Native Plant Diversity and Composition Across a Pinus radiata D.Don Plantation Landscape in South-Central Chile—The Impact of Plantation Age, Logging Roads and Alien Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Sampling
2.3. Data Analysis
3. Results
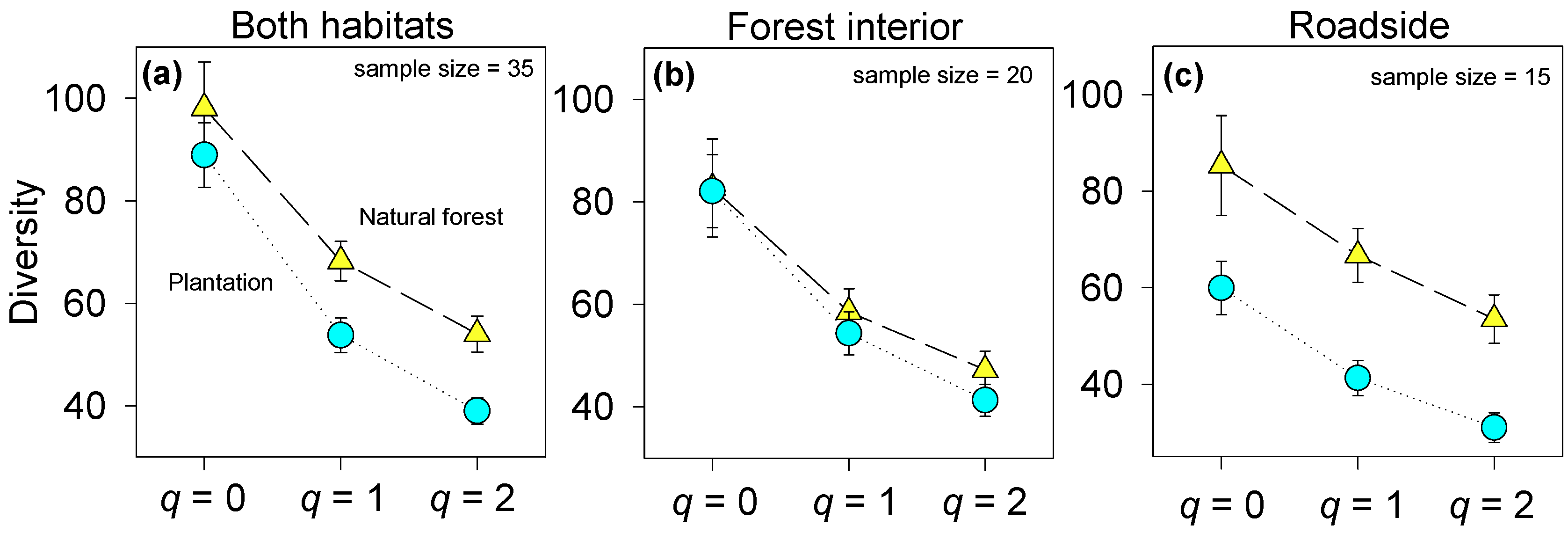
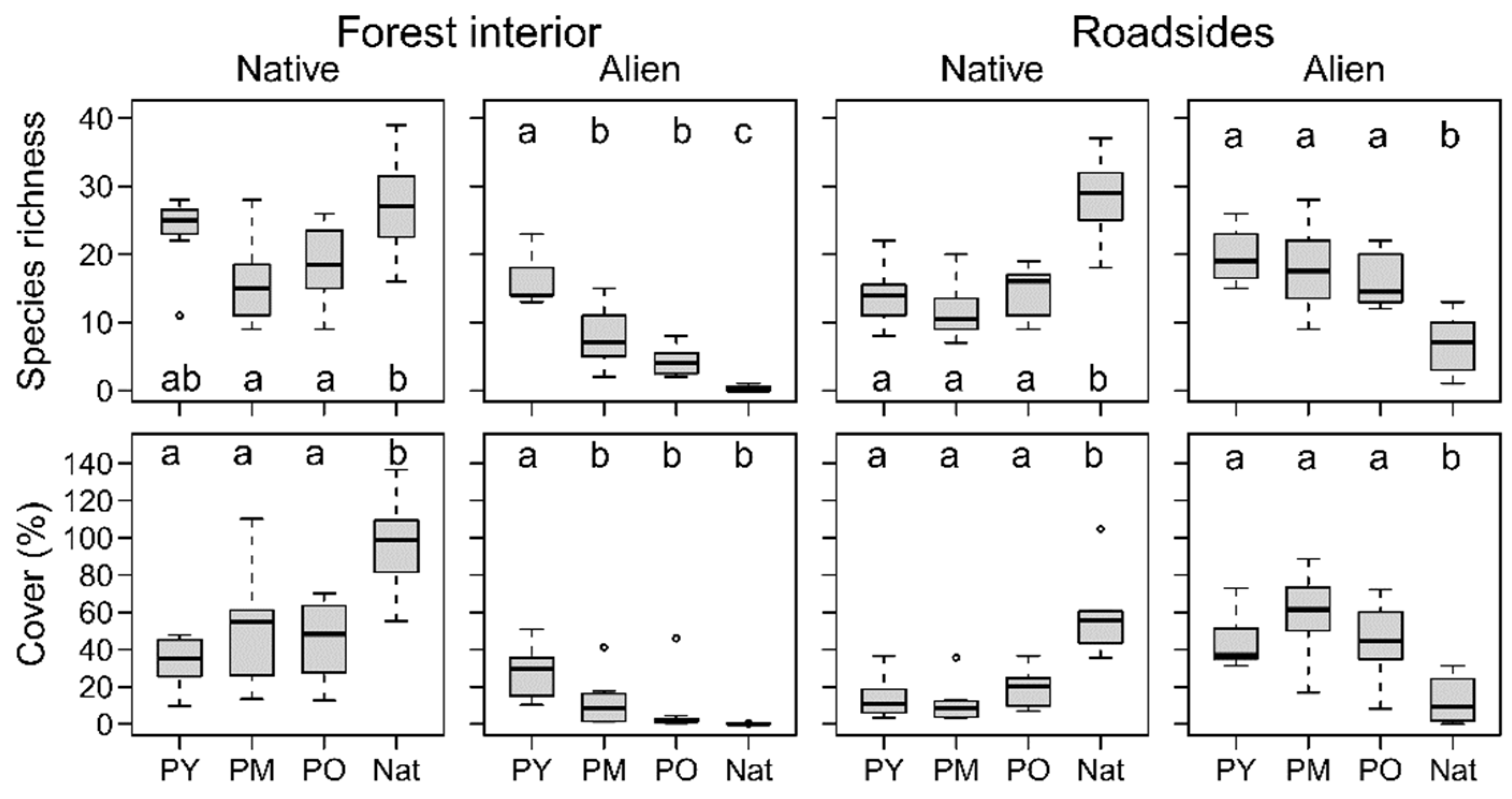
3.1. Native Plant Diversity in Plantations Compared to the Natural Forest
3.2. Differences in Environmental Conditions and Plant Species Richness among Age Classes
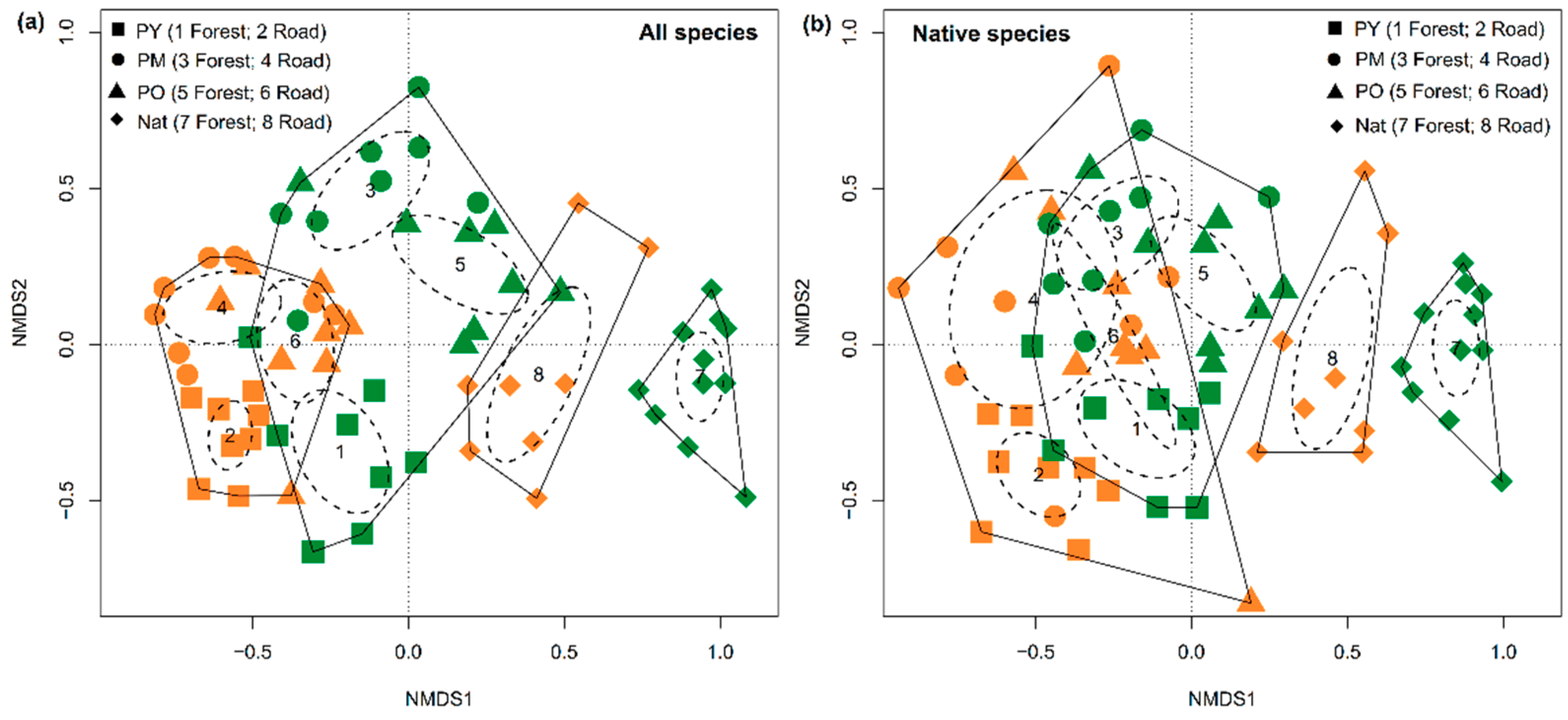
3.3. Species Composition of Forest Types and Habitats
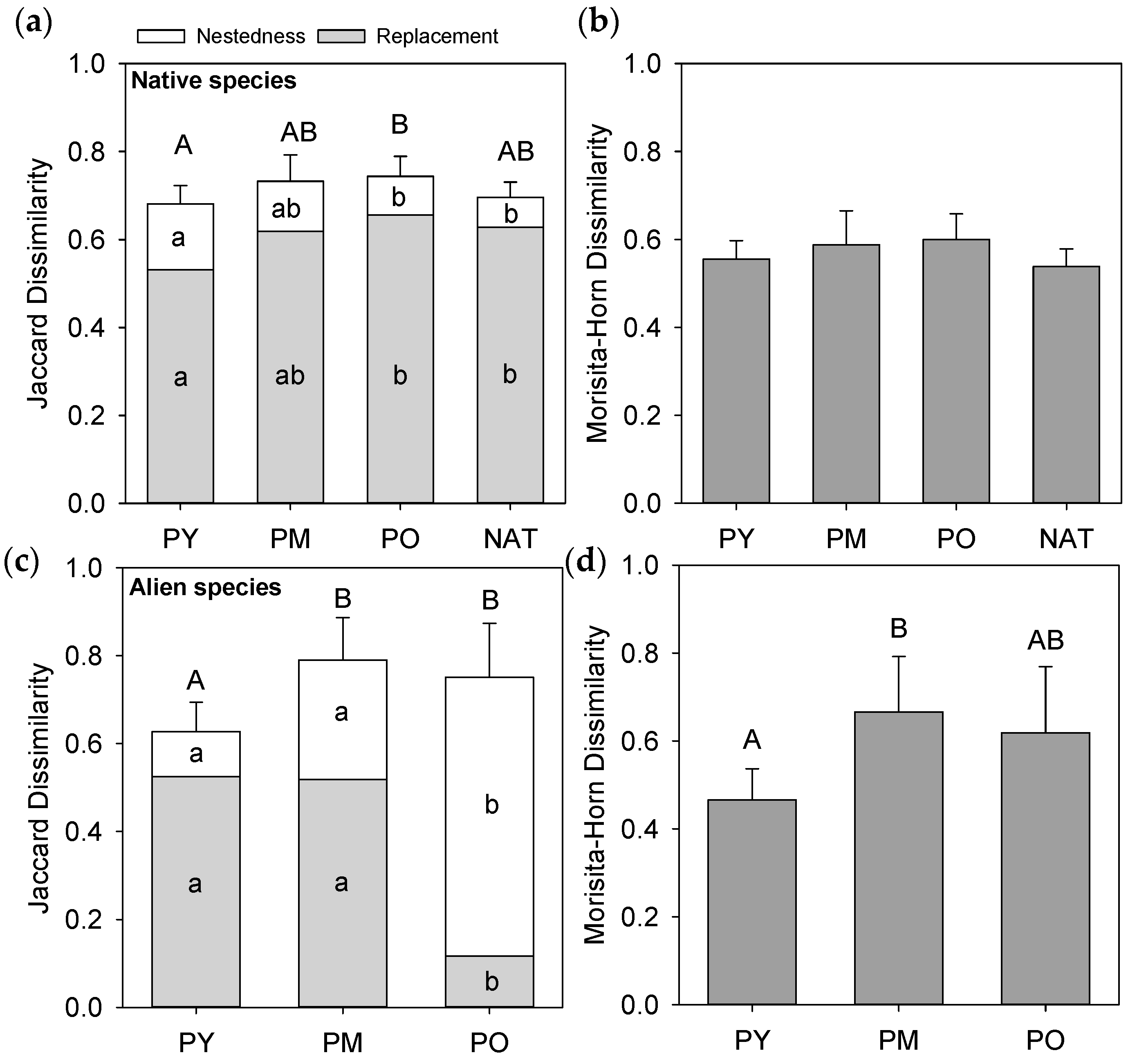
3.4. Dissimilarity between Forest Interiors and Roadsides
3.5. Determination of Indicator Species for Forest Interiors and Roadsides
4. Discussion
4.1. Effects of the Plantation Management Cycle on Native Plant Diversity
4.2. The Effect of Forest Roads on Native Plant Diversity in Plantations and Natural Forests
4.3. Alien Species within the Plantation Landscape
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Roadsides | Forest Interiors | |||||||
|---|---|---|---|---|---|---|---|---|
| PY | PM | PO | Nat | PY | PM | PO | Nat | |
| Distance to forest edge (m) | 1.1 (1.5) | 3.9 (2.0) | 3.1 (2.1) | 1.0 (0.5) | - | - | - | - |
| Road width (m) | 5.3 (1.0) | 4.9 (0.6) | 5.5 (0.6) | 4.9 (0.7) | - | - | - | - |
| Mean road use category (1–5) | 2.8 (1.5) ab | 2.1 (0.6) ab | 1.9 (0.8) a | 3.3 (0.7) b | - | - | - | - |
| Distance to road (m) | - | - | - | - | 55.3 (8.6) a | 67.6 (11.9) a | 79.8 (18.8) a | 290.1 (142.9) b |
| Distance to natural forest (m) | 425.1 (237.1) | 509.9 (350.4) | 558.8 (405.4) | - | 418.8 (261.6) | 534.0 (351.6) | 566.5 (387.9) | - |
| Nat | PO | PM | PY | |||||
|---|---|---|---|---|---|---|---|---|
| F (%) | mCv (%) | F (%) | mCv (%) | F (%) | mCv (%) | F (%) | mCv (%) | |
| n = 11 | n = 8 | n = 8 | n = 8 | |||||
| Alien species | ||||||||
| Pinus radiata | 27 | 0.1 | 100 | 0.5 | 38 | 0.5 | 100 | 13.9 |
| Rubus ulmifolius | 88 | 4.6 | 88 | 5.0 | 75 | 0.2 | ||
| Genista monspessulana | 63 | 1.0 | 38 | 2.5 | 38 | 0.1 | ||
| Agrostis capillaris | 38 | 0.7 | 75 | 2.9 | 88 | 2.2 | ||
| Rumex acetosella | 38 | 0.2 | 38 | 0.1 | 88 | 1.3 | ||
| Hypochaeris radicata | 25 | + | 50 | 0.1 | 50 | 0.2 | ||
| Plantago lanceolata | 25 | + | 13 | + | 38 | 0.4 | ||
| Sanguisorba minor | 25 | + | 13 | + | 38 | 0.5 | ||
| Hypericum perforatum | 13 | + | 50 | 0.3 | 13 | 0.1 | ||
| Rosa rubiginosa | 13 | 0.1 | 13 | + | ||||
| Lactuca serriola | 38 | + | 100 | 2.4 | ||||
| Euphorbia peplus | 38 | 0.1 | 50 | 0.1 | ||||
| Verbascum virgatum | 38 | 0.1 | 38 | 0.1 | ||||
| Briza minor | 25 | + | 88 | 0.9 | ||||
| Anagallis arvensis | 25 | + | 75 | 0.7 | ||||
| Cirsium vulgare | 25 | + | 75 | 0.3 | ||||
| Senecio sylvaticus | 25 | + | 63 | 0.3 | ||||
| Sonchus oleraceus | 25 | + | 63 | 0.2 | ||||
| Daucus carota | 25 | + | ||||||
| Trifolium dubium | 25 | 0.1 | ||||||
| Aira caryophyllea | 13 | + | 50 | 0.4 | ||||
| Arrhenaterum elatius ssp. bulbosus | 13 | + | ||||||
| Bromus hordeaceus | 13 | + | ||||||
| Holcus lanatus | 13 | + | ||||||
| Juncus cf. effusus | 13 | 0.1 | ||||||
| Logfia gallica | 13 | + | ||||||
| Poa pratensis | 13 | + | ||||||
| Trifolium arvense | 13 | + | ||||||
| Geranium robertianum | 63 | 0.7 | ||||||
| Vulpia bromoides | 63 | 0.7 | ||||||
| Carduus pycnocephalus | 38 | 0.1 | ||||||
| Galium aparine | 38 | 0.6 | ||||||
| Anthoxanthum odoratum | 25 | 0.5 | ||||||
| Avena fatua | 25 | 0.1 | ||||||
| Erodium cicutarium | 25 | 0.1 | ||||||
| Mentha pulegium | 25 | + | ||||||
| Silybum marianum | 25 | 0.2 | ||||||
| Taraxacum officinale | 25 | 0.1 | ||||||
| Cerastium fontanum ssp. vulgare | 13 | + | ||||||
| Cynosurus echinatus | 13 | + | ||||||
| Euphorbia lathyrus | 13 | + | ||||||
| Gastridium phleoides | 13 | + | ||||||
| Linum usitatissimum | 13 | + | ||||||
| Medicago lupulina | 13 | 0.1 | ||||||
| Medicago polymorpha | 13 | + | ||||||
| Sonchus asper | 13 | + | ||||||
| Vicia hirsuta | 13 | 0.1 | ||||||
| Total species number | 1 | 10 | 27 | 38 | ||||
| Native species | ||||||||
| Cryptocarya alba * | 100 | 3.9 | 50 | 2.2 | 38 | 1.5 | 63 | 4.1 |
| Ugni molinae | 100 | 23.4 | 50 | 3.0 | 13 | + | ||
| Gaultheria insana | 100 | 25.1 | 25 | 2.9 | ||||
| Azara integrifolia * | 91 | 3.0 | 75 | 1.4 | 63 | 0.1 | ||
| Lithraea caustica * | 91 | 5.6 | 50 | 0.9 | 13 | 0.4 | 50 | 0.6 |
| Relchela panicoides | 91 | 1.6 | 50 | 0.4 | 13 | + | 13 | + |
| Nothofagus glauca * | 91 | 1.9 | 38 | 4.7 | 13 | 0.9 | ||
| Lomatia hirsuta | 91 | 1.9 | 25 | 0.1 | 13 | 0.1 | ||
| Bomarea salsilla * | 82 | 1.6 | 50 | 0.2 | 13 | + | 50 | 0.1 |
| Lapageria rosea * | 82 | 4.1 | 13 | 0.1 | 13 | 0.5 | 38 | 0.1 |
| Podanthus ovatifolius * | 82 | 1.9 | 13 | + | 50 | 0.5 | ||
| Piptochaetium panicoides | 73 | 0.6 | 88 | 7.0 | 100 | 26.5 | 100 | 5.8 |
| Uncinia phleoides | 73 | 1.2 | 13 | + | 25 | 0.4 | ||
| Dioscorea bridgesii * | 64 | 0.1 | 100 | 0.7 | 88 | 0.2 | 63 | 0.3 |
| Peumus boldus * | 64 | 3.8 | 63 | 1.0 | 75 | 1.6 | 63 | 0.9 |
| Ribes punctatum | 64 | 0.6 | 25 | + | 63 | 0.1 | 25 | + |
| Galium cotinoides * | 64 | 0.3 | 13 | + | ||||
| Aristotelia chilensis | 55 | 0.4 | 88 | 11.7 | 100 | 11.0 | 88 | 1.4 |
| Gevuina avellana | 55 | 5.0 | 13 | 0.1 | 13 | + | 25 | 0.1 |
