High Thermal Diffusivity in Thermally Treated Filamentous Virus-Based Assemblies with a Smectic Liquid Crystalline Orientation
Abstract
:1. Introduction
2. Materials and Methods
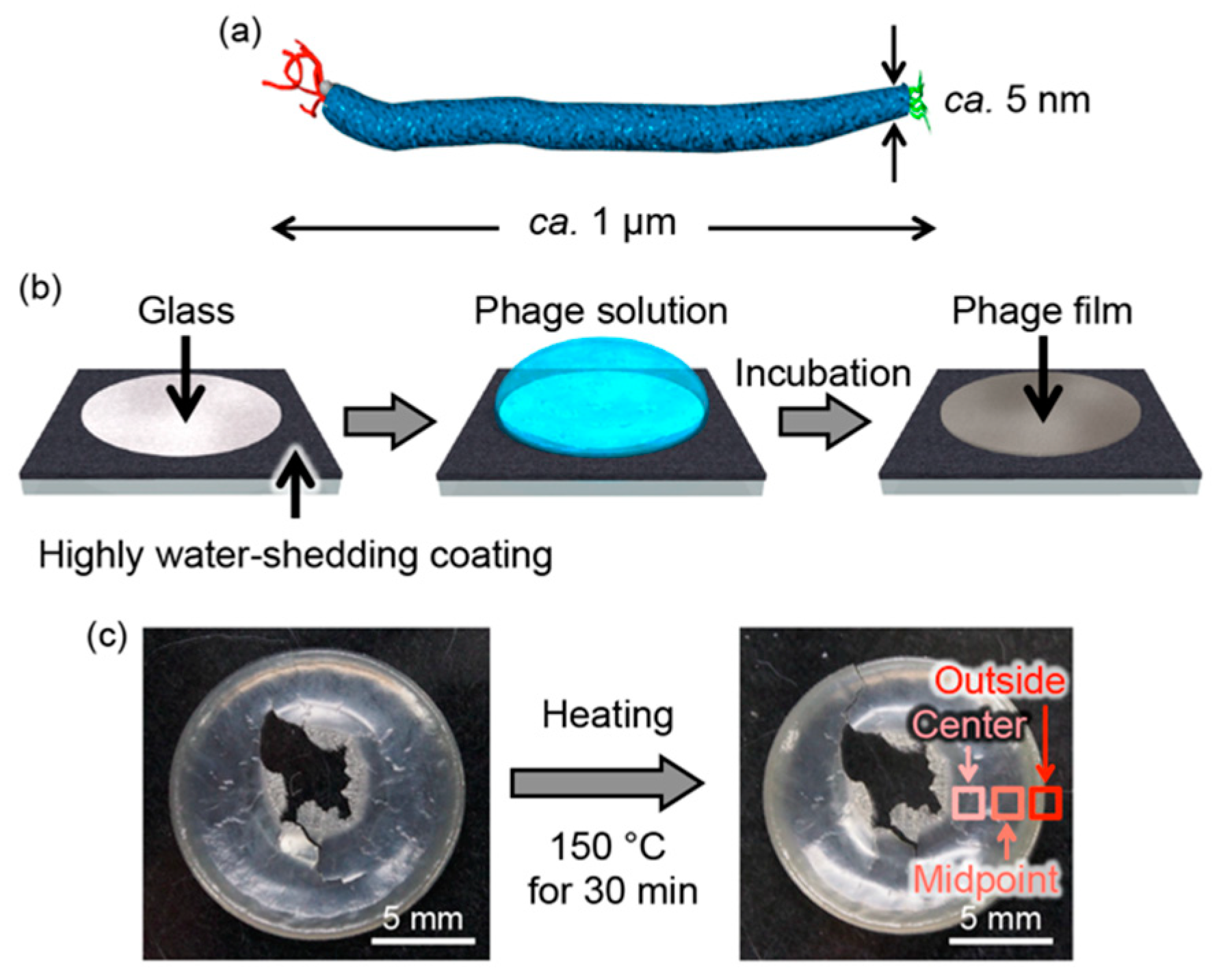
2.1. Preparation of M13 Phage-Based Films
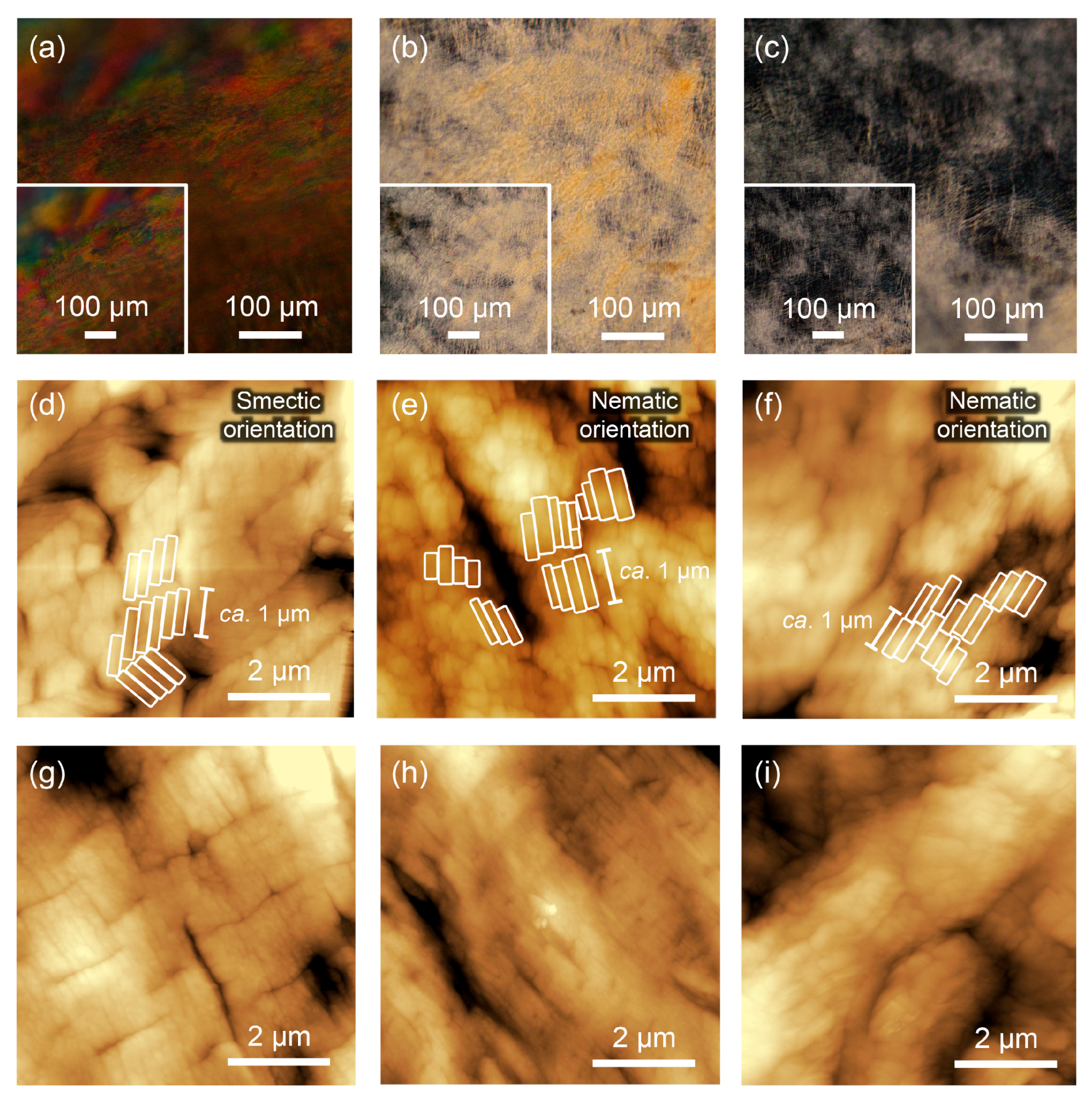
2.2. Polorized Optical Microscopy (POM) Observation
2.3. Atomic Force Microscopy (AFM) Observation
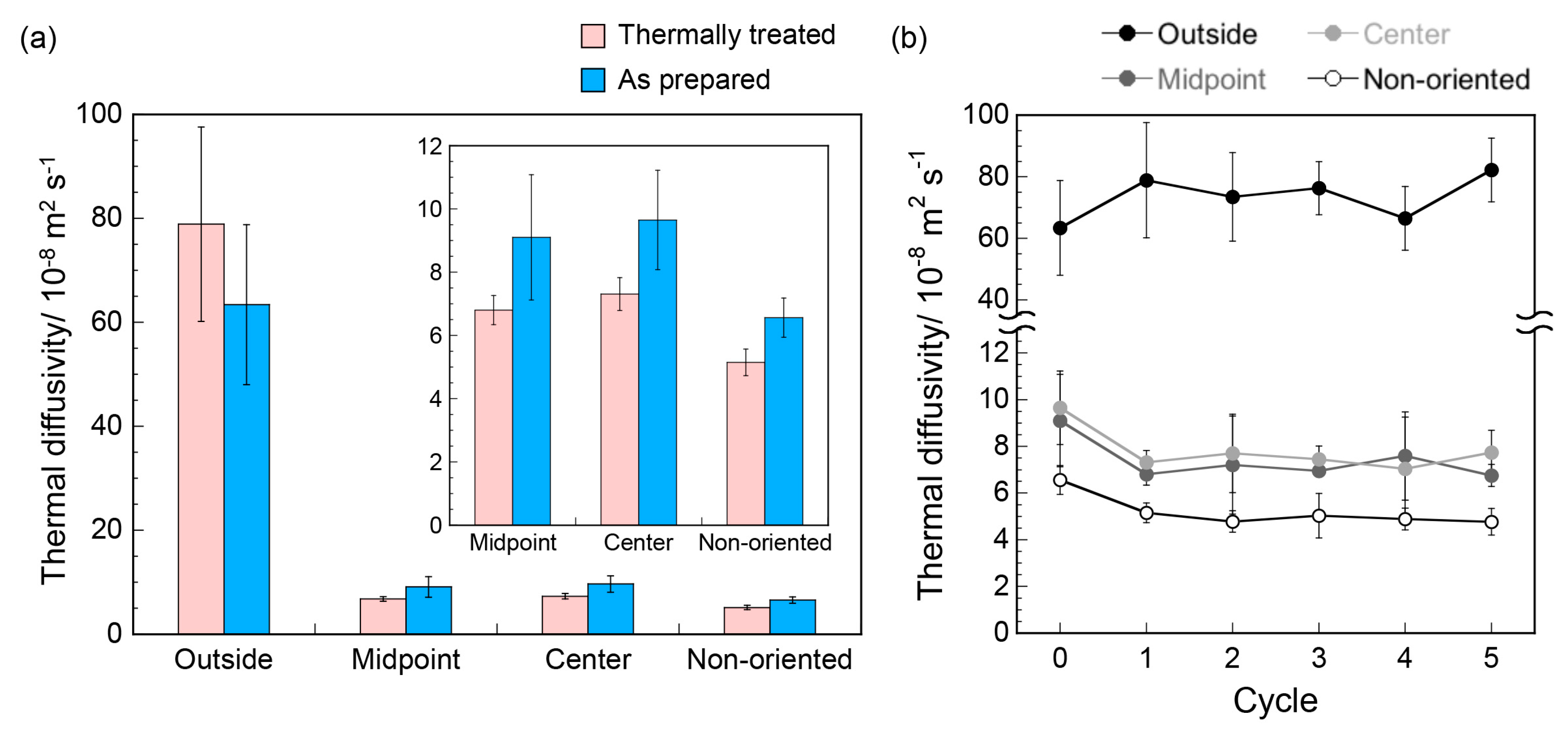
2.4. Thermal Diffusivity Measurements
2.5. SAXS Measurements
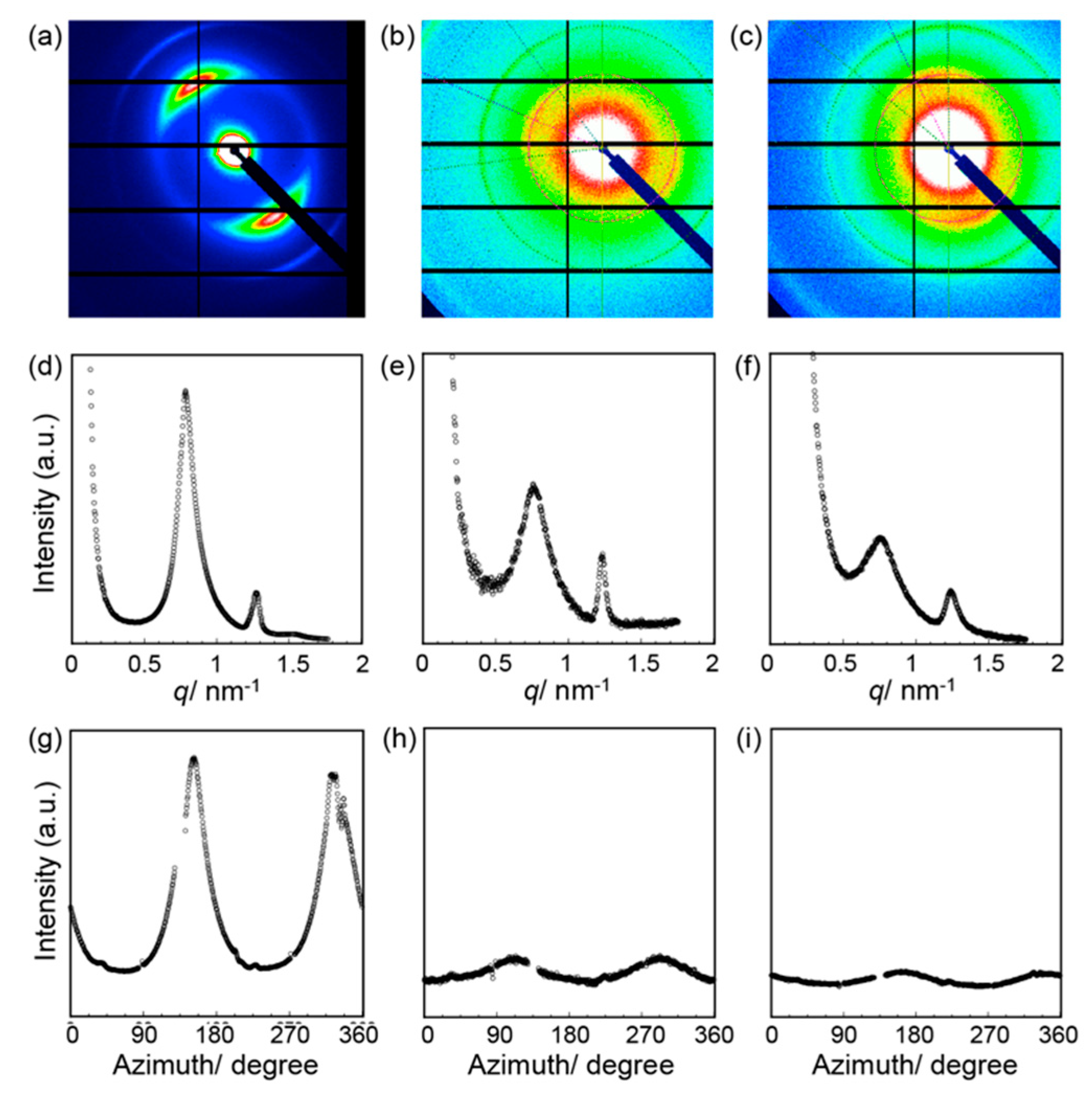
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chae, H.G.; Kumar, S. Materials science. Making strong fibers. Science 2008, 319, 908–909. [Google Scholar] [CrossRef] [PubMed]
- Winey, K.I.; Kashiwagi, T.; Mu, M.F. Improving electrical conductivity and thermal properties of polymers by the addition of carbon nanotubes as fillers. MRS Bull. 2007, 32, 348–353. [Google Scholar] [CrossRef]
- Wang, X.; Ho, V.; Segalman, R.A.; Cahill, D.G. Thermal conductivity of high-modulus polymer fibers. Macromolecules 2013, 46, 4937–4943. [Google Scholar] [CrossRef]
- Singh, V.; Bougher, T.L.; Weathers, A.; Cai, Y.; Bi, K.; Pettes, M.T.; McMenamin, S.A.; Lv, W.; Resler, D.P.; Gattuso, T.R.; et al. High thermal conductivity of chain-oriented amorphous polythiophene. Nat. Nanotechnol. 2014, 9, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.T.; Tan, E.P.S.; Ng, S.Y. Effects of crystalline morphology on the tensile properties of electrospun polymer nanofibers. Appl. Phys. Lett. 2008, 92, 141908. [Google Scholar] [CrossRef]
