Effect of Fertilization on Growth and Mortality of Jack Pine Growing on Poor, Sandy Soils in Michigan, USA: Implications for Sustainable Management
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Experimental Design
2.2. Field Measurements and Data Analysis
2.3. Nutrient Budgets
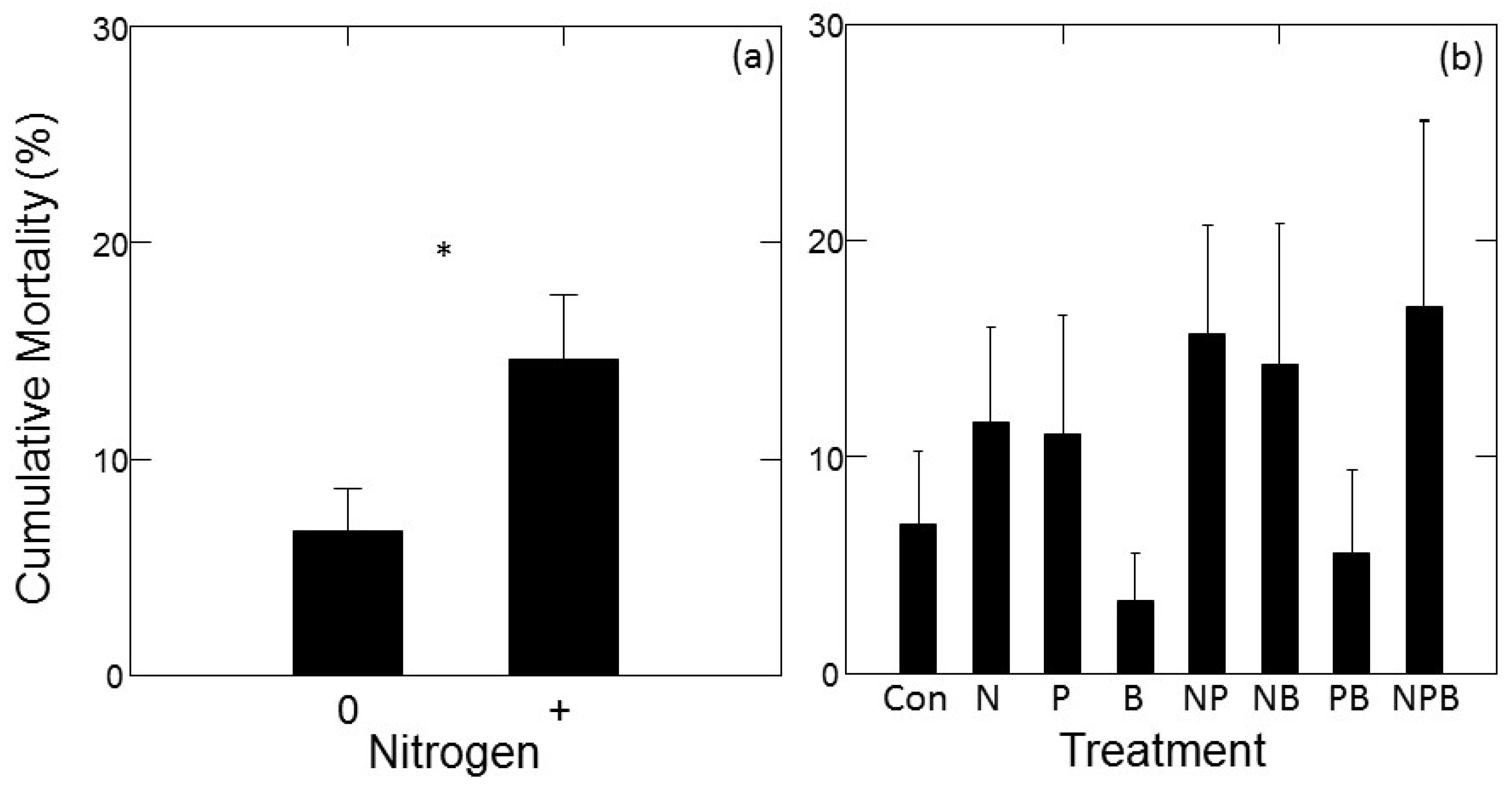
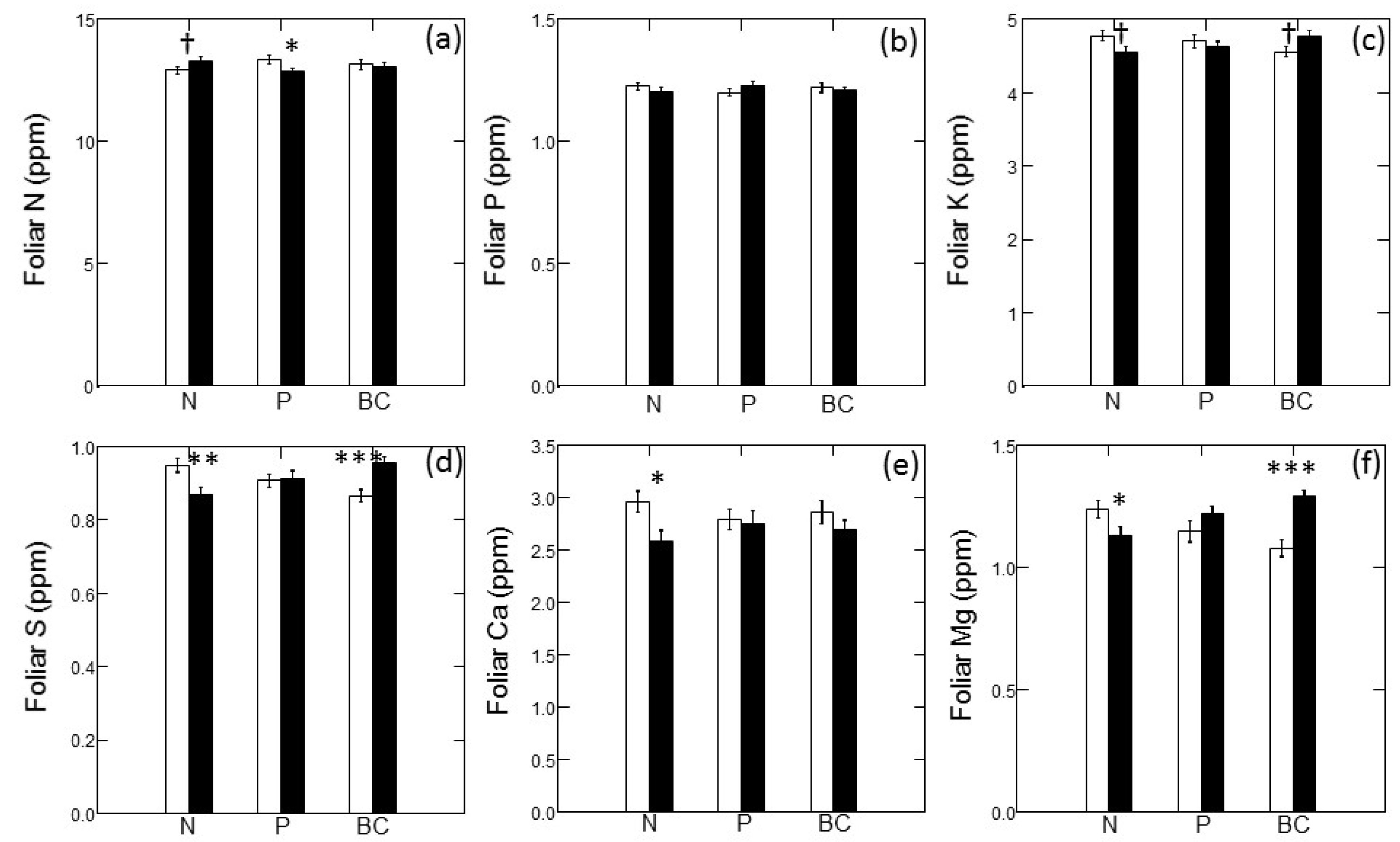
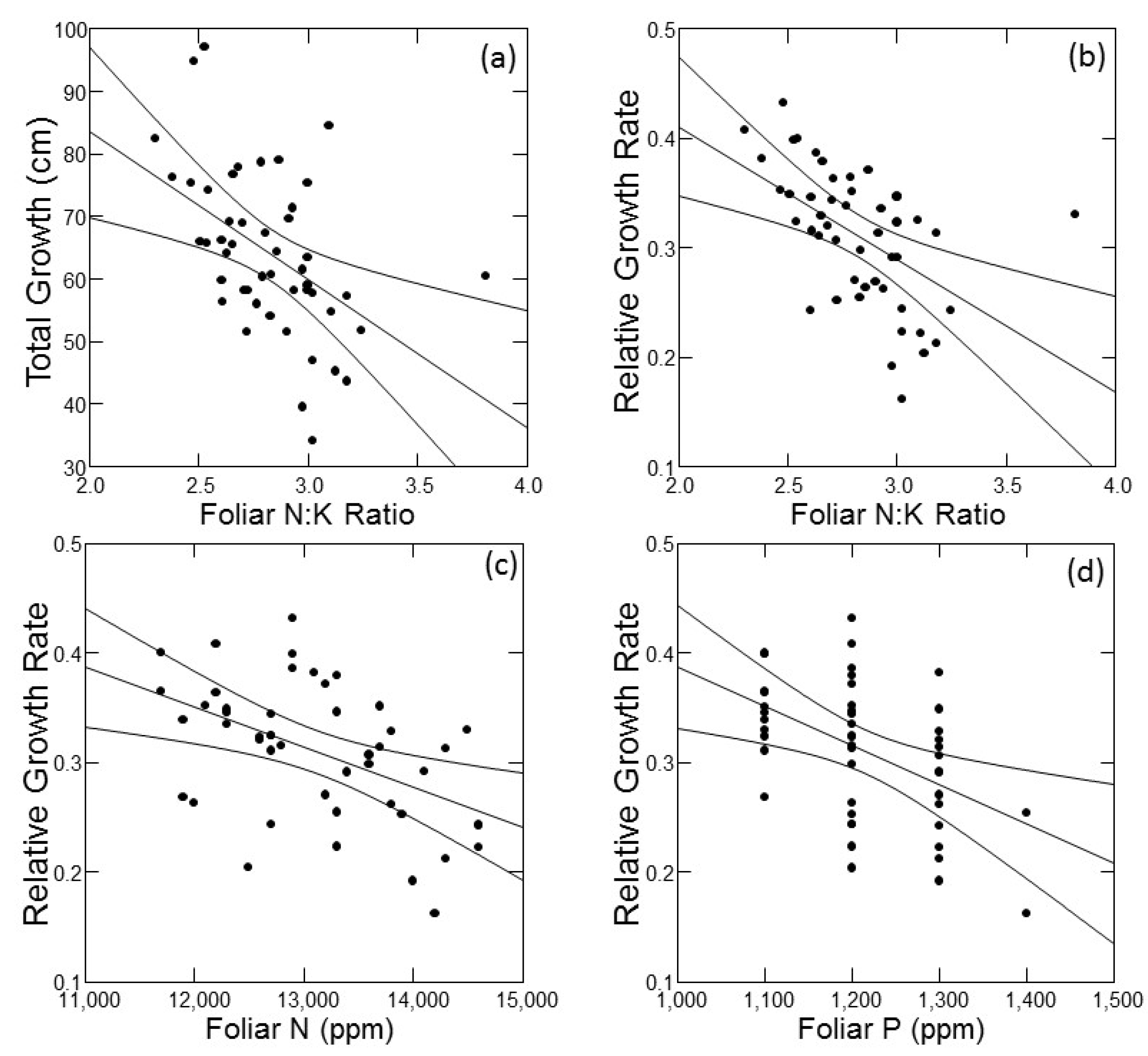
3. Results
3.1. Tree Growth and Mortality
3.2. Foliar Nutrition
3.3. Nutrient Budgets
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- LeBauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Uebbing, L.; de Vries, W. Global-scale impacts of nitrogen deposition on tree carbon sequestration in tropical, temperate, and boreal forests: A meta-analysis. Glob. Chang. Biol. 2018, 24, e416–e431. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen-phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Peñuelas, J. Potassium: A neglected nutrient in global change. Glob. Ecol. Biogeogr. 2015, 24, 261–275. [Google Scholar] [CrossRef]
- Mann, L.K.; Johnson, D.W.; West, D.C.; Cole, D.W.; Hornbeck, J.W.; Martin, C.W.; Riekerk, H.; Smith, C.T.; Swank, W.T.; Tritton, L.M.; et al. Effects of whole-tree and stem-only clearcutting on postharvest hydrologic losses, nutrient capital, and regrowth. For. Sci. 1988, 34, 412–428. [Google Scholar]
- Hendrickson, O.Q.; Chatarpaul, L.; Burgess, D. Nutrient cycling following whole-tree and conventional harvest in northern mixed forest. Can. J. For. Res. 1989, 19, 725–735. [Google Scholar] [CrossRef]
- Thiffault, E.; Hannam, K.D.; Paré, D.; Titus, B.D.; Hazlett, P.W.; Maynard, D.G.; Brais, S. Effects of forest biomass harvesting on soil productivity in boreal and temperate forests—A review. Environ. Rev. 2011, 19, 278–309. [Google Scholar] [CrossRef]