| Vicia nigricans | 55 | 0.3 | 13 | 0.4 | 25 | + | ||
| Persea lingue | 55 | 0.3 | 13 | 1.3 | 13 | 0.4 | ||
| Aextoxicon punctatum | 55 | 1.1 | ||||||
| Escallonia pulverulenta * | 45 | 0.5 | 63 | 3.8 | 88 | 0.7 | 63 | 0.7 |
| Colletia hystrix | 45 | 0.4 | 25 | + | 13 | + | 13 | + |
| Sophora macrocarpa * | 45 | 0.5 | 13 | + | ||||
| Citronella mucronata * | 45 | 0.4 | ||||||
| Teucrium bicolor * | 36 | + | 50 | 0.1 | 50 | 0.1 | 63 | 0.9 |
| Quillaja saponaria * | 36 | 0.6 | 50 | 1.7 | ||||
| Cynanchum pachyphyllum | 36 | 0.1 | 13 | + | ||||
| Blechnum hastatum | 36 | 0.3 | 13 | 0.1 | ||||
| Gilliesia montana * | 36 | 0.1 | ||||||
| Olsynium scirpoideum * | 36 | 0.1 | ||||||
| Solenomelus pedunculatus * | 27 | 0.2 | 38 | 0.2 | 13 | + | 25 | + |
| Tristagma bivalve * | 27 | + | 38 | 0.1 | 13 | + | 25 | + |
| Luma apiculata | 27 | 0.2 | 25 | 0.1 | 25 | 0.2 | 50 | 0.1 |
| Gochnatia foliolosa * | 27 | 0.1 | 25 | 0.9 | 13 | 0.1 | 13 | + |
| Oxalis arenaria * | 27 | + | 13 | + | ||||
| Alstroemeria ligtu * | 27 | + | 13 | 0.2 | ||||
| Gavilea venosa * | 27 | + | ||||||
| Podocarpus salignus * | 27 | 0.1 | ||||||
| Senna stipulacea * | 27 | 0.2 | ||||||
| Pasithaea coerulea | 18 | + | 63 | 0.2 | 13 | 0.5 | 50 | 0.1 |
| Lardizabala biternata * | 18 | + | 13 | + | 50 | 1.0 | ||
| Carex setifolia | 18 | + | 13 | + | ||||
| Lomatia dentata | 18 | 0.3 | 13 | 0.1 | 13 | 0.1 | ||
| Proustia pyrifolia * | 18 | 0.1 | 13 | + | ||||
| Baccharis racemosa | 18 | + | 13 | + | ||||
| Dioscorea humifusa * | 18 | 1.1 | ||||||
| Viola portalesia * | 18 | 0.1 | ||||||
| Adiantum chilense | 18 | + | ||||||
| Elytropus chilensis | 18 | 0.2 | ||||||
| Synammia feuillei | 18 | + | ||||||
| Sanicula crassicaulis | 18 | + | ||||||
| Galium hypocarpium | 9 | + | 88 | 0.2 | 88 | 0.2 | 100 | 1.4 |
| Baccharis rhomboidalis | 9 | + | 38 | 0.1 | 13 | 1.1 | 38 | 0.1 |
| Nassella laevissima | 9 | + | 13 | + | 38 | 0.1 | 63 | 1.3 |
| Margyricarpus pinnatus | 9 | + | 13 | + | 13 | + | ||
| Myoschilos oblongum | 9 | + | 13 | 0.1 | ||||
| Schinus polygama | 9 | 0.1 | 13 | 0.4 | ||||
| Chiropetulum tricuspidatum | 9 | 0.1 | 25 | + | ||||
| Dioscorea auriculata * | 9 | + | 13 | + | ||||
| Maytenus boaria | 9 | + | 13 | + | ||||
| Chusquea quila * | 9 | 0.3 | ||||||
| Greigia sphacelata * | 9 | + | ||||||
| Laurelia sempervirens * | 9 | 0.3 | ||||||
| Libertia sessiliflora * | 9 | + | ||||||
| Boquila trifoliolata | 9 | 0.2 | ||||||
| Calceolaria corymbosa | 9 | 0.1 | ||||||
| Cardamine vulgaris | 9 | + | ||||||
| Oxalis perdicaria | 9 | + | ||||||
| Rhaphithamnus spinosus | 9 | + | ||||||
| Senecio cymosus | 9 | 0.1 | ||||||
| Tristerix corymbosus | 9 | + | ||||||
| Muehlenbeckia hastulata | 75 | 0.3 | 100 | 2.1 | 88 | 1.2 | ||
| Danthonia chilensis var. aureofulva | 50 | 0.1 | 25 | 0.2 | 63 | 0.8 | ||
| Stachys gilliesii | 50 | 0.1 | 63 | 0.1 | ||||
| Gamochaeta coarctata | 38 | 0.2 | 38 | 0.1 | 100 | 0.3 | ||
| Lathyrus magellanicus | 38 | 0.1 | 25 | + | 25 | 0.1 | ||
| Conanthera bifolia * | 38 | 0.1 | 25 | 0.1 | ||||
| Piptochaetium montevidense | 25 | + | 13 | + | ||||
| Alstroemeria revoluta * | 25 | + | ||||||
| Acaena argentea | 13 | + | 50 | 0.5 | 75 | 1.5 | ||
| Lepechinia chamaedryoides * | 13 | + | 38 | + | ||||
| Cissus striata | 13 | + | 38 | 0.1 | ||||
| Chascolytrum subaristatum | 13 | + | 25 | 0.1 | 13 | 0.1 | ||
| Oxalis micrantha | 13 | + | 13 | + | 88 | 2.7 | ||
| Geranium core-core | 13 | + | 13 | + | 75 | 0.1 | ||
| Glandularia laciniata | 13 | + | 13 | + | 13 | + | ||
| Adesmia spec. | 13 | 0.4 | 13 | 0.1 | ||||
| Chloraea lamellata * | 13 | + | ||||||
| Raukaua valdiviensis * | 13 | + | ||||||
| Gnaphalium cheiranthifolium | 25 | 0.1 | 75 | 0.6 | ||||
| Hypericum caespitosum * | 25 | + | 25 | 3.6 | ||||
| Calceolaria dentata | 13 | + | 13 | 0.5 | ||||
| Eryngium paniculatum | 13 | 0.1 | 13 | + | ||||
| Lobelia tupa * | 13 | 0.1 | ||||||
| Berberis bidentata cf. | 13 | + | ||||||
| Bromus berteroanus | 13 | + | ||||||
| Carex phalaroides | 13 | + | ||||||
| Mutisia spinosa | 13 | 0.1 | ||||||
| Sisyrinchium graminifolium | 13 | + | ||||||
| Calandrinia compressa * | 50 | 0.1 | ||||||
| Oenothera stricta | 25 | + | ||||||
| Solanum crispum | 25 | + | ||||||
| Calceolaria purpurea * | 13 | + | ||||||
| Adenocaulon chilense | 13 | + | ||||||
| Cicendia quadrangularis | 13 | + | ||||||
| Juncus bufonius | 13 | + | ||||||
| Nothofagus obliqua | 13 | 0.4 | ||||||
| Soliva sessilis | 13 | 0.1 | ||||||
| Stellaria debilis | 13 | + | ||||||
| Total species numbers | 73 | 53 | 55 | 62 | ||||
| Nat | PO | PM | PY | |||||
|---|---|---|---|---|---|---|---|---|
| F (%) | mCv (%) | F (%) | mCv (%) | F (%) | mCv (%) | F (%) | mCv (%) | |
| n = 8 | n = 8 | n = 8 | n = 8 | |||||
| Alien species | ||||||||
| Pinus radiata | 100 | 3.5 | 100 | 0.6 | 88 | 2.4 | 88 | 8.0 |
| Agrostis capillaris | 88 | 7.0 | 100 | 20.9 | 100 | 24.8 | 88 | 15.8 |
| Genista monspessulana | 50 | 0.3 | 100 | 10.6 | 100 | 7.4 | 75 | 1.3 |
| Rumex acetosella | 38 | + | 100 | 0.3 | 88 | 0.7 | 88 | 0.3 |
| Hypochaeris radicata | 38 | + | 88 | 0.7 | 88 | 1.0 | 88 | 0.6 |
| Lactuca serriola | 38 | 0.1 | 63 | 0.1 | 38 | 0.1 | 100 | 0.5 |
| Briza minor | 38 | 0.1 | 50 | 0.1 | 75 | 0.2 | 88 | 2.9 |
| Avena fatua | 38 | 0.1 | 38 | 0.1 | 38 | 0.1 | 50 | 0.2 |