- Shen, S.; Henry, A.; Tong, J.; Zheng, R.; Chen, G. Polyethylene nanofibres with very high thermal conductivities. Nat. Nanotechnol. 2010, 5, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-H.; Lee, D.; Shanker, A.; Shao, L.; Kwon, M.; Gidley, D.; Kim, J.; Pipe, K.P. High thermal conductivity in amorphous polymer blends by engineered interchain interactions. Nat. Mater. 2015, 14, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, G.; Wang, X. New secrets of spider silk: Exceptionally high thermal conductivity and its abnormal change under stretching. Adv. Mater. 2012, 24, 1482–1486. [Google Scholar] [CrossRef] [PubMed]
- Henry, A. Thermal transport in polymers. Annu. Rev. Heat Transf. 2014, 17, 485–520. [Google Scholar] [CrossRef]
- Wu, L.; Lee, A.L.; Niu, Z.; Ghoshroy, S.; Wang, Q. Visualizing cell extracellular matrix (ECM) deposited by cells cultured on aligned bacteriophage M13 thin films. Langmuir 2011, 27, 9490–9496. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chung, W.-J.; McFarland, S.; Lee, S.-W. Assembly of bacteriophage into functional materials. Chem. Rec. 2013, 13, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, N.-M.M.; Klug, M.T.; Chen, P.-Y.Y.; Kooi, S.E.; Yun, D.S.; Hong, N.; Fang, N.X.; Belcher, A.M.; Hammond, P.T. Assembly of a bacteriophage-based template for the organization of materials into nanoporous networks. Adv. Mater. 2014, 26, 3398–3404. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, N.M.; Ghosh, D.; Belcher, A.M. Carbon nanotubes as in vivo bacterial probes. Nat. Commun. 