Signaling of Macrophage Inflammatory Protein (MIP)-3β Facilitates Dengue Virus-Induced Microglial Cell Migration
Abstract
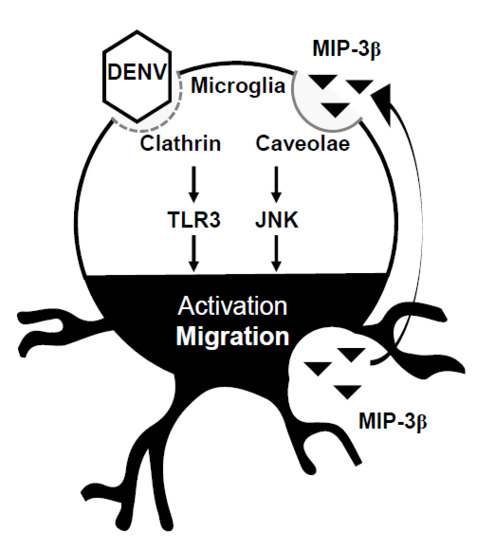
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Virus Culture
2.4. DENV Infection
2.5. Cell Viability and Cytotoxicity
2.6. Wound-Healing Assay
2.7. Cytokine Antibody Array
2.8. Statistical Analysis
3. Results
3.1. Conditioned Medium from DENV-Infected BV2 Microglia Cultures Stimulates Cell Migration
3.2. Blocking Lipid Rafts/Caveolae Retards DENV-Induced BV2 Microglial Cell Migration
3.3. Screening Cytokines/Chemokines in DENV-Infected BV2 Microglial Cells
3.4. A MIP-3β/c-Jun N-Terminal Kinase (JNK) Signaling Pathway Mediates DENV-Induced BV2 Microglial Cell Migration
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Guzman, M.G.; Gubler, D.J.; Izquierdo, A.; Martinez, E.; Halstead, S.B. Dengue infection. Nat. Rev. Dis. Primers 2016, 2, 16055. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.A.; Depelsenaire, A.C.; Young, P.R. Clinical and Laboratory Diagnosis of Dengue Virus Infection. J. Infect. Dis. 2017, 215, S89–S95. [Google Scholar] [CrossRef] [PubMed]
- Screaton, G.; Mongkolsapaya, J.; Yacoub, S.; Roberts, C. New insights into the immunopathology and control of dengue virus infection. Nat. Rev. Immunol. 2015, 15, 745–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martina, B.E. Dengue pathogenesis: A disease driven by the host response. Sci. Prog. 2014, 97 Pt 3, 197–214. [Google Scholar] [CrossRef]
- Torresi, J.; Ebert, G.; Pellegrini, M. Vaccines licensed and in clinical trials for the prevention of dengue. Hum. Vaccin. Immunother. 2017, 13, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Botta, L.; Rivara, M.; Zuliani, V.; Radi, M. Drug repurposing approaches to fight Dengue virus infection and related diseases. Front. Biosci. (Landmark Ed.) 2018, 23, 997–1019. [Google Scholar] [PubMed]
- Carod-Artal, F.J.; Wichmann, O.; Farrar, J.; Gascon, J. Neurological complications of dengue virus infection. Lancet Neurol. 2013, 12, 906–919. [Google Scholar] [CrossRef]
- Ramos, C.; Sanchez, G.; Pando, R.H.; Baquera, J.; Hernandez, D.; Mota, J.; Ramos, J.; Flores, A.; Llausas, E. Dengue virus in the brain of a fatal case of hemorrhagic dengue fever. J. Neurovirol. 1998, 4, 465–468. [Google Scholar] [CrossRef]
- Guzman, M.G.; Alvarez, M.; Rodriguez, R.; Rosario, D.; Vazquez, S.; Valdés, L.; Cabrera, M.V.; Kouri, G. Fatal dengue hemorrhagic fever in Cuba, 1997. Int. J. Infect. Dis. 1999, 3, 130–135. [Google Scholar] [CrossRef]
- Jhan, M.K.; HuangFu, W.C.; Chen, Y.F.; Kao, J.C.; Tsai, T.T.; Ho, M.R.; Shen, T.J.; Tseng, P.C.; Wang, Y.T.; Lin, C.F. Anti-TNF-alpha restricts dengue virus-induced neuropathy. J. Leukoc. Biol. 2018, 104, 961–968. [Google Scholar] [CrossRef]
- Salomao, N.G.; Rabelo, K.; Povoa, T.F.; Alves, A.M.B.; da Costa, S.M.; Goncalves, A.J.S.; Amorim, J.F.; Azevedo, A.S.; Nunes, P.C.G.; Basilio-de-Oliveira, C.A.; et al. BALB/c mice infected with DENV-2 strain 66985 by the intravenous route display injury in the central nervous system. Sci. Rep. 2018, 8, 9754. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.T.; Chen, C.L.; Lin, Y.S.; Chang, C.P.; Tsai, C.C.; Cheng, Y.L.; Huang, C.C.; Ho, C.J.; Lee, Y.C.; Lin, L.T.; et al. Microglia retard dengue virus-induced acute viral encephalitis. Sci. Rep. 2016, 6, 27670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velandia-Romero, M.L.; Acosta-Losada, O.; Castellanos, J.E. In vivo infection by a neuroinvasive neurovirulent dengue virus. J. Neurovirol. 2012, 18, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.R.; Tsai, T.T.; Chen, C.L.; Jhan, M.K.; Tsai, C.C.; Lee, Y.C.; Chen, C.H.; Lin, C.F. Blockade of dengue virus infection and viral cytotoxicity in neuronal cells in vitro and in vivo by targeting endocytic pathways. Sci. Rep. 2017, 7, 6910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Despres, P.; Frenkiel, M.P.; Ceccaldi, P.E.; Duarte Dos Santos, C.; Deubel, V. Apoptosis in the mouse central nervous system in response to infection with mouse-neurovirulent dengue viruses. J. Virol. 1998, 72, 823–829. [Google Scholar]
