Effects of Hardwood Content on Balsam Fir Defoliation during the Building Phase of a Spruce Budworm Outbreak
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Stand Sampling
2.2. Defoliation Measurements
2.3. The Simplified Linear Model
2.4. Analyzing the Relationship between Annual Balsam Fir Defoliation and Hardwood Content Using Generalized Linear Mixed-Effects Model
2.5. The Random Forests Model
2.6. Statistical Analyses
3. Results
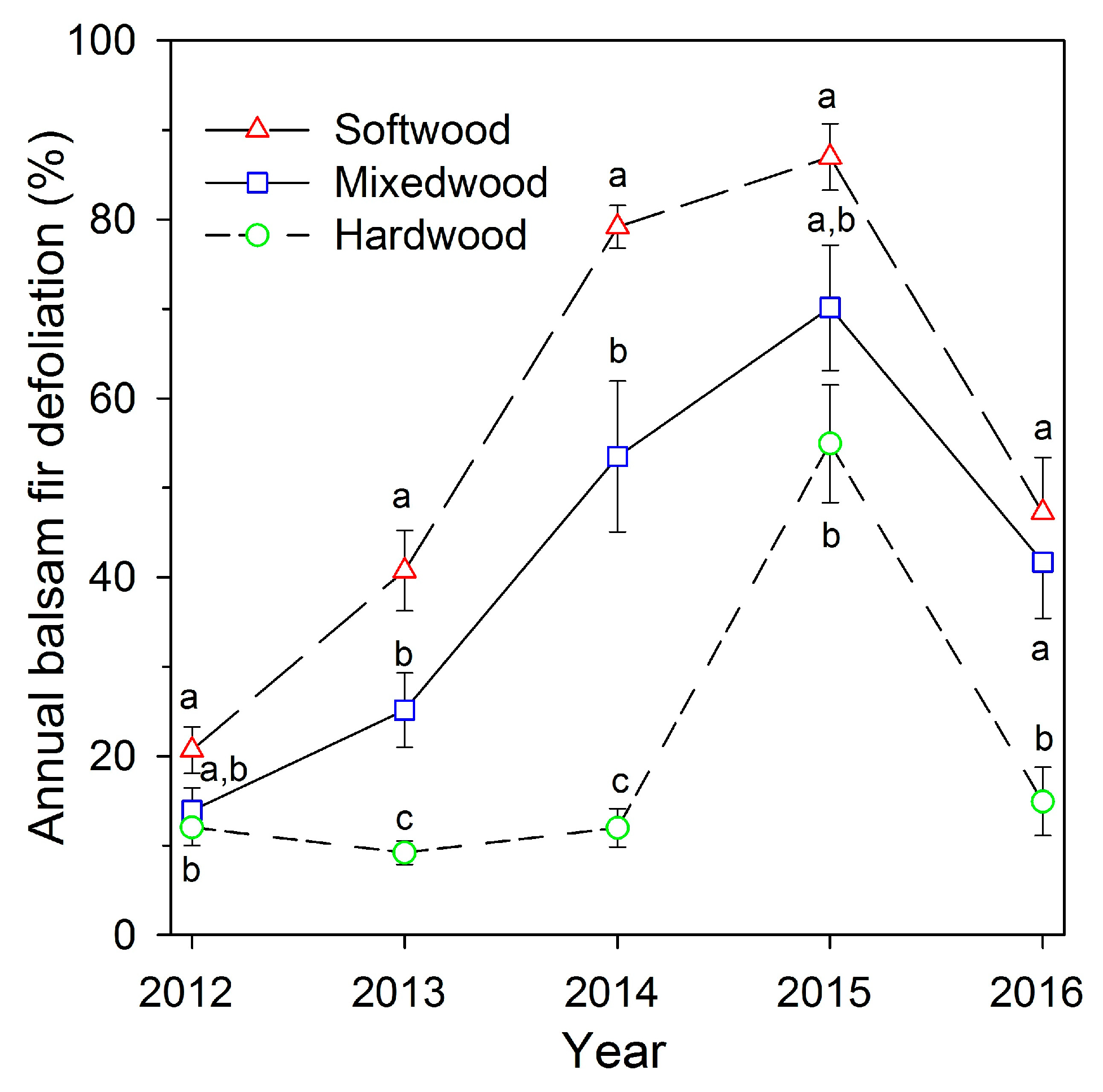
3.1. Relationship between Defoliation and Hardwood Content
3.2. Defoliation Estimated Using Three Model Formulations
4. Discussion
4.1. Relationships between Defoliation and Hardwood Content during Building Phase of a Spruce Budworm Outbreak
4.2. Which Model Provides the “Best” Defoliation Estimates?
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jactel, H.; Brockerhoff, E.G. Tree diversity reduces herbivory by forest insects. Ecol. Lett. 2007, 10, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.F.; Cheshire, W.F.; Miller, C.A.; Mott, D.G. The numerical response of avian and mammalian predators during a gradation of the spruce budworm. Ecology 1958, 39, 487–494. [Google Scholar] [CrossRef]
- Belle-Isle, J.; Kneeshaw, D. A stand and landscape comparison of the effects of a spruce budworm (Choristoneura fumiferana (Clem.)) outbreak to the combined effects of harvesting and thinning on forest structure. For. Ecol. Manag. 2007, 246, 163–174. [Google Scholar] [CrossRef]
- Morin, H.; Jardon, Y.; Gagnon, R. Relationship between spruce budworm outbreaks and forest dynamics in eastern North America. In Plant Disturbance Ecology: The Process and the Response; Johnson, E.A., Miyanishi, K., Eds.; Elsevier: New York, NY, USA, 2007; pp. 555–577. [Google Scholar]
- Royama, T. Population dynamics of the spruce budworm Choristoneura fumiferana. Ecol. Monogr. 1984, 54, 429–462. [Google Scholar] [CrossRef]
- Royama, T.; MacKinnon, W.E.; Kettela, E.G.; Carter, N.E.; Hartling, L.K. Analysis of spruce budworm outbreak cycles in New Brunswick, Canada, since 1952. Ecology 2005, 86, 1212–1224. [Google Scholar] [CrossRef]
- Piene, H.; MacLean, D.A. Spruce budworm defoliation and growth loss in young balsam fir: Patterns of shoot, needle and foliage weight production over a nine-year outbreak cycle. For. Ecol. Manag. 1999, 123, 115–133. [Google Scholar] [CrossRef]
- Hennigar, C.R.; MacLean, D.A.; Quiring, D.T.; Kershaw, J.A. Differences in spruce budworm defoliation among balsam fir and white, red, and black spruce. For. Sci. 2008, 54, 158–166. [Google Scholar] [CrossRef]
- Natural Resources Canada. Compendium of Canadian Forestry Statistics 1994; Canadian Council of Forest Ministers: Ottawa, ON, Canada, 1995; pp. 1–217. ISBN 0-662-21710-1.