2014, 5, 4918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moghimian, P.; Srot, V.; Rothenstein, D.; Facey, S.J.; Harnau, L.; Hauer, B.; Bill, J.; Aken, P.A.V. Adsorption and self-assembly of M13 phage into directionally organized structures on C and SiO2 films. Langmuir 2014, 30, 11428–11432. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jin, H.-E.; Desai, M.S.; Ren, S.; Kim, S.; Lee, S.-W. Biomimetic sensor design. Nanoscale 2015, 7, 18379–18391. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Asada, M.; Serizawa, T. Selective rare earth recovery employing filamentous viruses with chemically conjugated peptides. ChemistrySelect 2016, 1, 2712–2716. [Google Scholar] [CrossRef]
- Moghimian, P.; Harnau, L.; Srot, V.; de la Peña, F.; Bafi, N.; Facey, S.J.; van Aken, P.A. Controlled self-assembly of biomolecular rods on structured substrates. Soft Matter 2016, 12, 3177–3183. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, V.; Han, J.; Kim, C.; Kang, Y.-C.C.; Oh, J.-W.W. Self-assembled nanoporous biofilms from functionalized nanofibrous M13 bacteriophage. Viruses 2018, 10, 322. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.P.; Petrenko, V.A. Phage display. Chem. Rev. 1997, 97, 391–410. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.; Weiss, G.A. Chemically modifying viruses for diverse applications. ACS Chem. Biol. 2016, 11, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Dogic, Z.; Fraden, S. Ordered phases of filamentous viruses. Curr. Opin. Colloid Interface Sci. 2006, 11, 47–55. [Google Scholar] [CrossRef]
- Dogic, Z.; Sharma, P.; Zakhary, M.J. Hypercomplex liquid crystals. Annu. Rev. Condens. Matter Phys. 2014, 5, 137–157. [Google Scholar] [CrossRef]
