Antiviral Activity of Tannic Acid Modified Silver Nanoparticles: Potential to Activate Immune Response in Herpes Genitalis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of TA-AgNPs
2.2. Virus Preparation
2.3. Virus-AgNPs’ Interaction Analysis
2.4. Genital HSV-2 Infection and Virus Challenge
2.5. Virus Titration
2.6. Flow Cytometry Phenotypic Analysis
2.7. Neutralization Assay
2.8. Quantitative Reverse Transcriptase-Polymerase Chain Reaction
2.9. Statistical Methods
3. Results
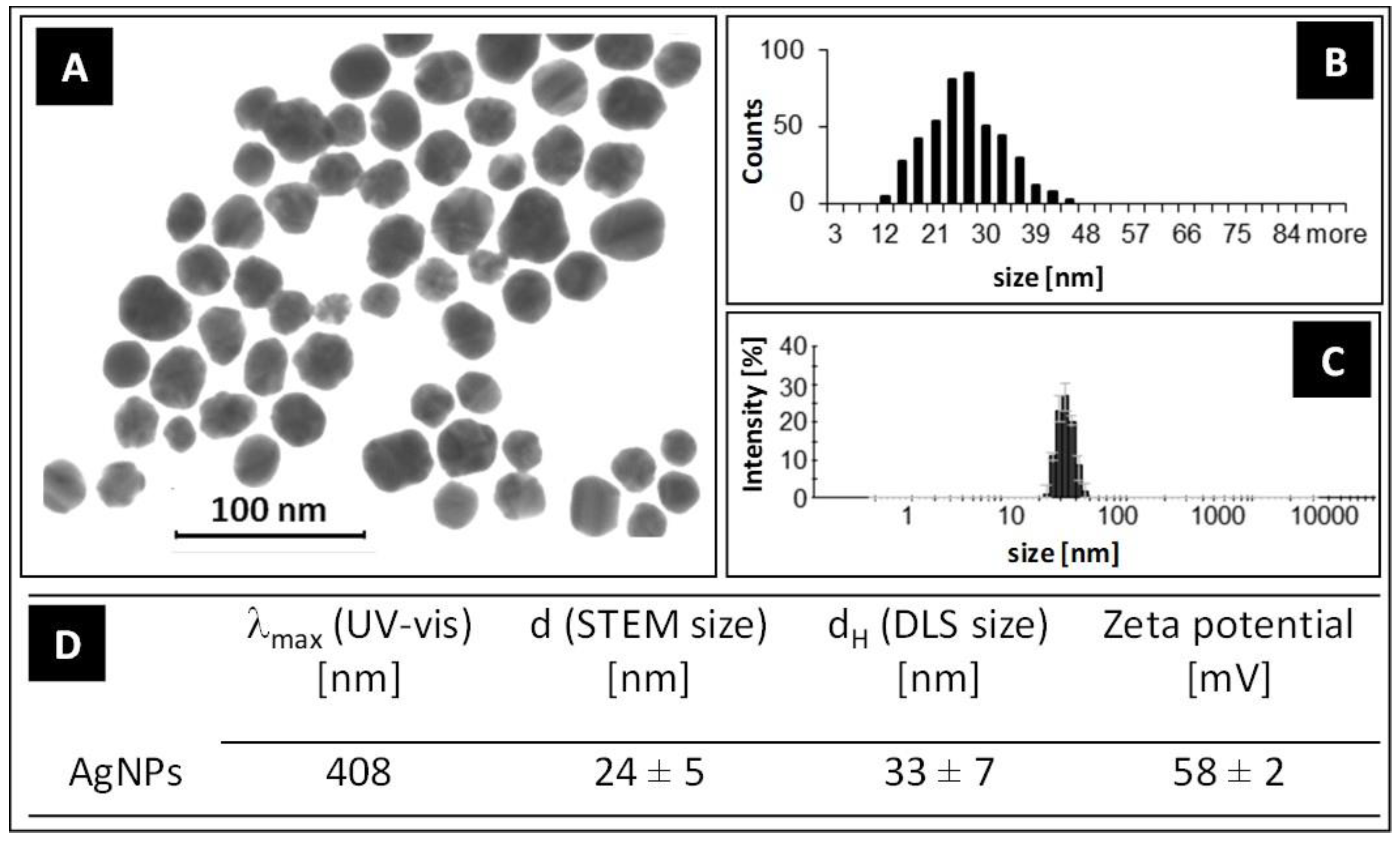
3.1. Characterization of AgNPs
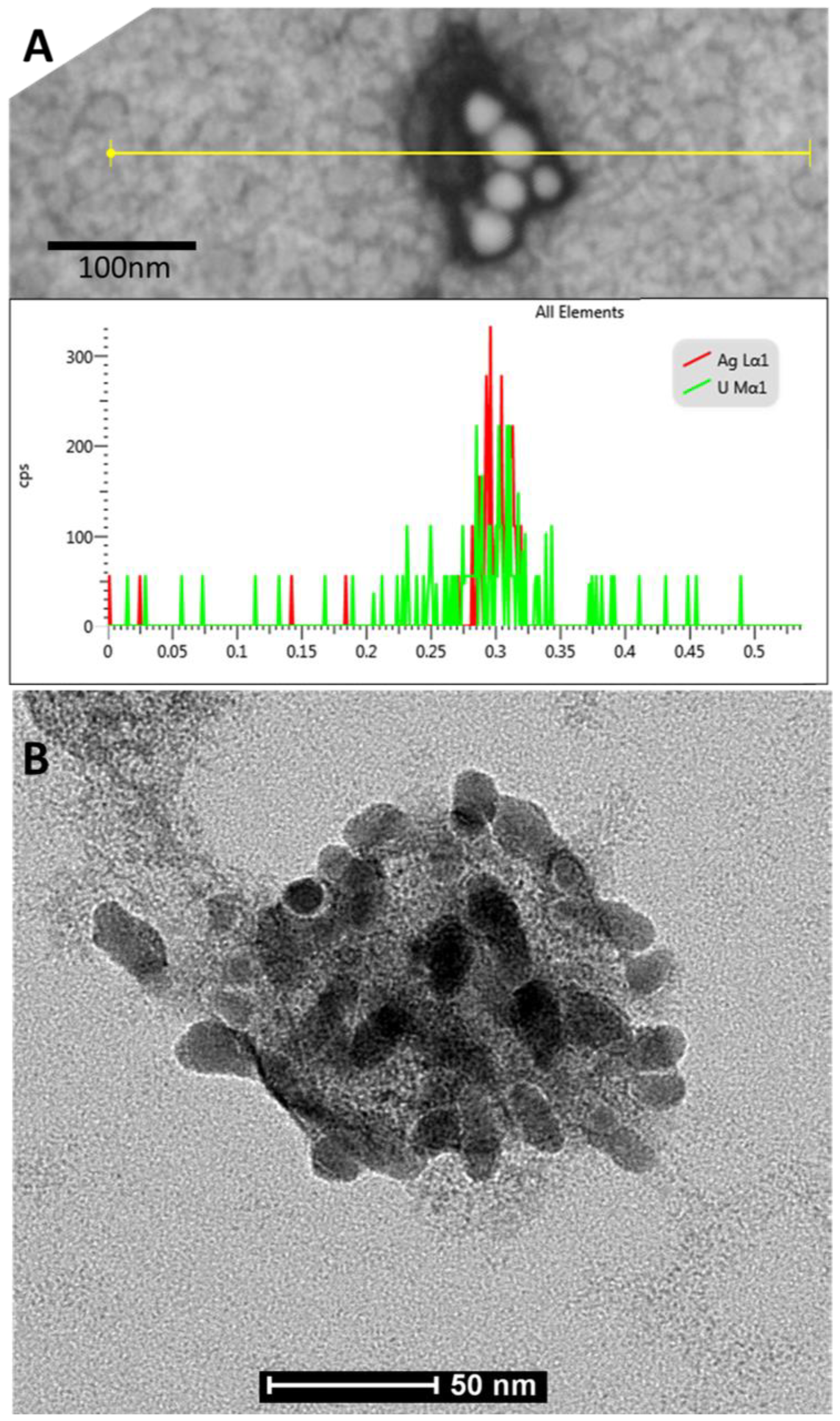
3.2. Virus-Nano Interaction
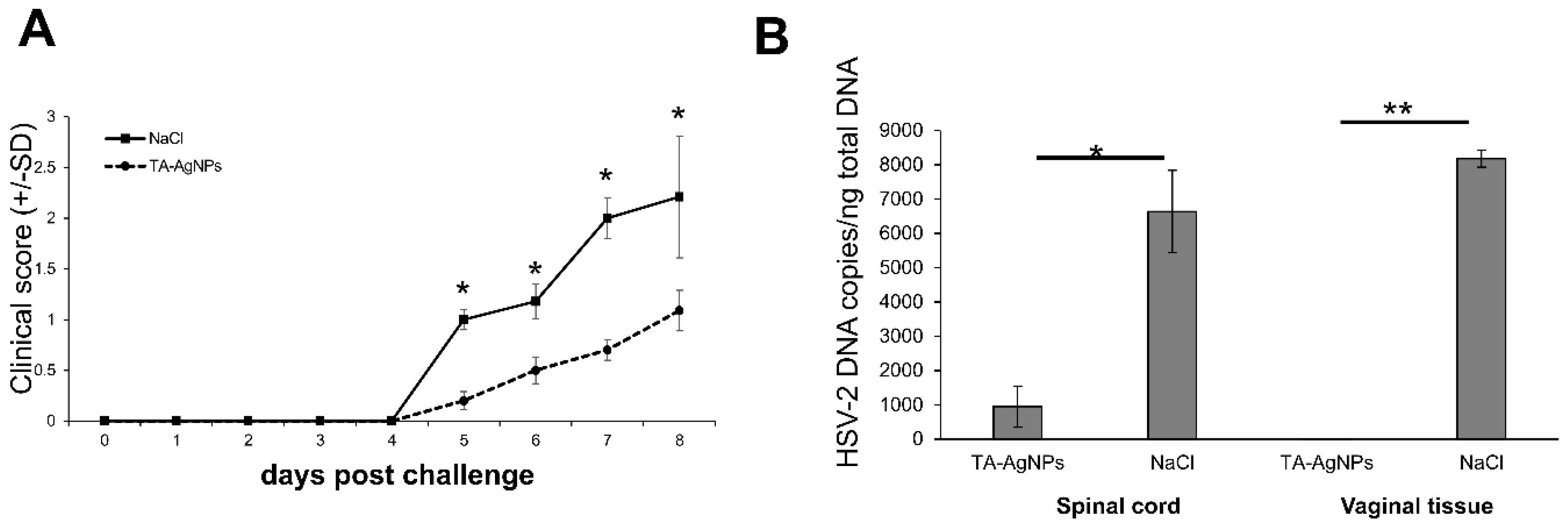
3.3. TA-AgNPs-Treated Mice Show Better Clinical Scores
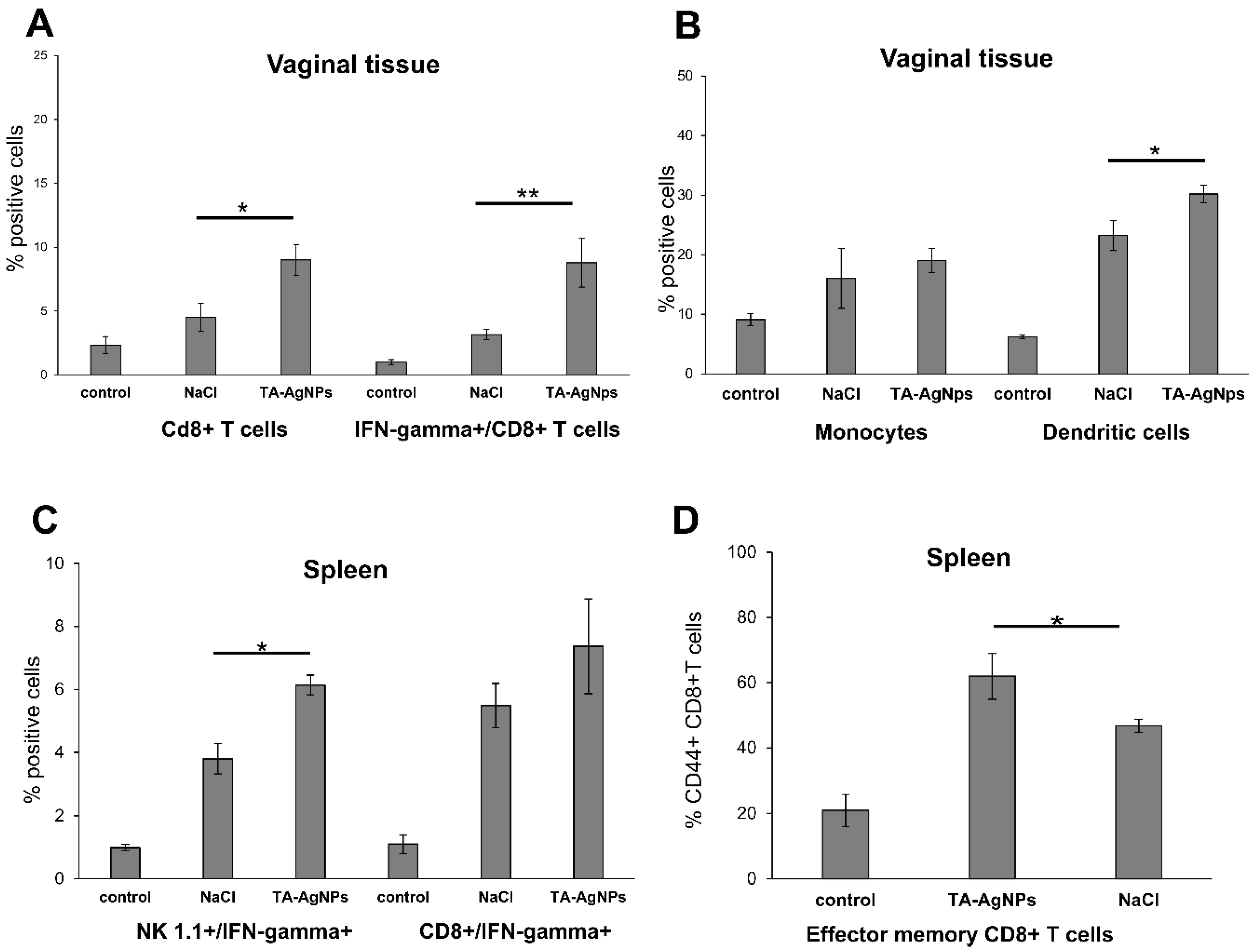
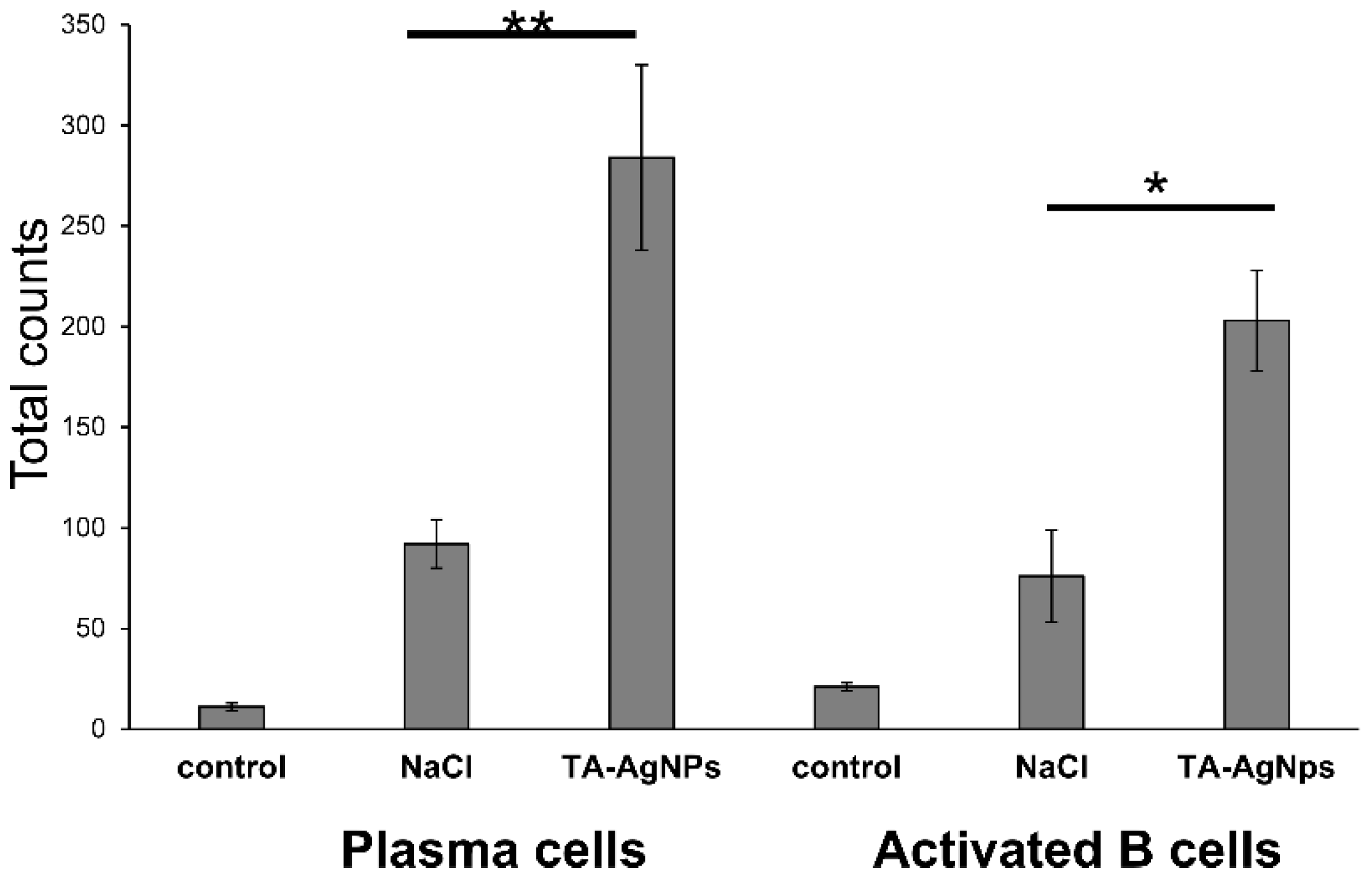
3.4. TA-AgNPs Help to Mount Early Immune Response
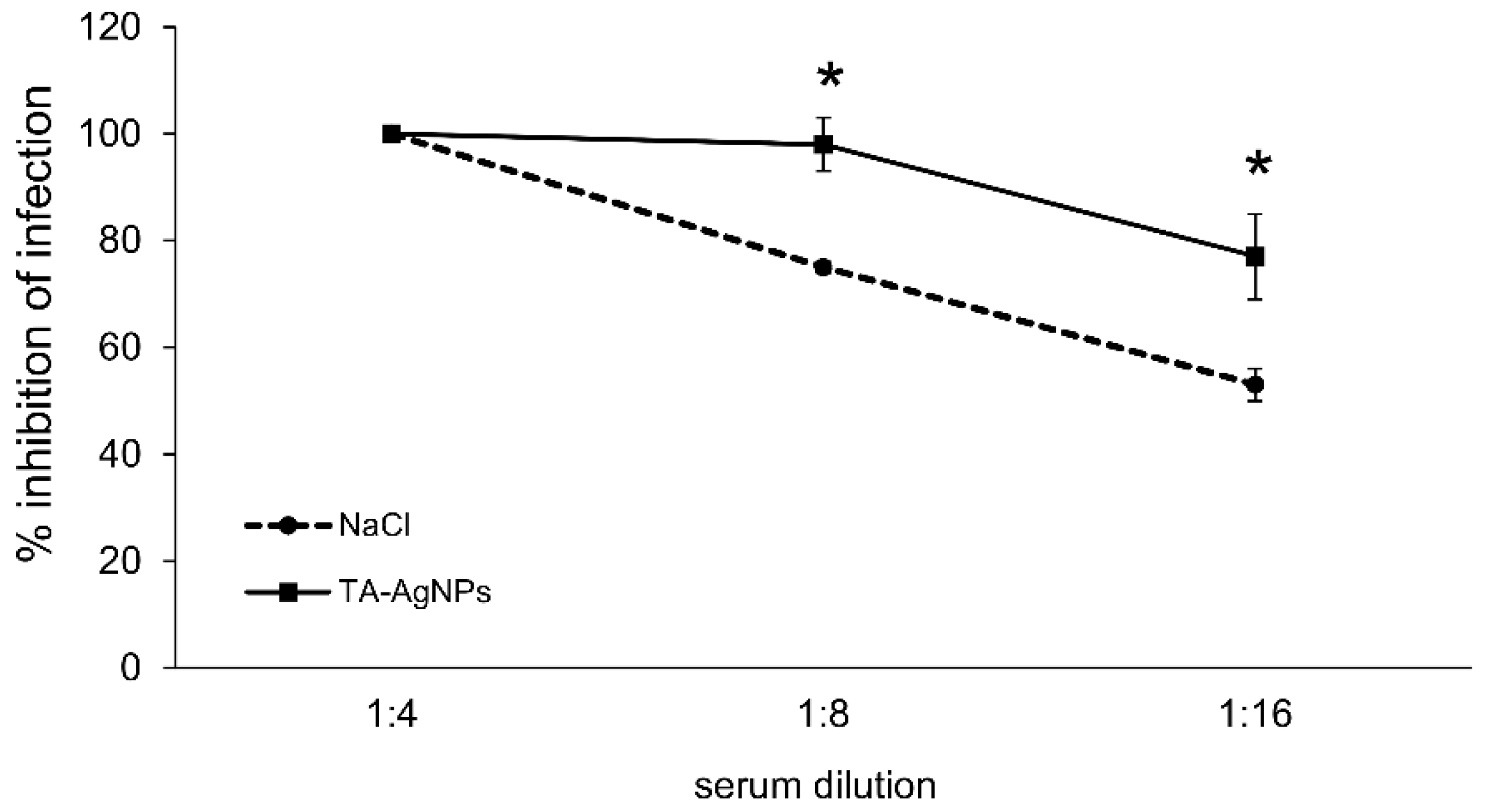
3.5. Treatment with TA-AgNPs Helps to Boost Anti-Viral Immunity
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Global estimates of prevalent and incident herpes global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS ONE 2015, 10, e0140765. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Warren, T.; Wald, A. Genital herpes. Lancet 2007, 22, 2127–2137. [Google Scholar] [CrossRef]
- Whitley, R. New approaches to the therapy of HSV infections. Herpes 2006, 13, 53–55. [Google Scholar] [PubMed]
- Roizman, B.; Whitley, R.J. An inquiry into the molecular basis of HSV latency and reactivation. Annu. Rev. Microbiol. 