- Sanders, C.J. A Summary of Current Techniques Used for Sampling Spruce Budworm Populations and Estimating Defoliation in Eastern Canada; Environment Canada, Canadian Forestry Service: Sault Ste. Marie, ON, Canada, 1980; pp. 1–33.
- MacLean, D.A.; MacKinnon, W.E. Sample sizes required to estimate defoliation of spruce and balsam fir caused by spruce budworm accurately. North. J. Appl. For. 1998, 15, 135–140. [Google Scholar] [CrossRef]
- Donovan, S.D.; MacLean, D.A.; Kershaw, J.A.; Lavigne, M.B. Quantification of forest canopy changes caused by spruce budworm defoliation using digital hemispherical imagery. Agric. For. Meteorol. 2018, 262, 89–99. [Google Scholar] [CrossRef]
- MacLean, D.A. Vulnerability of fir-spruce stands during uncontrolled spruce budworm outbreaks: A review and discussion. For. Chron. 1980, 56, 213–221. [Google Scholar] [CrossRef]
- Bergeron, Y.; Leduc, A.; Joyal, C.; Morin, H. Balsam fir mortality following the last spruce budworm outbreak in northwestern Quebec. Can. J. For. Res. 1995, 25, 1375–1384. [Google Scholar] [CrossRef]
- Su, Q.; Needham, T.D.; MacLean, D.A. The influence of hardwood content on balsam fir defoliation by spruce budworm. Can. J. For. Res. 1996, 26, 1620–1628. [Google Scholar] [CrossRef]
- Campbell, E.M.; MacLean, D.A.; Bergeron, Y. The severity of budworm-caused growth reductions in balsam fir/spruce stands varies with the hardwood content of surrounding forest landscapes. For. Sci. 2008, 54, 195–205. [Google Scholar] [CrossRef]
- MacLean, D.A. Effects of spruce budworm outbreaks on the productivity and stability of balsam fir forests. For. Chron. 1984, 60, 273–279. [Google Scholar] [CrossRef]
- Nealis, V.G.; Régnière, J. Insect host relationships influencing disturbance by the spruce budworm in a boreal mixedwood forest. Can. J. For. Res. 2004, 34, 1870–1882. [Google Scholar] [CrossRef]
- Riihimäki, J.; Kaitaniemi, P.; Koricheva, J.; Vehviläinen, H. Testing the enemies hypothesis in forest stands: The important role of tree species composition. Oecologia 2005, 142, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Siemann, E.; Tilman, D.; Haarstad, J.; Ritchie, M. Experimental tests of the dependence of arthropod diversity on plant diversity. Am. Nat. 1998, 152, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Quayle, D.; Régnière, J.; Cappuccino, N.; Dupont, A. Forest composition, host-population density, and parasitism of spruce budworm Choristoneura fumiferana eggs by Trichogramma minutum. Entomol. Exp. Appl. 2003, 107, 215–227. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Srivastava, D.S.; Duffy, J.E.; Wright, J.P.; Downing, A.L.; Sankaran, M.; Jouseau, C. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 2006, 443, 989–992. [Google Scholar] [CrossRef] [PubMed]