| Plantago lanceolata | 25 | + | 100 | 0.6 | 100 | 2.5 | 100 | 4.6 |
| Senecio sylvaticus | 25 | + | 63 | 0.1 | 25 | + | 75 | 0.4 |
| Anagallis arvensis | 25 | + | 25 | 0.1 | 25 | + | 50 | 0.7 |
| Acacia dealbata | 25 | 0.6 | 13 | 2.1 | 75 | 8.1 | 13 | 0.4 |
| Cirsium vulgare | 25 | + | 13 | + | 63 | 0.3 | ||
| Taraxacum officinale | 25 | + | 13 | + | ||||
| Sanguisorba minor | 13 | + | 100 | 1.2 | 75 | 1.4 | 63 | 0.8 |
| Hypericum perforatum | 13 | + | 75 | 0.2 | 100 | 0.8 | 100 | 0.4 |
| Lotus pedunculatus | 13 | 0.1 | 38 | 0.1 | 38 | + | 13 | + |
| Eucalyptus globulus | 13 | 0.1 | 25 | + | 25 | + | ||
| Briza maxima | 13 | 0.1 | 13 | 0.2 | 25 | + | 25 | 0.1 |
| Sherardia arvensis | 13 | + | 13 | + | 13 | + | 13 | + |
| Crepis capillaris | 13 | + | 13 | + | ||||
| Galega officinalis | 13 | 0.4 | 13 | + | ||||
| Rubus ulmifolius | 75 | 5.9 | 50 | 4.2 | 50 | 0.7 | ||
| Rosa rubiginosa | 63 | 0.2 | 25 | 0.5 | 13 | + | ||
| Vulpia bromoides | 50 | 0.1 | 75 | 1.9 | 63 | 2.9 | ||
| Aira caryophyllea | 38 | 0.2 | 50 | 0.3 | 75 | 0.2 | ||
| Mentha pulegium | 38 | + | 25 | 0.1 | ||||
| Bromus hordeaceus | 25 | 0.1 | 63 | 0.9 | 13 | 0.1 | ||
| Linum usitatissimum | 25 | + | 38 | 0.1 | 63 | 0.5 | ||
| Sonchus oleraceus | 25 | + | 13 | + | 50 | 0.1 | ||
| Logfia gallica | 25 | + | 13 | + | 50 | 0.6 | ||
| Cynosurus echinatus | 25 | 0.1 | 13 | + | 25 | + | ||
| Trifolium arvense | 13 | 0.1 | 50 | 0.2 | 13 | + | ||
| Holcus lanatus | 13 | + | 50 | 0.3 | ||||
| Verbascum virgatum | 13 | + | 38 | 0.1 | 50 | 0.1 | ||
| Euphorbia peplus | 13 | 0.1 | 25 | 0.1 | 25 | + | ||
| Geranium robertianum | 13 | + | 13 | + | ||||
| Vicia hirsuta | 13 | + | 13 | + | ||||
| Anthoxanthum odoratum | 13 | + | 50 | 0.5 | ||||
| Carduus pycnocephalus | 13 | + | 38 | + | ||||
| Medicago lupulina | 13 | + | ||||||
| Daucus carota | 38 | 0.3 | ||||||
| Chamaemelum mixtum | 25 | + | 13 | + | ||||
| Tolpis barbata | 25 | + | ||||||
| Arrhenaterum elatius ssp. bulbosus | 25 | 0.2 | ||||||
| Dactylis glomerata | 25 | 0.8 | ||||||
| Gastridium phleoides | 13 | + | 13 | + | ||||
| Erodium cicutarium | 63 | 0.3 | ||||||
| Silybum marianum | 25 | 0.4 | ||||||
| Cerastium fontanum ssp. vulgare | 13 | + | ||||||
| Galium aparine | 13 | + | ||||||
| Sonchus asper | 13 | + | ||||||
| Barbarea verna cf. | 13 | 0.1 | ||||||
| Total species numbers | 22 | 38 | 39 | 44 | ||||
| Native species | ||||||||
| Piptochaetium panicoides | 100 | 1.0 | 75 | 3.4 | 100 | 2.6 | 100 | 4.2 |
| Ugni molinae | 100 | 3.4 | 38 | 0.3 | ||||
| Bomarea salsilla * | 100 | 0.8 | 25 | + | 13 | + | 25 | + |
| Cryptocarya alba * | 100 | 8.6 | 13 | + | 13 | + | 25 | 0.1 |
| Aristotelia chilensis | 88 | 5.7 | 100 | 7.7 | 75 | 4.0 | 63 | 0.3 |
| Gaultheria insana | 88 | 6.4 | 25 | 0.5 | 25 | + | ||
| Azara integrifolia * | 88 | 2.2 | 13 | + | 13 | + | ||
| Gevuina avellana | 88 | 4.6 | ||||||
| Galium hypocarpium | 75 | 0.3 | 88 | 0.3 | 38 | 0.1 | 100 | 0.3 |
| Solenomelus pedunculatus * | 75 | 0.6 | 50 | 0.1 | 13 | + | 13 | + |
| Peumus boldus * | 75 | 4.1 | 38 | 1.0 | 13 | + | ||
| Ribes punctatum | 75 | 0.2 | 25 | 0.1 | ||||
| Lapageria rosea * | 75 | 0.8 | 13 | 0.1 | ||||
| Nothofagus obliqua | 75 | 4.7 | ||||||
| Gamochaeta coarctata | 63 | 0.2 | 75 | 0.2 | 63 | 0.1 | 100 | 0.6 |
| Podanthus ovatifolius * | 63 | 2.4 | 25 | 0.4 | ||||
| Danthonia chilensis var. aureofulva | 50 | + | 50 | 0.1 | 50 | 0.2 | 88 | 0.5 |
| Teucrium bicolor * | 50 | 0.1 | 50 | 0.2 | 25 | + | 63 | 0.1 |
| Baccharis rhomboidalis | 50 | 0.3 | 13 | + | 13 | 0.1 | 25 | 0.1 |
| Alstroemeria ligtu * | 50 | 0.3 | 13 | + | 25 | 0.1 | ||
| Lithraea caustica * | 50 | 3.3 | 13 | 0.1 | 13 | + | ||
| Lardizabala biternata * | 50 | 0.2 | 13 | 0.4 | ||||
| Lomatia hirsuta | 50 | 0.9 | 13 | + | ||||
| Dioscorea bridgesii * | 38 | 0.1 | 75 | 0.3 | 63 | 0.1 | 13 | + |
| Lathyrus magellanicus | 38 | + | 25 | + | 25 | + | ||
| Oxalis arenaria * | 38 | 0.1 | 13 | + | ||||
| Gochnatia foliolosa * | 38 | 0.2 | 13 | 0.1 | ||||
| Colletia hystrix | 38 | + | 13 | + | ||||
| Quillaja saponaria * | 38 | 0.9 | 13 | + | ||||
| Olsynium scirpoideum * | 38 | + | ||||||
| Mutisia spinosa | 38 | + | ||||||
| Oxalis micrantha | 25 | + | 63 | 0.1 | 13 | + | 75 | 3.5 |
| Tristagma bivalve * | 25 | + | 50 | + | 13 | + | ||
| Escallonia pulverulenta * | 25 | 0.6 | 38 | 2.7 | 88 | 1.5 | ||
| Oxalis perdicaria | 25 | + | 38 | + | 13 | + | ||
| Gnaphalium cheiranthifolium | 25 | + | 25 | + | 13 | + | 88 | 0.4 |
| Nassella laevissima | 25 | 0.1 | 25 | + | 13 | + | 63 | 1.9 |
| Pasithaea coerulea | 25 | 0.1 | 25 | 0.1 | ||||
| Alstroemeria revoluta * | 25 | + | 13 | + | 25 | + | ||
| Luma apiculata | 25 | 0.1 | 13 | 0.8 | 13 | 0.1 | 13 | + |
| Relchela panicoides | 25 | 0.1 | 13 | + | 13 | + | ||
| Adiantum chilense | 25 | 0.2 | 13 | 0.1 | 13 | + | ||
| Nothofagus glauca * | 25 | 1.1 | 13 | + | 13 | 0.1 | ||
| Proustia pyrifolia * | 25 | + | ||||||
| Sophora macrocarpa * | 25 | + | ||||||
| Gavilea venosa * | 25 | 0.1 | ||||||
| Gilliesia montana * | 25 | 0.1 | ||||||
| Galium cotinoides * | 25 | 0.1 | ||||||
| Blechnum hastatum | 25 | 0.1 | ||||||
| Lomatia dentata | 25 | 0.1 | ||||||
| Boquila trifoliolata | 25 | 0.1 | ||||||
| Vicia nigricans | 25 | 0.1 | ||||||
| Uncinia phleoides | 25 | 0.1 | ||||||
| Tristerix corymbosus | 25 | 0.2 | ||||||