2013, 67, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.; Fidler, S. Sexual transmission of HIV-1. Antivir. Res. 2010, 85, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.P.; Muhammad, Z.S.; Wang, J.G.; Lin, W.; Guo, S.K.; Zhang, W. HSV-2 vaccine: Current status and insight into factors for developing an efficient vaccine. Viruses 2014, 6, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Rebbapragada, A.; Wachihi, C.; Pettengell, C.; Sunderji, S.; Huibner, S.; Jaoko, W.; Ball, B.; Fowke, K.; Mazzulli, T.; Plummer, F.A.; et al. Negative mucosal synergy between Herpes simplex type 2 and HIV in the female genital biochemistry. AIDS 2007, 21, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Oakley, C.; Epstein, J.B.; Sherlock, C.H. Reactivation of oral herpes simplex virus: Implications for clinical management of herpes simplex virus recurrence during radiotherapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1997, 84, 272–278. [Google Scholar] [CrossRef]
- Vermerris, W. Chemical Properties of Phenolic Compounds. In Phenolic Compound Biochemistry, 1st ed.; Vermerris, W., Nicholson, R., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 35–61. ISBN 1-4020-5163-8. [Google Scholar]
- Lin, L.T.; Chen, T.Y.; Chung, C.Y.; Noyce, R.S.; Grindley, T.B.; McCormick, C.; Lin, T.C.; Wang, G.H.; Lin, C.C.; Richardson, C.D. Hydrolyzable tannins (chebulagic acid and punicalagin) target viral glycoprotein-glycosaminoglycan interactions to inhibit herpes simplex virus 1 entry and cell-to-cell spread. J. Virol. 2011, 85, 4386–4398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Dai, Y.C.; Zhong, W.; Tan, M.; Lv, Z.P.; Zhou, Y.C.; Jiang, X. Tannic acid inhibited norovirus binding to HBGA receptors, a study of 50 Chinese medicinal herbs. Bioorg. Med. Chem. 2012, 20, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Theisen, L.L.; Erdelmeier, C.A.; Spoden, G.A.; Boukhallouk, F.; Sausy, A.; Florin, L.; Muller, C.P. Tannins from Hamamelis virginiana bark extract: Characterization and improvement of the antiviral efficacy against influenza A virus and human papillomavirus. PLoS ONE 2014, 9, e88062. [Google Scholar] [CrossRef] [PubMed]