- Zhang, Z.; Grelet, E. Tuning chirality in the self-assembly of rod-like viruses by chemical surface modifications. Soft Matter 2012, 9, 1015–1024. [Google Scholar] [CrossRef]
- Zan, T.; Wu, F.; Pei, X.; Jia, S.; Zhang, R.; Wu, S.; Niu, Z.; Zhang, Z. Into the polymer brush regime through the “grafting-to” method: Densely polymer-grafted rodlike viruses with an unusual nematic liquid crystal behavior. Soft Matter 2015, 12, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.-J.; Oh, J.-W.; Kwak, K.; Lee, B.; Meyer, J.; Wang, E.; Hexemer, A.; Lee, S.-W. Biomimetic self-templating supramolecular structures. Nature 2011, 478, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Zhang, J.; Zueger, C.; Chung, W.-J.; Yoo, S.; Wang, E.; Meyer, J.; Ramesh, R.; Lee, S.-W. Virus-based piezoelectric energy generation. Nat. Nanotechnol. 2012, 7, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Serizawa, T. Immobilization of highly-oriented filamentous viruses onto polymer substrates. J. Mater. Chem. B 2013, 1, 149–152. [Google Scholar] [CrossRef]
- Sawada, T.; Kang, S.; Watanabe, J.; Mihara, H.; Serizawa, T. Hybrid hydrogels composed of regularly assembled filamentous viruses and gold nanoparticles. ACS Macro Lett. 2014, 3, 341–345. [Google Scholar] [CrossRef]
- Sawada, T.; Chen, H.; Shirakawa, N.; Kang, S.; Watanabe, J.; Serizawa, T. Regular assembly of filamentous viruses and gold nanoparticles by specific interactions and subsequent chemical crosslinking. Polym. J. 2014, 46, 511–515. [Google Scholar] [CrossRef]