| Muehlenbeckia hastulata | 13 | + | 63 | 0.2 | 63 | 0.7 | 25 | 0.1 |
| Acaena argentea | 13 | + | 63 | 0.2 | 50 | 0.1 | 38 | 0.5 |
| Stachys gilliesii | 13 | 0.1 | 38 | 0.1 | 25 | + | ||
| Geranium core-core | 13 | + | 13 | + | 25 | + | ||
| Citronella mucronata * | 13 | + | 13 | + | ||||
| Oenothera stricta | 13 | + | 38 | 0.1 | 13 | + | ||
| Conanthera bifolia * | 13 | + | 25 | + | 13 | + | ||
| Cissus striata | 13 | + | 13 | + | 13 | + | ||
| Schinus polygama | 13 | + | 13 | + | ||||
| Chloraea lamellata * | 13 | + | ||||||
| Calandrinia compressa * | 13 | + | ||||||
| Libertia sessiliflora * | 13 | + | ||||||
| Chusquea quila * | 13 | + | ||||||
| Podocarpus salignus * | 13 | 0.1 | ||||||
| Dioscorea humifusa * | 13 | 1.3 | ||||||
| Cynanchum pachyphyllum | 13 | + | ||||||
| Chiropetalum tricuspidatum | 13 | + | ||||||
| Calceolaria corymbosa | 13 | + | ||||||
| Soliva sessilis | 13 | + | ||||||
| Myoschilos oblongum | 13 | 0.1 | ||||||
| Rhaphithamnus spinosus | 13 | 0.1 | ||||||
| Persea lingue | 13 | 0.1 | ||||||
| Carex setifolia | 13 | 0.1 | ||||||
| Baccharis racemosa | 25 | 0.1 | 25 | + | 25 | + | ||
| Sisyrinchium graminifolium | 25 | + | ||||||
| Glandularia laciniata | 13 | + | 13 | + | 13 | + | ||
| Hypericum caespitosum * | 13 | + | 13 | + | ||||
| Embothrium coccineum | 13 | + | ||||||
| Maytenus boaria | 13 | + | ||||||
| Bromus lithobius | 13 | + | ||||||
| Eryngium paniculatum | 13 | 0.1 | ||||||
| Margyricarpus pinnatus | 50 | 0.1 | ||||||
| Chascolytrum subaristatum | 38 | 0.1 | 38 | 0.1 | ||||
| Adesmia spec. | 38 | 0.1 | 25 | 0.1 | ||||
| Lobelia tupa * | 13 | 0.4 | 13 | + | ||||
| Piptochaetium montevidense | 13 | + | 13 | 0.1 | ||||
| Stachys ochroleuca * | 13 | + | ||||||
| Hypochaeris scorzonerae cf. * | 13 | + | ||||||
| Bromus berteroanus | 13 | + | ||||||
| Calceolaria dentata | 25 | + | ||||||
| Baccharis linearis * | 13 | 0.1 | ||||||
| Carex phalaroides | 13 | + | ||||||
| Conyza bonariensis | 13 | + | ||||||
| Solanum crispum | 13 | + | ||||||
| Collomia biflora | 13 | + | ||||||
| Total species numbers | 77 | 46 | 40 | 45 | ||||

References
- Sala, O.E.; Chapin, F.S., III; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Börger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global effects of land use on local terrestrial biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keenan, R.J.; Reams, G.A.; Achard, F.; De Freitas, J.V.; Grainger, A.; Lindquist, E. Dynamics of global forest area: Results from the FAO Global Forest Resources Assessment 2015. For. Ecol. Manag. 2015, 352, 9–20. [Google Scholar] [CrossRef]
- Paritsis, J.; Aizen, M.A. Effects of exotic conifer plantations on biodiversity of understory plants, epigeal beetles and birds in Nothofagus dombeyi forests. For. Ecol. Manag. 2008, 255, 1575–1583. [Google Scholar] [CrossRef]
- Bremer, L.L.; Farley, K.A. Does plantation forestry restore biodiversity or create green deserts? A synthesis of the effects of land-use transitions on plant species richness. Biodivers. Conserv. 2010, 19, 3893–3915. [Google Scholar] [CrossRef] [Green Version]
- Orellana, I.A.; Raffaele, E. Effect of Pseudotsuga Menziesii Plantations on Vascular Plants Diversity in Northwest Patagonia, Argentina. Available online: https://www.intechopen.com/books/biodiversity-enrichment-in-a-diverse-world/effect-of-pseudotsuga-menziesii-plantations-on-vascular-plants-diversity-in-northwest-patagonia-arge (accessed on 29 August 2012).
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- San Martín, J.; Donoso, C. Estructura florística e impacto antrópico en el bosque Maulino de Chile. In Ecología de los Bosques Nativos de Chile; Armesto, J.J., Villagrán, C., Arroyo, M.K., Eds.; Editorial Universitaria: Santiago, Chile, 1995; pp. 153–168. [Google Scholar]
- Litton, C.; Santelices, R. Comparación de las comunidades vegetales en bosques Nothofagus glauca (Phil.) Krasser en la Séptima Región de Chile. Bosque 1996, 17, 77–86. [Google Scholar] [CrossRef]
- Muñoz, F.; Muñoz, C.; Uribe, M.; Martín, M.A.; Molina, J.R.; Herrera, M.Á.; Álvarez, J.B.; Martín, L.M. Composición, estructura y diversidad de poblaciones de Nothofagus glauca ubicadas en la zona mediterránea de Chile. Gayana Bot. 2013, 70, 82–91. [Google Scholar] [CrossRef]
- Camus, G.P. Ambiente, Bosques y Gestión Forestal en Chile 1541–2005; Centro de Investigaciones Diego Barros Arana: Santiago, Chile, 2006; ISBN 956-244-179-2. [Google Scholar]
- Echeverría, C.; Coomes, D.; Salas, J.; Rey-Benayas, J.M.; Lara, A.; Newton, A. Rapid deforestation and fragmentation of Chilean Temperate Forests. Biol. Conserv. 2006, 130, 481–494. [Google Scholar] [CrossRef]
- Niklitschek, M.E. Trade liberalization and land use changes: Explaining the expansion of afforested land in Chile. For. Sci. 2007, 53, 385–394. [Google Scholar]
- Heilmayr, R.; Echeverría, C.; Fuentes, R.; Lambin, E.F. A plantation-dominated forest transition in Chile. Appl. Geogr. 2016, 75, 71–82. [Google Scholar] [CrossRef]
- Salas, C.; Donoso, P.J.; Vargas, R.; Arriagada, C.A.; Pedraza, R.; Soto, D.P. The forest sector in Chile: An overview and current challenges. J. For. 2016, 114, 562–571. [Google Scholar] [CrossRef]
- Peralta, G.; Frost, C.M.; Didham, R.K. Plant, herbivore and parasitoid community composition in native Nothofagaceae forests vs. exotic pine plantations. J. Appl. Ecol. 2018, 55, 1265–1275. [Google Scholar] [CrossRef]