- Asgary, V.; Shoari, A.; Baghbani-Arani, F.; Sadat Shandiz, S.A.; Khosravy, M.S.; Janani, A.; Bigdeli, R.; Bashar, R.; Cohan, R.A. Green synthesis and evaluation of silver nanoparticles as adjuvant in rabies veterinary vaccine. Int. J. Nanomed. 2016, 11, 3597–3605. [Google Scholar] [CrossRef]
- Kim, T.Y.; Cha, S.H.; Cho, S.; Park, Y. Tannic acid-mediated green synthesis of antibacterial silver nanoparticles. Arch. Pharm. Res. 2016, 39, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Sun, R.W.; Chen, R.; Hui, C.K.; Ho, C.M.; Luk, J.M.; Lau, G.K.; Che, C.M. Silver nanoparticles inhibit hepatitis B virus replication. Antivir. Ther. 2008, 13, 253–262. [Google Scholar] [PubMed]
- Lara, H.H.; Ayala-Nunez, N.V.; Ixtepan-Turrent, L.; Rodriguez-Padilla, C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnol. 2010, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Baram-Pinto, D.; Shukla, S.; Perkas, N.; Gedanken, A.; Sarid, R. Inhibition of herpes simplex virus type 1 infection by silver nanoparticles capped with mercaptoethanesulfonate. Bioconjug. Chem. 2009, 19, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Zheng, Y.; Duan, W.; Li, X.; Yin, J.; Shigdar, S.; O’Connor, M.L.; Marappan, M.; Zhao, X.; Miao, Y.; et al. Inhibition of A/Human/Hubei/3/2005 (H3N2) influenza virus infection by silver nanoparticles in vitro and in vivo. Int. J. Nanomed. 2013, 8, 4103–4114. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, P.; Tomaszewska, E.; Gniadek, M.; Baska, P.; Nowakowska, J.; Sokolowska, J.; Nowak, Z.; Donten, M.; Celichowski, G.; Grobelny, J.; et al. A Tannic acid modified silver nanoparticles show antiviral activity in herpes simplex virus type 2 infection. PLoS ONE 2014, 9, e104113. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Orłowski, P.; Winnicka, K.; Tomaszewska, E.; Bąska, P.; Celichowski, G.; Grobelny, J.; Basa, A.; Krzyżowska, M. Multifunctional tannic acid/silver nanoparticle-based mucoadhesive hydrogel for improved local treatment of HSV infection: In vitro and in vivo studies. Int. J. Mol. Sci. 2018, 19, 387. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, P.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Gniadek, M.; Labedz, O.; Malewski, T.; Nowakowska, J.; Chodaczek, G.; Celichowski, G.; Grobelny, J.; et al. Tannic acid-modified silver and gold nanoparticles as novel stimulators of dendritic cells activation. Front. Immunol. 2018, 9, 1115. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, P.; Soliwoda, K.; Tomaszewska, E.; Bien, K.; Fruba, A.; Gniadek, M.; Labedz, O.; Nowak, Z.; Celichowski, G.; Grobelny, J.; et al. Toxicity of tannic acid-modified silver nanoparticles in keratinocytes: Potential for immunomodulatory applications. Toxicol. In Vitro 2016, 35, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Seth, P.; Rawls, W.E.; Duff, R.; Rapp, F.; Adam, E.; Melnick, J.L. Antigenic differences between isolates of herpesvirus type 2. Intervirology 1974, 3, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Namvar, L.; Olofsson, S.; Bergstrom, T.; Lindh, M. Detection and typing of herpes simplex virus (HSV) in mucocutaneous samples by TaqMan PCR targeting a gB segment homologous for HSV types 1 and 2. J. Clin. Microbiol. 2005, 43, 2058–2064. [Google Scholar] [CrossRef] [PubMed]
