730. [Google Scholar]
- Yang, J.; Yang, F.; Campos, L.; Mansfield, W.; Skelton, H.; Hooks, Y.; Liu, P. Quenching Autofluorescence in Tissue Immunofluorescence [Version 1; Referees: 2 Approved with Reservations, 1 Not Approved]. Wellcome Open Res. 2017, 2, 79. [Google Scholar] [CrossRef]
- Stanberry, L.R.; Kit, S.; Myers, M.G. Thymidine kinase-deficient herpes simplex virus type 2 genital infection in guinea pigs. J. Virol. 1985, 55, 322–328. [Google Scholar] [PubMed]
- Bourne, N.; Bravo, F.J.; Francotte, M.; Bernstein, D.I.; Myers, M.G.; Slaoui, M.; Stanberry, L.R. Herpes simplex virus (HSV) type 2 glycoprotein d subunit vaccines and protection against genital HSV-1 or HSV-2 disease in guinea pigs. J. Inf. Dis. 2003, 187, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Sawtell, N.M.; Thompson, R.L. Comparison of herpes simplex virus reactivation in ganglia in vivo and in explants demonstrates quantitative and qualitative differences. J. Virol. 2004, 78, 7784–7794. [Google Scholar] [CrossRef] [PubMed]
- Steiner, I.; Spivack, J.G.; Deshmane, S.L.; Ace, C.I.; Preston, C.M.; Fraser, N.W. A herpes simplex virus type 1 mutant containing a nontransinducing Vmw65 protein establishes latent infection in vivo in the absence of viral replication and reactivates efficiently from explanted trigeminal ganglia. J. Virol. 1990, 64, 1630–1638. [Google Scholar] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pieknik, J.R.; Bertke, A.S.; Tang, S.; Krause, P.R. A VP26-mNeonGreen Capsid Fusion HSV-2 Mutant Reactivates from Viral Latency in the Guinea Pig Genital Model with Normal Kinetics. Viruses 2018, 10, 246. https://doi.org/10.3390/v10050246
Pieknik JR, Bertke AS, Tang S, Krause PR. A VP26-mNeonGreen Capsid Fusion HSV-2 Mutant Reactivates from Viral Latency in the Guinea Pig Genital Model with Normal Kinetics. Viruses. 2018; 10(5):246. https://doi.org/10.3390/v10050246
Chicago/Turabian StylePieknik, Julianna R., Andrea S. Bertke, Shuang Tang, and Philip R. Krause. 2018. "A VP26-mNeonGreen Capsid Fusion HSV-2 Mutant Reactivates from Viral Latency in the Guinea Pig Genital Model with Normal Kinetics" Viruses 10, no. 5: 246. https://doi.org/10.3390/v10050246