- Kemp, W.P.; Simmons, G.A. Influence of stand factors on survival of early instar spruce budworm. Environ. Entomol. 1979, 8, 993–996. [Google Scholar] [CrossRef]
- Cappuccino, N.; Lavertu, D.; Bergeron, Y.; Régnière, J. Spruce budworm impact, abundance and parasitism rate in a patchy landscape. Oecologia 1998, 114, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, K. Biodiversity and stability of herbivore populations: Influences of the spatial sparseness of food plants. Popul. Ecol. 2002, 44, 33–40. [Google Scholar] [CrossRef]
- Needham, T.; Kershaw, J.A.; MacLean, D.A.; Su, Q. Effects of mixed stand management to reduce impacts of spruce budworm defoliation on balsam fir stand-level growth and yield. North. J. Appl. For. 1999, 16, 19–24. [Google Scholar] [CrossRef]
- Sainte-Marie, G.B.; Kneeshaw, D.D.; MacLean, D.A.; Hennigar, C.R. Estimating forest vulnerability to the next spruce budworm outbreak: Will past silvicultural efforts pay dividends? Can. J. For. Res. 2014, 45, 314–324. [Google Scholar] [CrossRef]
- Stoszek, K.J.; Mika, P.G.; Moore, J.A.; Osborne, H.L. Relationships of Douglas-fir tussock moth defoliation to site and stand characteristics in northern Idaho. For. Sci. 1981, 27, 431–442. [Google Scholar] [CrossRef]
- De Somviele, B.; Lyytikäinen-Saarenmaa, P.; Niemelä, P. Sawfly (Hym., Diprionidae) outbreaks on Scots pine: Effect of stand structure, site quality and relative tree position on defoliation intensity. For. Ecol. Manag. 2004, 194, 305–317. [Google Scholar] [CrossRef]
- MacKinnon, W.E.; MacLean, D.A. The influence of forest and stand conditions on spruce budworm defoliation in New Brunswick, Canada. For. Sci. 2003, 49, 657–667. [Google Scholar] [CrossRef]
- Colford-Gilks, A.K.; MacLean, D.A.; Kershaw, J.A.; Béland, M. Growth and mortality of balsam fir-and spruce-tolerant hardwood stands as influenced by stand characteristics and spruce budworm defoliation. For. Ecol. Manag. 2012, 280, 82–92. [Google Scholar] [CrossRef]
- MacLean, D.A.; MacKinnon, W.E. Effects of stand and site characteristics on susceptibility and vulnerability of balsam fir and spruce to spruce budworm in New Brunswick. Can. J. For. Res. 1997, 27, 1859–1871. [Google Scholar] [CrossRef]
- Rowe, J.S. Forest Regions of Canada; Environment Canada, Canadian Forestry Service: Ottawa, ON, Canada, 1972; pp. 1–172.
- Ministère des Forêts de la Faune et des Parcs. Aires Infestées Par la Tordeuse des Bourgeons de L’épinette au Québec en 2012-Version 1.1; Gouvernement du Québec, Direction de la Protection des Forêts: Québec, QC, Canada, 2012; pp. 1–19.