- Ranoszek-Soliwoda, K.; Tomaszewska, E.; Socha, E.; Krzyczmonik, P.; Ignaczak, A.; Orlowski, P.; Krzyzowska, M.; Celichowski, G.; Grobelny, J. The role of tannic acid and sodium citrate in the synthesis of silver nanoparticles. J. Nanopart. Res. 2017, 19, 273. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; Puupponen-Pimia, R.; Dauer, A.; Aura, A.M.; Saura-Calixto, F. Tannins: Current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food. Res. 2009, 53 (Suppl. 2), S310–S329. [Google Scholar] [CrossRef] [PubMed]
- Buzzini, P.; Arapitsas, P.; Goretti, M.; Branda, E.; Turchetti, B.; Pinelli, P.; Ieri, F.; Romani, A. Antimicrobial and antiviral activity of hydrolysable tannins. Mini. Rev. Med. Chem. 2008, 8, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Pitchaimani, V.; Sreedhar, R.; Afrin, R.; Harima, M.; Suzuki, H.; Nomoto, M.; Miyashita, S.; et al. Tannic acid modulates NFκB signaling pathway and skin inflammation in NC/Nga mice through PPARγ expression. Cytokine 2015, 76, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Obreque-Slier, E.; Mateluna, C.; Peña-Neira, A.; López-Solís, R. Quantitative determination of interactions between tannic acid and a model protein using diffusion and precipitation assays on cellulose membranes. J. Agric. Food Chem. 2010, 58, 8375–8379. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, R.; Hagedorn, C.H. Tannic acid inhibits hepatitis C virus entry into Huh7.5 cells. PLoS ONE 2015, 10, e0131358. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, A.; Polachira, S.K.; Nair, R.; Agarwal, A.; Mishra, N.N.; Gupta, S.K. Anti-HSV-2 activity of Terminalia chebula Retz extract and its constituents, chebulagic and chebulinic acids. BMC Complement. Altern. Med. 2017, 17, 110. [Google Scholar] [CrossRef] [PubMed]