- Achat, D.L.; Deleuze, C.; Landmann, G.; Pousse, N.; Ranger, J.; Augusto, L. Quantifying consequences of removing harvesting residues on forest soils and tree growth—A meta-analysis. For. Ecol. Manag. 2015, 348, 124–141. [Google Scholar] [CrossRef]
- Adams, P.W.; Boyle, J.R. Soil fertility changes following clearcut and whole-tree harvesting and burning in central michigan 1. Soil Sci. Soc. Am. J. 1982, 46, 638–640. [Google Scholar] [CrossRef]
- Silkworth, D.R.; Grigal, D.F. Determining and evaluating nutrient losses following whole-tree harvesting of aspen 1. Soil Sci. Soc. Am. J. 1982, 46, 626–631. [Google Scholar] [CrossRef]
- Herrick, S.; Kovach, J.; Padley, E.; Wagner, C.; Zastrow, D. Wisconsin’s Forestland Woody Biomass Harvesting Guidelines; WI DNR Division of Forestry and Wisconsin Council on Forestry: Madison, WI, USA, 2009.
- Michigan Department of Natural Resources and Environment Forest Management Division. Michigan Woody Biomass Harvesting Guidance. 2010. Available online: https://www.michigan.gov/documents/dnr/WGBH_321271_7.pdf (accessed on 9 July 2018).
- Newton, P.F.; Amponsah, I.G. Systematic review of short-term growth responses of semi-mature black spruce and jack pine stands to nitrogen-based fertilization treatments. For. Ecol. Manag. 2006, 237, 1–14. [Google Scholar] [CrossRef]
- Vitousek, P.M. Beyond global warming: Ecology and global change. Ecology 1994, 75, 1861–1876. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Magnani, F.; Mencuccini, M.; Borghetti, M.; Berbigier, P.; Berninger, F.; Delzon, S.; Grelle, A.; Hari, P.; Jarvis, P.G.; Kolari, P.; et al. The human footprint in the carbon cycle of temperate and boreal forests. Nature 2007, 447, 849. [Google Scholar] [CrossRef] [PubMed]
- Solberg, S.; Dobbertin, M.; Reinds, G.J.; Lange, H.; Andreassen, K.; Fernandez, P.G.; Hildingsson, A.; de Vries, W. Analyses of the impact of changes in atmospheric deposition and climate on forest growth in European monitoring plots: A stand growth approach. For. Ecol. Manag. 2009, 258, 1735–1750. [Google Scholar] [CrossRef]