- Bhatt, R.S.; Kothari, S.T.; Gohil, D.J.; D’Souza, M.; Chowdhary, A.S. Novel evidence of microglial immune response in impairment of Dengue infection of CNS. Immunobiology 2015, 220, 1170–1176. [Google Scholar] [CrossRef]
- Jhan, M.K.; Tsai, T.T.; Chen, C.L.; Tsai, C.C.; Cheng, Y.L.; Lee, Y.C.; Ko, C.Y.; Lin, Y.S.; Chang, C.P.; Lin, L.T.; et al. Dengue virus infection increases microglial cell migration. Sci. Rep. 2017, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Neel, N.F.; Schutyser, E.; Sai, J.; Fan, G.H.; Richmond, A. Chemokine receptor internalization and intracellular trafficking. Cytokine Growth Factor Rev. 2005, 16, 637–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iijima, N.; Yanagawa, Y.; Clingan, J.M.; Onoe, K. CCR7-mediated c-Jun N-terminal kinase activation regulates cell migration in mature dendritic cells. Int. Immunol. 2005, 17, 1201–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miner, J.J.; Diamond, M.S. Mechanisms of restriction of viral neuroinvasion at the blood-brain barrier. Curr. Opin. Immunol. 2016, 38, 18–23. [Google Scholar] [CrossRef]
- Das Sarma, J. Microglia-mediated neuroinflammation is an amplifier of virus-induced neuropathology. J. Neurovirol. 2014, 20, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.L.; Wang, M.Y.; Ho, L.J.; Huang, C.Y.; Lai, J.H. Up-regulation of galectin-9 induces cell migration in human dendritic cells infected with dengue virus. J. Cell. Mol. Med. 2015, 19, 1065–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, Y.L.; Wang, M.Y.; Ho, L.J.; Lai, J.H. Dengue virus infection induces interferon-lambda1 to facilitate cell migration. Sci. Rep. 2016, 6, 24530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dijkstra, I.M.; de Haas, A.H.; Brouwer, N.; Boddeke, H.W.; Biber, K. Challenge with innate and protein antigens induces CCR7 expression by microglia in vitro and in vivo. Glia 2006, 54, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Forster, R.; Davalos-Misslitz, A.C.; Rot, A. CCR7 and its ligands: Balancing immunity and tolerance. Nat. Rev. Immunol. 2008, 8, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.; Endo, M.; Ochi, H.; Hojo, H.; Miyasaka, M.; Hayasaka, H. Regulation of CCR7-dependent cell migration through CCR7 homodimer formation. Sci. Rep. 2017, 7, 8536. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jhan, M.-K.; Shen, T.-J.; Tseng, P.-C.; Wang, Y.-T.; Lin, C.-F. Signaling of Macrophage Inflammatory Protein (MIP)-3β Facilitates Dengue Virus-Induced Microglial Cell Migration. Viruses 2018, 10, 690. https://doi.org/10.3390/v10120690
Jhan M-K, Shen T-J, Tseng P-C, Wang Y-T, Lin C-F. Signaling of Macrophage Inflammatory Protein (MIP)-3β Facilitates Dengue Virus-Induced Microglial Cell Migration. Viruses. 2018; 10(12):690. https://doi.org/10.3390/v10120690
Chicago/Turabian StyleJhan, Ming-Kai, Ting-Jing Shen, Po-Chun Tseng, Yung-Ting Wang, and Chiou-Feng Lin. 2018. "Signaling of Macrophage Inflammatory Protein (MIP)-3β Facilitates Dengue Virus-Induced Microglial Cell Migration" Viruses 10, no. 12: 690. https://doi.org/10.3390/v10120690