- Dwevedi, A.; Dwivedi, R.; Sharma, Y.K. Exploration of phytochemicals found in Terminalia sp. and their antiretroviral activities. Pharmacogn. Rev. 2016, 10, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.T.; Chen, T.Y.; Lin, S.C.; Chung, C.Y.; Lin, T.C.; Wang, G.H.; Anderson, R.; Lin, C.C.; Richardson, C.D. Broad-spectrum antiviral activity of chebulagic acid and punicalagin against viruses that use glycosaminoglycans for entry. BMC Microbiol. 2013, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Le Bourvellec, C.; Renard, C.M. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef] [PubMed]
- Luck, G.; Liao, H.; Murray, N.J.; Grimmer, H.R.; Warminski, E.E.; Williamson, M.P.; Lilley, T.H.; Haslam, E. Polyphenols, astringency and proline-rich proteins. Phytochemistry 1994, 37, 357–371. [Google Scholar] [CrossRef]
- Gianni, T.; Amasio, M.; Campadelli-Fiume, G. Herpes simplex virus gD forms distinct complexes with fusion executors gB and gH/gL in part through the C-terminal profusion domain. J. Biol. Chem. 2009, 284, 17370–17382. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Truong, N.R.; James, V.; Bosnjak, L.; Sandgren, K.J.; Harman, A.N.; Nasr, N.; Bertram, K.M.; Olbourne, N.; Sawleshwarkar, S.; et al. Relay of herpes simplex virus between Langerhans cells and dermal dendritic cells in human skin. PLoS Pathog. 2015, 11, e1004812. [Google Scholar] [CrossRef] [PubMed]
- Cappel, R. Comparison of the humoral and cellular immune responses after immunization with live, UV inactivated herpes simplex virus and a subunit vaccine and efficacy of these immunizations. Arch. Virol. 1976, 52, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Hernández-Ruiz, M.; Khan, A.A.; Fouladi, M.A.; Kim, G.J.; Ly, V.T.; Yamada, T.; Lam, C.; Sarain, S.A.B.; Boldbaatar, U.; et al. CXCL17 chemokine-dependent mobilization of CXCR8+CD8+ effector memory and tissue-resident memory T cells in the vaginal mucosa is asssociated with protection against genital herpes. J. Immunol. 