- Vergara, P.M.; Simonetti, J.A. Abundance and movement of understory birds in a maulino forest fragmented by pine plantations. Biodivers. Conserv. 2006, 15, 3937–3947. [Google Scholar] [CrossRef]
- Simonetti, J.A.; Grez, A.A.; Estades, C.F. Providing habitat for native mammals through understory enhancement in forestry plantations. Conserv. Biol. 2013, 27, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Cerda, Y.; Grez, A.A.; Simonetti, J.A. The role of the understory on the abundance, movement and survival of Ceroglossus chilensis in pine plantations: An experimental test. J. Insect Conserv. 2015, 19, 119–127. [Google Scholar] [CrossRef]
- Poch, T.J.; Simonetti, J.A. Insectivory in Pinus radiata plantations with different degree of structural complexity. For. Ecol. Manag. 2013, 304, 132–136. [Google Scholar] [CrossRef]
- Wimberly, M.C. Species dynamics in disturbed landscapes: When does a shifting habitat mosaic enhance connectivity? Landsc. Ecol. 2006, 21, 35–46. [Google Scholar] [CrossRef]
- Harrison, S.; Hohn, C.; Ratay, S. Distribution of exotic plants along roads in a peninsular nature reserve. Biol. Invasions 2002, 4, 425–430. [Google Scholar] [CrossRef]
- Watkins, R.Z.; Chen, J.; Pickens, J.; Brosofske, K.D. Effects of forest roads on understorey plants in a managed hardwood landscape. Conserv. Biol. 2003, 17, 411–419. [Google Scholar] [CrossRef]
- Gelbard, J.L.; Belnap, J. Roads as conduits for exotic plant invasions in a semiarid landscape. Conserv. Biol. 2003, 17, 420–432. [Google Scholar] [CrossRef]
- Christen, D.; Matlack, G. The role of roadsides in plant invasions: A demographic approach. Conserv. Biol. 2006, 20, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.; Finckh, M. Impactos de las plantaciones de pino oregón sobre la vegetación y el suelo en la zona centro-sur de Chile. Rev. Chil. Hist. Nat. 1997, 70, 191–211. [Google Scholar]
- Becerra, P.I.; Simonetti, J.A. Patterns of exotic species richness of different taxonomic groups in a fragmented landscape of central Chile. Bosque 2013, 34, 45–51. [Google Scholar] [CrossRef]
- Suárez-Esteban, A.; Delibes, M.; Fedriani, J.M. Barriers or corridors? The overlooked role of unpaved roads in endozoochorous seed dispersal. J. Appl. Ecol. 2013, 50, 767–774. [Google Scholar] [CrossRef] [Green Version]
- Rost, J.; Pons, P.; Bas, J.M. Seed dispersal by carnivorous mammals into burnt forests: An opportunity for non-indigenous and cultivated plant species. Basic Appl. Ecol. 2012, 13, 623–630. [Google Scholar] [CrossRef]
- Damschen, E.I.; Haddad, N.M.; Orrock, J.L.; Tewksbury, J.J.; Levey, D.J. Corridors increase plant species richness at large scales. Science 2006, 313, 1284–1286. [Google Scholar] [CrossRef] [PubMed]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Estades, C.F.; Escobar, M.A. Los ecosistemas de las plantaciones de pino de la Cordillera de la Costa. In Historia, Biodiversidad y Ecología de los Bosques Costeros de Chile; Smith-Ramírez, C., Armesto, J.J., Valdovinos, C., Eds.; Editorial Universitaria: Santiago, Chile, 2005; pp. 600–616. ISBN 956-11-1777-0. [Google Scholar]
- Onaindia, M.; Mitxelena, A. Potential use of pine plantations to restore native forests in a highly fragmented river basin. Ann. For. Sci. 2009, 66, 13–37. [Google Scholar] [CrossRef]
- Lust, N.; Kongs, T.; Nachtergale, L.; De Keersmaeker, L. Spontaneous ingrowth of tree species in poplar plantations in Flanders. Ann. For. Sci. 2001, 58, 861–868. [Google Scholar] [CrossRef] [Green Version]
- Onaindia, M.; Ametzaga-Arregi, I.; San Sebastián, M.; Mitxelena, A.; Rodríguez-Loinaz, G.; Peña, L.; Alday, J.G. Can understorey native woodland plant species regenerate under exotic pine plantations using natural succession? For. Ecol. Manag. 2013, 308, 136–144. [Google Scholar] [CrossRef]
- McFadden, T.N.; Dirzo, R. Opening the silvicultural toolbox: A new framework for conserving biodiversity in Chilean timber plantations. For. Ecol. Manag. 2018, 425, 75–84. [Google Scholar] [CrossRef]
- Foster, D.; Swanson, F.; Aber, J.; Burke, I.; Brokaw, N.; Tilman, D.; Knapp, A. The importance of land-use legacies to ecology and conservation. BioScience 2003, 53, 77–88. [Google Scholar] [CrossRef]
- Gómez, P.; Hahn, S.; San Martín, J. Estructura y composición florística de un matorral bajo plantaciones de Pinus radiata D.Don en Chile central. Gayana Bot. 2009, 66, 256–268. [Google Scholar] [CrossRef]
- Simonetti, J.A. Conservación de la biodiversidad en ambientes fragmentados: El caso del bosque maulino. In Biodiversidad en Ambientes Fragmentados de Chile: Patrones y Procesos a Diferentes Escalas; Grez, A.A., Simonetti, J.A., Bustamante, R.O., Eds.; Editorial Universitaria: Santiago, Chile, 2006; pp. 215–231. ISBN 9789561119123. [Google Scholar]
- Cubbage, F.; Diaz, D.; Yapura, P.; Dube, F. Impacts of forest management certification in Argentina and Chile. For. Policy Econ. 2010, 12, 497–504. [Google Scholar] [CrossRef]
- Lara, A.; Little, C.; Urrutia, R.; McPhee, J.; Álvarez-Garretón, C.; Oyarzún, C.; Soto, D.; Donoso, P.; Nahuelhual, L.; Pino, M.; Arismendi, I. Assessment of ecosystem services as an opportunity for the conservation management of native forests in Chile. For. Ecol. Manag. 2009, 258, 415–424. [Google Scholar] [CrossRef]