- Sawada, T.; Otsuka, H.; Yui, H.; Serizawa, T. Preparation and characterization of hybrid hydrogels composed of physically cross-linked gelatin and liquid-crystalline filamentous viruses. Polym. Bull. 2015, 72, 1487–1496. [Google Scholar] [CrossRef]
- Sawada, T.; Yanagimachi, M.; Serizawa, T. Controlled release of antibody proteins from liquid crystalline hydrogels composed of genetically engineered filamentous viruses. Mater. Chem. Front. 2017, 1, 146–151. [Google Scholar] [CrossRef]
- Sawada, T. Filamentous virus-based soft materials based on controlled assembly through liquid crystalline formation. Polym. J. 2017, 49, 639–647. [Google Scholar] [CrossRef]
- Sawada, T.; Serizawa, T. Filamentous viruses as building blocks for hierarchical self-assembly toward functional soft materials. Bull. Chem. Soc. Jpn. 2018, 91, 455–466. [Google Scholar] [CrossRef]
- Sawada, T.; Murata, Y.; Marubayashi, H.; Nojima, S.; Morikawa, J.; Serizawa, T. Filamentous virus-based assembly: Their oriented structures and thermal diffusivity. Sci. Rep. 2018, 8, 5412. [Google Scholar] [CrossRef] [PubMed]
- Poklar, N.; Lah, J.; Salobir, M.; Maček, P.; Vesnaver, G. pH and temperature-induced molten globule-like denatured states of equinatoxin II: A study by UV-melting, DSC, far- and near-UV CD spectroscopy, and ANS fluorescence. Biochemistry 1997, 36, 14345–14352. [Google Scholar] [CrossRef] [PubMed]
- Borzova, V.A.; Markossian, K.A.; Chebotareva, N.A.; Kleymenov, S.; Poliansky, N.B.; Muranov, K.O.; Stein-Margolina, V.A.; Shubin, V.V.; Markov, D.I.; Kurganov, B.I. Kinetics of thermal denaturation and aggregation of bovine serum albumin. PLoS ONE 2016, 11, e0153495. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, J.; Hashimoto, T. Thermal diffusivity of aromatic polyimide thin films by temperature wave analysis. J. Appl. Phys. 2009, 105, 113506. [Google Scholar] [CrossRef]