- Thomas, R.Q.; Canham, C.D.; Weathers, K.C.; Goodale, C.L. Increased tree carbon storage in response to nitrogen deposition in the US. Nat. Geosci. 2010, 3, 13. [Google Scholar] [CrossRef]
- Nilsen, P. Effect of nitrogen on drought strain and nutrient uptake in Norway spruce (Picea abies (L.) Karst.) trees. Plant Soil 1995, 172, 73–85. [Google Scholar] [CrossRef]
- Weetman, G.F. Canadian fertilization: Some history and basic understandings. In Enhanced Forest Management: Fertilization and Economics; Bamsey, C., Ed.; Clear Lake Ltd.: Edmonton, AB, Canada, 2001; pp. 4–10. [Google Scholar]
- Xia, J.; Wan, S. Global response patterns of terrestrial plant species to nitrogen addition. New Phytol. 2008, 179, 428–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenn, M.E.; Poth, M.A.; Aber, J.D.; Baron, J.S.; Bormann, B.T.; Johnson, D.W.; Lemly, A.D.; McNulty, S.G.; Ryan, D.F.; Stottlemyer, R. Nitrogen excess in North American ecosystems: Predisposing factors, ecosystem responses, and management strategies. Ecol. Appl. 1998, 8, 706–733. [Google Scholar] [CrossRef]
- Linder, S.; Benson, M.L.; Myers, B.J.; Raison, R.J. Canopy dynamics and growth of Pinus radiata.: I. Effects of irrigation and fertilization during a drought. Can. J. For. Res. 1987, 17, 1157–1165. [Google Scholar] [CrossRef]
- Tan, W.; Hogan, G.D. Physiological and morphological responses to nitrogen limitation in jack pine seedlings: potential implications for drought tolerance. New For. 1997, 14, 19–31. [Google Scholar] [CrossRef]