- Agromet. Red Agrometereológica de INIA. Available online: http://agromet.inia.cl/estaciones.php (accessed on 5 July 2018).
- Casanova, M.; Salazar, O.; Seguel, O.; Luzio, W. The soils of Chile; Springer: Dodrecht, The Netherlands, 2013; ISBN 978-94-007-5949-7. [Google Scholar]
- Mead, D.J. Sustainable Management of Pinus radiata Plantations; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013; ISBN 9789251076347. [Google Scholar]
- Gardner, M.F.; Hechenleitner, P.; Thomas, P.I.; Echeverria, C.; Escobar, B.; Brownless, P.; Martinez, A.C. Threatened Plants of Central and South Chile: Distribution, Conservation and Propagation, 1st ed.; Universidad Austral de Chile: Valdivia, Chile; Royal Botanic Garden: Edinburgh, UK, 2006; pp. 1–188. ISBN 1 872291 25 2. [Google Scholar]
- Guerrero, P.C.; Bustamante, R.O. Abiotic alterations caused by forest fragmentation affect tree regeneration: A shade and drought tolerance gradient in the remnants of Coastal Maulino Forest. Rev. Chil. Hist. Nat. 2009, 82, 413–424. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensoziologie: Grundzüge der Vegetationskunde, 2nd ed.; Springer: Wien, Austria, 1964; ISBN 978-3-7091-8110-2. [Google Scholar]
- Zuloaga, F.O.; Morrone, O.; Belgrano, M. Catálogo de las Plantas Vasculares del Cono Sur: Argentina, Sur de Brasil, Chile, Paraguay y Uruguay; Missouri Botanical Garden Press: St. Louis, MS, USA, 2008; Volume 107, ISBN-13: 9781930723702. [Google Scholar]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef] [Green Version]
- Baselga, A. The relationship between species replacement, dissimilarity derived from nestedness and nestedness. Glob. Ecol. Biogeogr. 2012, 21, 1223–1232. [Google Scholar] [CrossRef]
- Horn, H.S. Measurement of “Overlap” in comparative ecological studies. Am. Nat. 1966, 100, 419–424. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OH, USA, 2002; ISBN 0972129006. [Google Scholar]
- Luebert, F.; Pliscoff, P. Sinopsis Bioclimática y Vegetacional de Chile; Editorial Universitaria: Santiago, Chile, 2006; ISBN 956-11-1832-7. [Google Scholar]
- Matthei, O. Manual de las Malezas Que Crecen en Chile; Alfabeta Impresores: Santiago, Chile, 1995; ISBN 956-272-214-7. [Google Scholar]
- Fuentes, N.; Sánchez, P.; Pauchard, A.; Urrutia, J.; Cavieres, L.; Marticorena, A. Plantas Invasoras del Centro-Sur de Chile: Una Guía de Campo; Laboratorio de Invasiones Biológicas (LIB): Concepción, Chile, 2014; ISBN 978-956-358-031-0. [Google Scholar]
- Fischer, M.; Stöcklin, J. Local extinctions of plants in remnants of extensively used calcareous grasslands 1950–1985. Conserv. Biol. 1997, 11, 727–737. [Google Scholar] [CrossRef]
- Vellend, M.; Verheyen, K.; Jacquemyn, H.; Kolb, A.; Van Calster, H.; Peterken, G.; Hermy, M. Extinction debt of forest plants persists for more than a century following habitat fragmentation. Ecology 2006, 87, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.T.; Sax, D.F. Balancing biodiversity in a changing environment: Extinction debt, immigration credit and species turnover. Trends Ecol. Evol. 2010, 25, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; May, R.M.; Lehman, C.L.; Nowak, M.A. Habitat destruction and the extinction debt. Nature 1994, 371, 65–66. [Google Scholar] [CrossRef]
- Kuussaari, M.; Bommarco, R.; Heikkinen, R.K.; Helm, A.; Krauss, J.; Lindborg, R.; Öckinger, E.; Pärtel, M.; Pino, J.; Rodà, F.; et al. Extinction debt: A challenge for biodiversity conservation. Trends Ecol. Evol. 2009, 24, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Frank, D. Umweltauswirkungen des Landnutzungswandels in der IX. Region Chiles: Untersuchung von Waldökosystemen und forstlichen Monokulturen am Beispiel der Umgebung Temucos; Mensch&Buch Verlag: Berlin, Germany, 1998; ISBN 3-933346-42-8. [Google Scholar]
- Heinrichs, S.; Pauchard, A. Struggling to maintain native plant diversity in a peri-urban reserve surrounded by a highly anthropogenic matrix. Biodivers. Conserv. 2015, 24, 2769–2788. [Google Scholar] [CrossRef]
- García, V.; Simonetti, J.; Becerra, P. Llluvia de semillas, depredación de semillas y germinación de especies nativas en plantaciones de Pinus radiata en Chile centro-sur: Efecto de la distancia a bosque native y presencia de sotobosque. Bosque 2016, 37, 359–367. [Google Scholar] [CrossRef]
- Frank, D.; Finckh, M.; Wirth, C. Impacts of land use on habitat functions of old-growth forests and their biodiversity. In Old-Growth Forests: Function, Fate and Value; Wirth, C., Gleixner, G., Heimann, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 207, pp. 429–450. ISBN 978-3-540-92706-8. [Google Scholar]
- Valdivia, C.E.; Simonetti, J.A.; Henríquez, C.A. Depressed pollination of Lapageria rosea Ruiz et. Pav. (Philesiaceae) in the fragmented temperate rainforest of southern South America. Biodivers. Conserv. 2006, 15, 1845–1856. [Google Scholar] [CrossRef]
- Lusk, C.H.; Chazdon, R.L.; Hofmann, G. Abounded null model explains juvenile tree community structure along light availability gradients in a temperate rain forest. Oikos 2006, 112, 131–137. [Google Scholar] [CrossRef]
- Bergès, L.; Chevalier, R.; Avon, C. Influence of forest road, road-surfacing material and stand age on floristic diversity and composition in a nutrient-poor environment. Appl. Veg. Sci. 2013, 16, 470–479. [Google Scholar] [CrossRef]