2018, 200, 2915–2926. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Koelle, D.M.; Cao, J.; Vazquez, J.; Huang, M.L.; Hladik, F.; Wald, A.; Corey, L. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J. Exp. Med. 2007, 204, 595–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebhardt, T.; Wakim, L.M.; Eidsmo, L.; Reading, P.C.; Heath, W.R.; Carbone, F.R. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 2009, 10, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Halford, W.P.; Geltz, J.; Messer, R.J.; Hasenkrug, K.J. Antibodies are required for complete vaccine-induced protection against herpes simplex virus 2. PLoS ONE 2015, 10, e0145228. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.R.; Brais, L.J.; Evelegh, M.J. Mucosal and systemic antiviral antibodies in mice inoculated intravaginally with herpes simplex virus type 2. J. Gen. Virol. 1990, 71, 1497–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milligan, G.N.; Bernstein, D.I. Generation of humoral immune responses against herpes simplex virus type 2 in the murine female genital tract. Virology 1995, 206, 234–241. [Google Scholar] [CrossRef]
- Parr, M.B.; Parr, E.L. Protective immunity against HSV-2 in the mouse vagina. J. Reprod. Immunol. 1997, 36, 77–92. [Google Scholar] [CrossRef]
- Parr, E.L.; Parr, M.B. Immune responses and protection against vaginal infection after nasal or vaginal immunization with attenuated herpes simplex virus type-2. Immunology 1999, 98, 639–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Control (Uninfected) | HSV-2 + NaCl Treatment | HSV-2 + TA-AgNP Treatment |
|---|---|---|
| CD4+ T cells | ||
| 2.5 ± 0.5 | 14.89 ± 3.52 | 19.2 ± 4.7 |
| % of CD69+ CD4+ T cells | ||