- Lim, H.; Oren, R.; Palmroth, S.; Torngern, P.; Mörling, T.; Näsholm, T.; Lundmark, T.; Leppälammi-Kujansuu, J.; Linder, S. Inter-annual variability of precipitation constrains the production response of boreal Pinus sylvestris to nitrogen fertilization. For. Ecol. Manag. 2015, 348, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Albert, D.A. Regional Landscape Ecosystems of Michigan, Minnesota and Wisconsin: A Working Map and Classification; General Technical Report for North Central Forest Experiment Station: Saint Paul, MN, USA, 1995. [Google Scholar]
- Kepler, C.B.; Irvine, G.W.; DeCapita, M.E.; Weinrich, J. The conservation management of Kirtland’s Warbler Dendroica kirtlandii. Bird Conserv. Int. 1996, 6, 11–22. [Google Scholar] [CrossRef]
- Alban, D.H. Nutrient Accumulation in Planted Red and Jack Pine; North Central Forest Experiment Station: Saint Paul, MN, USA, 1988; p. 6. [Google Scholar]
- NADP 2018. Available online: http://nadp.slh.wisc.edu/committees/tdep/tdepmaps/ (accessed on 18 February 2018).
- Schwede, D.B.; Lear, G.G. A novel hybrid approach for estimating total deposition in the United States. Atmos. Environ. 2014, 92, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Koseva, I.S.; Watmough, S.A.; Aherne, J. Estimating base cation weathering rates in Canadian forest soils using a simple texture-based model. Biogeochemistry 2010, 101, 183–196. [Google Scholar] [CrossRef]
- Soil Survey Staff 2018. Natural Resources Conservation Service, United States Department of Agriculture; Web Soil Survey. Available online: https://websoilsurvey.sc.egov.usda.gov/ (accessed on 18 February 2018).
- Rothstein, D.E.; Spaulding, S.E. Replacement of wildfire by whole-tree harvesting in jack pine forests: Effects on soil fertility and tree nutrition. For. Ecol. Manag. 2010, 260, 1164–1174. [Google Scholar] [CrossRef]
- Newman, E.I. Phosphorus inputs to terrestrial ecosystems. J. Ecol. 1995, 83, 713–726. [Google Scholar] [CrossRef]
- Son, Y. Non-symbiotic nitrogen fixation in forest ecosystems. Ecol. Res. 2001, 16, 183–196. [Google Scholar] [CrossRef]