- González, M.E.; Veblen, T.T.; Donoso, C.; Valeria, L. Tree regeneration responses in a lowland Nothofagus-dominated forest after bamboo dieback in South-Central Chile. Plant Ecol. 2002, 161, 59–73. [Google Scholar] [CrossRef]
- Piper, F.I. Patterns of carbon storage in relation to shade tolerance in southern South American species. Am. J. Bot. 2015, 102, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Fernández, Q.T.; Alquicira-Artega, M.L.; Flores-Palacios, A. Is orchid species richness and abundance related to the conservation status of oak forest? Plant Ecol. 2011, 212, 1091–1099. [Google Scholar] [CrossRef]
- Sánchez, E.P.; Armenteras, D.; Retana, J. Edge influence on diversity of orchids in Andean cloud forests. Forests 2016, 7, 63. [Google Scholar] [CrossRef]
- Peterken, G.G.; Francis, J.L. Open spaces as habitats for vascular ground flora species in the woods of central Lincolnshire, UK. Biol. Conserv. 1999, 91, 55–72. [Google Scholar] [CrossRef]
- García, R.A.; Pauchard, A.; Cavieres, L.A.; Peña, E.; Rodriguez, M.F. El fuego favorece la invasión de Teline monspessulana (Fabacea) al aumentar su germinación. Rev. Chil. Hist. Nat. 2010, 83, 443–452. [Google Scholar] [CrossRef]
- Gómez, P.; Bustamante, R.O.; San Martín, J. Estructura poblacional de Teline monspessulana (L.) K. Koch en fragmentos de bosque maulino en Chile central. Gayana Bot. 2012, 69, 197–200. [Google Scholar] [CrossRef]
- Tilman, D. Community invasibility, recruitment limitation, and grassland biodiversity. Ecology 1997, 78, 81–92. [Google Scholar] [CrossRef]
- Arévalo, J.R.; Otto, R.; Escudero, C.; Fernández-Lugo, S.; Arteaga, M.; Delgado, J.D.; Fernández-Palacios, J.M. Do anthropogenic corridors homogenize plant communities at a local scale? A case studied in Tenerife (Canary Islands). Plant Ecol. 2010, 209, 23–35. [Google Scholar] [CrossRef]
- Badalamenti, E.; da Silveira Bueno, R.; Campo, O.; Gallo, M.; La Mela Veca, D.S.; Pasta, S.; Sala, G.; La mantia, T. Pine stand density influences the regeneration of Acacia saligna Labill. H. L. Wendl. and native woody species in a Mediterranean coastal pine plantation. Forests 2018, 9, 359. [Google Scholar] [CrossRef]
- Puettmann, K.J.; Wilson, S.M.; Baker, S.C.; Donoso, P.J.; Drössler, L.; Amente, G.; Harvey, B.D.; Knoke, T.; Lu, Y.; Nocentini, S.; et al. Silvicultural alternatives to conventional even-aged forest management—What limits global adoption? For. Ecosyst. 2015, 2, 8. [Google Scholar] [CrossRef]
- Bustamante, R.O.; Simonetti, J.A.; Grez, A.A.; San Martín, J. Fragmentación y dinámica de regeneración del bosque maulino: Diagnóstico acutal y perspectivas futuras. In Historia, Biodiversidas y Ecología de los Bosques Costeros de Chile; Smith-Ramírez, C., Armesto, J.J., Valdovinos, C., Eds.; Editorial Universitaria: Santiago, Chile, 2005; pp. 555–564. ISBN 956-11-1777-0. [Google Scholar]

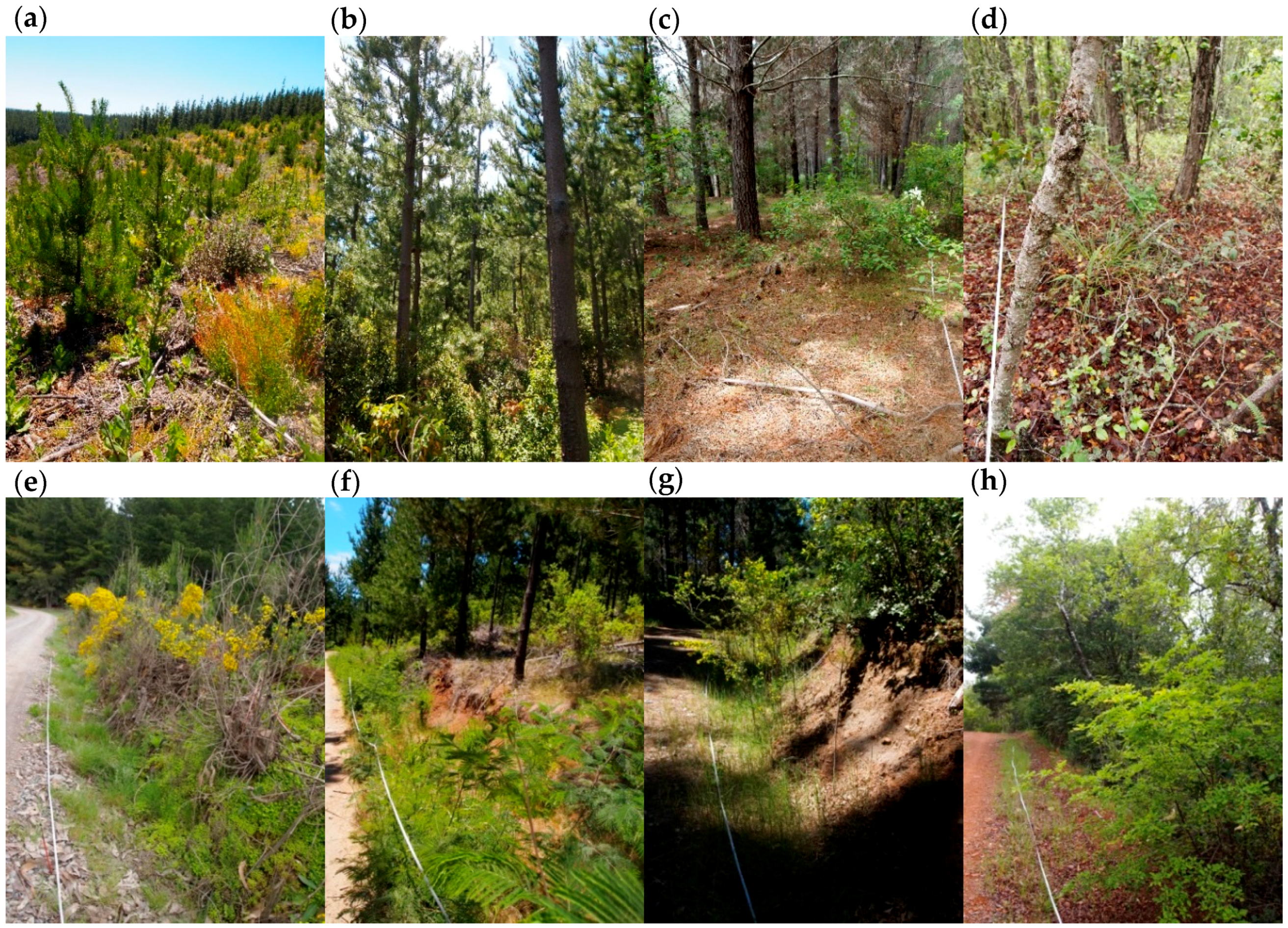

| Forest Interior | Roadside | |||||||
|---|---|---|---|---|---|---|---|---|
| PY | PM | PO | Nat | PY | PM | PO | Nat | |
| Structure | ||||||||
| Cover tree layer (%) | 2.9 (8.1) a | 57.6 (15.1) b | 61.3 (12.1) b | 78.6 (7.6) c | - a | 12.6 (12.6) ab | 28.6 (19.9) b | 48.6 (20.8) c |
| Cover shrub layer (%) | 22.9 (18.4) a | 19.3 (19.7) a | 31.3 (11.2) a | 59.1 (16.8) b | 10.2 (16.2) a | 21.2 (19.8) a | 25.8 (14.8) a | 46.8 (10.0) b |
| Cover herb layer (%) | 32.3 (11.1) ab | 39.5 (19.4) a | 17.6 (12.8) b | 21.1 (10.4) b | 44.1 (13.9) a | 47.3 (25.8) a | 36.7 (14.4) a | 14.7 (8.4) b |
| Environment | ||||||||
| Light (%) | 84.6 (11.5) a | 16.9 (7.3) b | 12.7 (5.7) b | 8.9 (3.9) b | 82.3 (10.1) a | 50.9 (9.4) b | 29.9 (14.9) c | 27.6 (18.6) c |
| Litter (cm) | 0.2 (0.2) a | 2.4 (1.0) b | 3.6 (0.5) b | 6.3 (2.5) d | 0.3 (0.4) a | 0.3 (0.3) a | 1.1 (0.6) a | 2.5 (1.4) b |
| pH | 5.3 (0.2) | 5.4 (0.1) | 5.2 (0.2) | 5.2 (0.3) | 5.3 (0.1) | 5.2 (0.2) | 5.2 (0.1) | 5.4 (0.3) |
| C/N | 23.1 (9.5) | 22.6 (3.2) | 27.1 (8.5) | 24.3 (2.8) | 16.0 (5.4) | 13.7 (3.8) | 14.6 (4.2) | 15.3 (4.6) |
| Cluster | ISA1 | ISA2 | Origin | LC | GF | Forest | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pl. vs. Nat | Pl Age Classes | |||||||||
| IV | p | IV | p | |||||||
| Natural forest interior | ||||||||||
| Ugni molinae | Nat (I)→PO (I) | 0.840 | 0.001 | → | 0.454 | 0.007 | N | w | s | 1 |
| Gaultheria insana | Nat (I) | 0.770 | 0.001 | N | w | s | 3 | |||
| Relchela panicoides | Nat (I)→PO (I) | 0.768 | 0.001 | → | 0.403 | 0.009 | N | p | g | 1 |
| Lapageria rosea | Nat (I) | 0.658 | 0.001 | E | p | cl | 1 | |||
| Lomatia hirsuta | Nat (I) | 0.614 | 0.001 | N | w | t | 1 | |||
| Uncinia phleoides | Nat (I) | 0.611 | 0.001 | N | p | g | 1 | |||
| Aextoxicon punctatum | Nat (I) | 0.546 | 0.001 | N | w | t | 1 | |||
| Lithraea caustica | Nat (I) | 0.535 | 0.001 | E | w | t | 2 | |||
| Bomarea salsilla | Nat (I) | 0.512 | 0.009 | E | p | h | 1 | |||
| Galium cotinoides | Nat (I) | 0.485 | 0.001 | E | p | h | nd | |||
| Azara integrifolia | Nat (I)→PO (I) | 0.478 | 0.006 | → | 0.679 | 0.001 | E | w | t | 1 |
| Ribes punctatum | Nat (I)→PM (I) | 0.463 | 0.009 | → | 0.313 | 0.021 | N | w | s | 1 |
| Sophora macrocarpa | Nat (I) | 0.434 | 0.003 | E | w | t | 1 | |||