- Marubayashi, H.; Asai, S.; Sumita, M. Complex crystal formation of poly(l-lactide) with solvent molecules. Macromolecules 2012, 45, 1384–1397. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Lu, G.; Hong, W.; Tong, L.; Bai, H.; Wei, Y.; Shi, G. Drying enhanced adhesion of polythiophene nanotubule arrays on smooth surfaces. ACS Nano 2008, 2, 2342–2348. [Google Scholar] [CrossRef] [PubMed]
- Haris, P.I.; Chapman, D. The conformational analysis of peptides using Fourier transform IR spectroscopy. Biopolymers 1995, 37, 251–263. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawada, T.; Murata, Y.; Marubayashi, H.; Nojima, S.; Morikawa, J.; Serizawa, T. High Thermal Diffusivity in Thermally Treated Filamentous Virus-Based Assemblies with a Smectic Liquid Crystalline Orientation. Viruses 2018, 10, 608. https://doi.org/10.3390/v10110608
Sawada T, Murata Y, Marubayashi H, Nojima S, Morikawa J, Serizawa T. High Thermal Diffusivity in Thermally Treated Filamentous Virus-Based Assemblies with a Smectic Liquid Crystalline Orientation. Viruses. 2018; 10(11):608. https://doi.org/10.3390/v10110608
Chicago/Turabian StyleSawada, Toshiki, Yuta Murata, Hironori Marubayashi, Shuichi Nojima, Junko Morikawa, and Takeshi Serizawa. 2018. "High Thermal Diffusivity in Thermally Treated Filamentous Virus-Based Assemblies with a Smectic Liquid Crystalline Orientation" Viruses 10, no. 11: 608. https://doi.org/10.3390/v10110608