- McCullough, D.G.; Kulman, H.M. Effects of nitrogen fertilization on young jack pine (Pinus banksiana) and on its suitability as a host for jack pine budworm (Choristoneura pinus pinus) (Lepidoptera: Tortricidae). Can. J. For. Res. 1991, 21, 1447–1458. [Google Scholar] [CrossRef]
- Hember, R.A.; Coops, N.C.; Spittlehouse, D.L. Spatial and Temporal Variability of Potential Evaporation across North American Forests. Hydrology 2017, 4, 5. [Google Scholar] [CrossRef]
- Vangansbeke, P.; de Schrijver, A.; de Frenne, P.; Verstraeten, A.; Gorissen, L.; Verheyen, K. Strong negative impacts of whole tree harvesting in pine stands on poor, sandy soils: A long-term nutrient budget modelling approach. For. Ecol. Manag. 2015, 356, 101–111. [Google Scholar] [CrossRef]
- Tripler, C.E.; Kaushal, S.S.; Likens, G.E.; Todd Walter, M. Patterns in potassium dynamics in forest ecosystems. Ecol. Lett. 2006, 9, 451–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skeffington, R.A.; Wilson, E.J. Excess nitrogen deposition: Issues for consideration. Environ. Pollut. 1988, 54, 159–184. [Google Scholar] [CrossRef]
- Aber, J.D.; Nadelhoffer, K.J.; Steudler, P.; Melillo, J.M. Nitrogen saturation in northern forest ecosystems. BioScience 1989, 39, 378–387. [Google Scholar] [CrossRef]
- Janowiak, M.K.; Iverson, L.R.; Mladenoff, D.J.; Peters, E.; Wythers, K.R.; Xi, W.; Brandt, L.A.; Butler, P.R.; Handler, S.D.; Shannon, P.D.; et al. Forest Ecosystem Vulnerability Assessment and Synthesis for Northern Wisconsin and Western Upper Michigan: A Report from the Northwoods Climate Change Response Framework Project; General Technical Report for Northern Research Station: Newtown Square, PA, USA, 2014. [Google Scholar]


| Absolute Growth (cm) | r | p |
|---|---|---|
| Foliar N (ppm) | −0.425 | 0.118 |
| Foliar P (ppm) | −0.351 | 0.647 |
| Foliar K (ppm) | 0.248 | 1.000 |
| Foliar S (ppm) | −0.055 | 1.000 |