| Citronella mucronata | Nat (I) | 0.408 | 0.002 | E | w | t | 1 | |||
| Colletia hystrix | Nat (I) | 0.399 | 0.005 | N | w | s | 1 | |||
| Nothofagus glauca | Nat (I)→PO (I) | 0.354 | 0.024 | → | 0.309 | 0.038 | E | w | t | 1 |
| Podanthus ovatifolius | Nat (I) | 0.337 | 0.044 | E | w | s | 3 | |||
| Vicia nigricans | Nat (I) | 0.305 | 0.013 | N | p | h | 1 | |||
| Olsynium scirpoideum | Nat (I) | 0.290 | 0.025 | E | p | h | 3 | |||
| Cynanchum pachyphyllum | Nat (I) | 0.288 | 0.016 | N | p | h | 1 | |||
| Blechnum hastatum | Nat (I) | 0.286 | 0.010 | N | p | f | 1 | |||
| Senna stipulacea | Nat (I) | 0.273 | 0.007 | E | w | s | 1 | |||
| Gilliesia montana | Nat (I) | 0.231 | 0.022 | E | p | h | nd | |||
| Elytropus chilensis | Nat (I) | 0.182 | 0.031 | N | p | cl | 1 | |||
| Synammia feuillei | Nat (I) | 0.182 | 0.037 | N | p | f | 1 | |||
| Sanicula crassicaulis | Nat (I) | 0.182 | 0.040 | N | p | h | 1 | |||
| Viola portalesia | Nat (I) | 0.182 | 0.035 | E | w | s | 1 | |||
| Nothofagus obliqua | Nat (R) | 0.730 | 0.001 | N | w | t | 1 | |||
| Cryptocarya alba | Nat (R) | 0.571 | 0.001 | E | w | t | 2 | |||
| Solenomelus pedunculatus | Nat (R) | 0.490 | 0.002 | E | p | h | 2 | |||
| Gevuina avellana | Nat (R) | 0.418 | 0.008 | N | w | t | 1 | |||
| Alstroemeria ligtu | Nat (R) | 0.340 | 0.016 | E | p | h | 2 | |||
| Oxalis arenaria | Nat (R) | 0.258 | 0.028 | E | p | h | nd | |||
| Tristerix corymbosus | Nat (R) | 0.206 | 0.036 | N | w | s | 2 | |||
| Plantation interior | ||||||||||
| Lactuca serriola | PY (I) | 0.779 | 0.001 | A | s | h | 3 | |||
| Geranium robertianum | PY (I) | 0.612 | 0.001 | A | s | h | 2 | |||
| Galium hypocarpium | P (I)→PY (I) | 0.472 | 0.035 | → | 0.559 | 0.001 | N | p | h | 1 |
| Pinus radiata | PY (I) | 0.538 | 0.008 | A | w | t | nd | |||
| Calandrinia compressa | PY (I) | 0.500 | 0.003 | E | s | h | 3 | |||
| Gnaphalium cheiranthifolium | PY (I) | 0.438 | 0.016 | N | p | h | 3 | |||
| Geranium core-core | PY (I) | 0.427 | 0.005 | N | p | h | 3 | |||
| Lardizabala biternata | PY (I) | 0.363 | 0.035 | E | w | cl | 1 | |||
| Galium aparine | PY (I) | 0.360 | 0.040 | A | s | h | 3 | |||
| Cirsium vulgare | PY (I) | 0.356 | 0.025 | A | s | h | 3 | |||
| Sonchus oleraceus | PY (I) | 0.285 | 0.047 | A | s | h | 3 | |||
| Piptochaetium panicoides | P (I)→PM (I) | 0.695 | 0.001 | → | 0.537 | 0.001 | N | p | g | nd |
| Muehlenbeckia hastulata | P (I) →PM (I) | 0.687 | 0.001 | → | 0.450 | 0.011 | N | w | s | 2 |
| Quillaja saponaria | PO (I) | 0.496 | 0.001 | E | w | t | 2 | |||
| Dioscorea bridgesii | P (I)→PO (I) | 0.439 | 0.007 | → | 0.438 | 0.003 | E | p | cl | nd |
| Plantation Road | ||||||||||
| Hypochaeris radicata | P (R) | 0.737 | 0.001 | A | p | h | 3 | |||
| Sanguisorba minor | P (R) | 0.670 | 0.001 | A | p | h | 3 | |||
| Vulpia bromoides | P (R) | 0.546 | 0.005 | A | s | g | 3 | |||
| Aira caryophyllea | P (R) | 0.346 | 0.029 | A | s | g | 3 | |||
| Rosa rubiginosa | P (R) | 0.288 | 0.037 | A | w | s | 3 | |||
| Rumex acetosella | P (R)→PY (I) | 0.405 | 0.045 | → | 0.397 | 0.044 | A | p | h | 3 |
| Plantago lanceolata | P (R)→PY (R) | 0.931 | 0.001 | → | 0.560 | 0.002 | A | p | h | 2 |
| Linum usitatissimum | P (R)→PY (R) | 0.409 | 0.017 | → | 0.492 | 0.011 | A | s | h | 3 |
| Logfia gallica | P (R)→PY (R) | 0.291 | 0.027 | → | 0.474 | 0.005 | A | s | h | 3 |
| Erodium cicutarium | PY (R) | 0.459 | 0.002 | A | s | h | 3 | |||
| Oxalis micrantha | PY (R) | 0.409 | 0.048 | N | s | h | 3 | |||
| Gamochaeta coarctata | P (R)→PY (R) | 0.341 | 0.036 | → | 0.399 | 0.001 | N | s | h | 3 |
| Senecio sylvaticus | PY (R) | 0.352 | 0.030 | A | s | h | 3 | |||
| Acacia dealbata | P (R)→PM(R) | 0.282 | 0.029 | → | 0.572 | 0.001 | A | w | s | 3 |
| Bromus hordeaceus | P (R)→PM(R) | 0.326 | 0.007 | → | 0.489 | 0.001 | A | s | g | 3 |
| Holcus lanatus | PM (R) | 0.450 | 0.005 | A | s | g | 3 | |||
| Hypericum perforatum | P (R)→PM(R) | 0.704 | 0.001 | → | 0.427 | 0.003 | A | p | h | 3 |
| Margyricarpus pinnatus | PM (R) | 0.389 | 0.021 | N | w | s | nd | |||
| Agrostis capillaris | P (R)→PM(R) | 0.668 | 0.001 | → | 0.369 | 0.011 | A | p | g | 3 |
| Daucus carota | PM (R) | 0.326 | 0.037 | A | s | h | 3 | |||
| Trifolium arvense | P (R)→PM(R) | 0.222 | 0.036 | → | 0.279 | 0.029 | A | s | h | 3 |
| Genista monspessulana | P (R)→PO(R) | 0.744 | 0.001 | → | 0.464 | 0.022 | A | w | s | 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heinrichs, S.; Pauchard, A.; Schall, P. Native Plant Diversity and Composition Across a Pinus radiata D.Don Plantation Landscape in South-Central Chile—The Impact of Plantation Age, Logging Roads and Alien Species. Forests 2018, 9, 567. https://doi.org/10.3390/f9090567
Heinrichs S, Pauchard A, Schall P. Native Plant Diversity and Composition Across a Pinus radiata D.Don Plantation Landscape in South-Central Chile—The Impact of Plantation Age, Logging Roads and Alien Species. Forests. 2018; 9(9):567. https://doi.org/10.3390/f9090567
Chicago/Turabian StyleHeinrichs, Steffi, Aníbal Pauchard, and Peter Schall. 2018. "Native Plant Diversity and Composition Across a Pinus radiata D.Don Plantation Landscape in South-Central Chile—The Impact of Plantation Age, Logging Roads and Alien Species" Forests 9, no. 9: 567. https://doi.org/10.3390/f9090567