| 8.8 ± 1.1 | 40.2 ± 4.24 | 47.6 ± 2.4 |
| CD8+ T cells | ||
| 2.12 ± 0.45 | 11.15 ± 2.25 | 13.8 ± 4.48 |
| % of CD69+ CD8+ T cells | ||
| 9.2 ± 2.1 | 52.2 ± 6.12 | 49.4 ± 4.05 |
| NK cells | ||
| 2.54 ± 0.67 | 9.82 ± 2.62 | 9.4 ± 2.69 |
| Monocytes | ||
| 8.34 ± 0.9 | 18.8 ± 1.1 | 25.4 ± 2.8 * |
| Dendritic cells | ||
| 5.41 ± 1.2 | 16.4 ± 0.67 | 26.8 ± 2.7 * |
| HSV-2 + NaCl Treatment | HSV-2 + TA-AgNPs Treatment |
|---|---|
| IFN-alpha | |
| 8192 ± 54 | 8902 ± 67 |
| IFN-gamma | |
| 25 ± 4.3 | 19.42 ± 5.1 |
| CXCL9 | |
| 430 ± 51 | 317 ± 56 |
| CXCL10 | |
| 1243 ± 121 | 1024 ± 99 |
| CXCL17 | |
| 2048 ± 128 | 33,454,432 ± 53 ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orłowski, P.; Kowalczyk, A.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Węgrzyn, A.; Grzesiak, J.; Celichowski, G.; Grobelny, J.; Eriksson, K.; Krzyzowska, M. Antiviral Activity of Tannic Acid Modified Silver Nanoparticles: Potential to Activate Immune Response in Herpes Genitalis. Viruses 2018, 10, 524. https://doi.org/10.3390/v10100524
Orłowski P, Kowalczyk A, Tomaszewska E, Ranoszek-Soliwoda K, Węgrzyn A, Grzesiak J, Celichowski G, Grobelny J, Eriksson K, Krzyzowska M. Antiviral Activity of Tannic Acid Modified Silver Nanoparticles: Potential to Activate Immune Response in Herpes Genitalis. Viruses. 2018; 10(10):524. https://doi.org/10.3390/v10100524
Chicago/Turabian StyleOrłowski, Piotr, Andrzej Kowalczyk, Emilia Tomaszewska, Katarzyna Ranoszek-Soliwoda, Agnieszka Węgrzyn, Jakub Grzesiak, Grzegorz Celichowski, Jarosław Grobelny, Kristina Eriksson, and Malgorzata Krzyzowska. 2018. "Antiviral Activity of Tannic Acid Modified Silver Nanoparticles: Potential to Activate Immune Response in Herpes Genitalis" Viruses 10, no. 10: 524. https://doi.org/10.3390/v10100524