| Foliar Ca (ppm) | 0.071 | 1.000 |
| Foliar Mg(ppm) | 0.131 | 1.000 |
| Foliar N:P Ratio | −0.050 | 1.000 |
| Foliar N:K Ratio | −0.490 | 0.018 |
| Foliar N:S Ratio | −0.190 | 0.585 |
| Foliar N:Ca Ratio | −0.216 | 1.000 |
| Foliar N:Mg Ratio | −0.178 | 1.000 |
| Relative Growth Rate | ||
| Foliar N (ppm) | −0.493 | 0.017 |
| Foliar P (ppm) | −0.481 | 0.024 |
| Foliar K (ppm) | 0.240 | 1.000 |
| Foliar S (ppm) | −0.220 | 0.398 |
| Foliar Ca (ppm) | −0.118 | 1.000 |
| Foliar Mg (ppm) | 0.200 | 1.000 |
| Foliar N:P Ratio | 0.021 | 1.000 |
| Foliar N:K Ratio | −0.526 | 0.005 |
| Foliar N:S Ratio | −0.059 | 1.000 |
| Foliar N:Ca Ratio | −0.053 | 1.000 |
| Foliar N:Mg Ratio | −0.248 | 1.000 |
| Nutrient Leaching Scenarios | |||
|---|---|---|---|
| Whole-Tree Harvest | 100% Retention | 2/3 Retention | 1/3 Retention |
| Nitrogen | 20 | 28 | 48 |
| Phosphorus | 27 | 33 | 43 |
| Potassium | 73 | 90 | 117 |
| Calcium | 19 | 23 | 31 |
| Magnesium | 25 | 34 | 53 |
| Stem-only Harvest | |||
| Nitrogen | 8 | 11 | 19 |
| Phosphorus | 10 | 12 | 15 |
| Potassium | 35 | 42 | 55 |
| Calcium | 12 | 15 | 20 |
| Magnesium | 14 | 19 | 30 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
E. Rothstein, D. Effect of Fertilization on Growth and Mortality of Jack Pine Growing on Poor, Sandy Soils in Michigan, USA: Implications for Sustainable Management. Forests 2018, 9, 549. https://doi.org/10.3390/f9090549
E. Rothstein D. Effect of Fertilization on Growth and Mortality of Jack Pine Growing on Poor, Sandy Soils in Michigan, USA: Implications for Sustainable Management. Forests. 2018; 9(9):549. https://doi.org/10.3390/f9090549
Chicago/Turabian StyleE. Rothstein, David. 2018. "Effect of Fertilization on Growth and Mortality of Jack Pine Growing on Poor, Sandy Soils in Michigan, USA: Implications for Sustainable Management" Forests 9, no. 9: 549. https://doi.org/10.3390/f9090549