- Ministère des Forêts de la Faune et des Parcs. Aires Infestées Par la Tordeuse des Bourgeons de L’épinette au Québec en 2016-Version 1.0; Gouvernement du Québec, Direction de la Protection des Forêts: Québec, QC, Canada, 2016; pp. 1–16. ISBN 978-2-550-7474-6.
- MacLean, D.A.; Lidstone, R.G. Defoliation by spruce budworm: Estimation by ocular and shoot-count methods and variability among branches, trees, and stands. Can. J. For. Res. 1982, 12, 582–594. [Google Scholar] [CrossRef]
- Morris, R.F. The development of sampling techniques for forest insect defoliators, with particular reference to the spruce budworm. Can. J. Zool. 1955, 33, 225–294. [Google Scholar] [CrossRef]
- Blais, J.R. Effects of the destruction of the current year’s foliage of balsam fir on the fecundity and habits of flight of the spruce budworm. Can. Entomol. 1953, 85, 446–448. [Google Scholar] [CrossRef]
- McCullagh, P.; Nelder, J.A. Generalized Linear Models, 2nd ed.; Chapman Hall/CRC Press: London, UK, 1989; Volume 37, pp. 476–478. ISBN 0412317605. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Candau, J.N.; Fleming, R.A. Forecasting the response of spruce budworm defoliation to climate change in Ontario. Can. J. For. Res. 2011, 41, 1948–1960. [Google Scholar] [CrossRef]
- Penner, M.; Pitt, D.G.; Woods, M.E. Parametric vs. nonparametric LiDAR models for operational forest inventory in boreal Ontario. Can. J. Remote Sens. 2013, 39, 426–443. [Google Scholar] [CrossRef]
- Lopatin, J.; Dolos, K.; Hernández, H.J.; Galleguillos, M.; Fassnacht, F.E. Comparing Generalized Linear Models and random forest to model vascular plant species richness using LiDAR data in a natural forest in central Chile. Remote Sens. Environ. 2016, 173, 200–210. [Google Scholar] [CrossRef]
- Chen, X.; Ishwaran, H. Random forests for genomic data analysis. Genomics 2012, 99, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, A.M.; Iverson, L.R.; Liaw, A. Newer classification and regression tree techniques: Bagging and random forests for ecological prediction. Ecosystems 2006, 9, 181–199. [Google Scholar] [CrossRef]
- Cutler, D.R.; Edwards, T.C.; Beard, K.H.; Cutler, A.; Hess, K.T.; Gibson, J.; Lawler, J.J. Random forests for classification in ecology. Ecology 2007, 88, 2783–2792. [Google Scholar] [CrossRef] [PubMed]
- De’ath, G.; Fabricius, K.E. Classification and regression trees: A powerful yet simple technique for ecological data analysis. Ecology 2000, 81, 3178–3192. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Murphy, M.A.; Evans, J.S.; Storfer, A. Quantifying Bufo boreas connectivity in Yellowstone National Park with landscape genetics. Ecology 2010, 91, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models; R package version 3.1-137; 2018. Available online: https://CRAN.R-project.org/package=nlme (accessed on 25 June 2018).
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Royama, T.; Eveleigh, E.S.; Morin, J.R.B.; Pollock, S.J.; McCarthy, P.C.; McDougall, G.A.; Lucarotti, C.J. Mechanisms underlying spruce budworm outbreak processes as elucidated by a 14-year study in New Brunswick, Canada. Ecol. Monogr. 2017, 84, 600–631. [Google Scholar] [CrossRef]
- Eveleigh, E.S.; McCann, K.S.; McCarthy, P.C.; Pollock, S.J.; Lucarotti, C.J.; Morin, B.; McDougall, G.A.; Strongman, D.B.; Huber, J.T.; Umbanhowar, J.; et al. Fluctuations in density of an outbreak species drive diversity cascades in food webs. Proc. Natl. Acad. Sci. USA 2007, 104, 16976–16981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Křivan, V.; Schmitz, O.J. Adaptive foraging and flexible food web topology. Evol. Ecol. Res. 2003, 5, 623–652. [Google Scholar]
- Régnière, J.; Nealis, V.G. The fine-scale population dynamics of spruce budworm: Survival of early instars related to forest condition. Ecol. Entomol. 2008, 33, 362–373. [Google Scholar] [CrossRef]
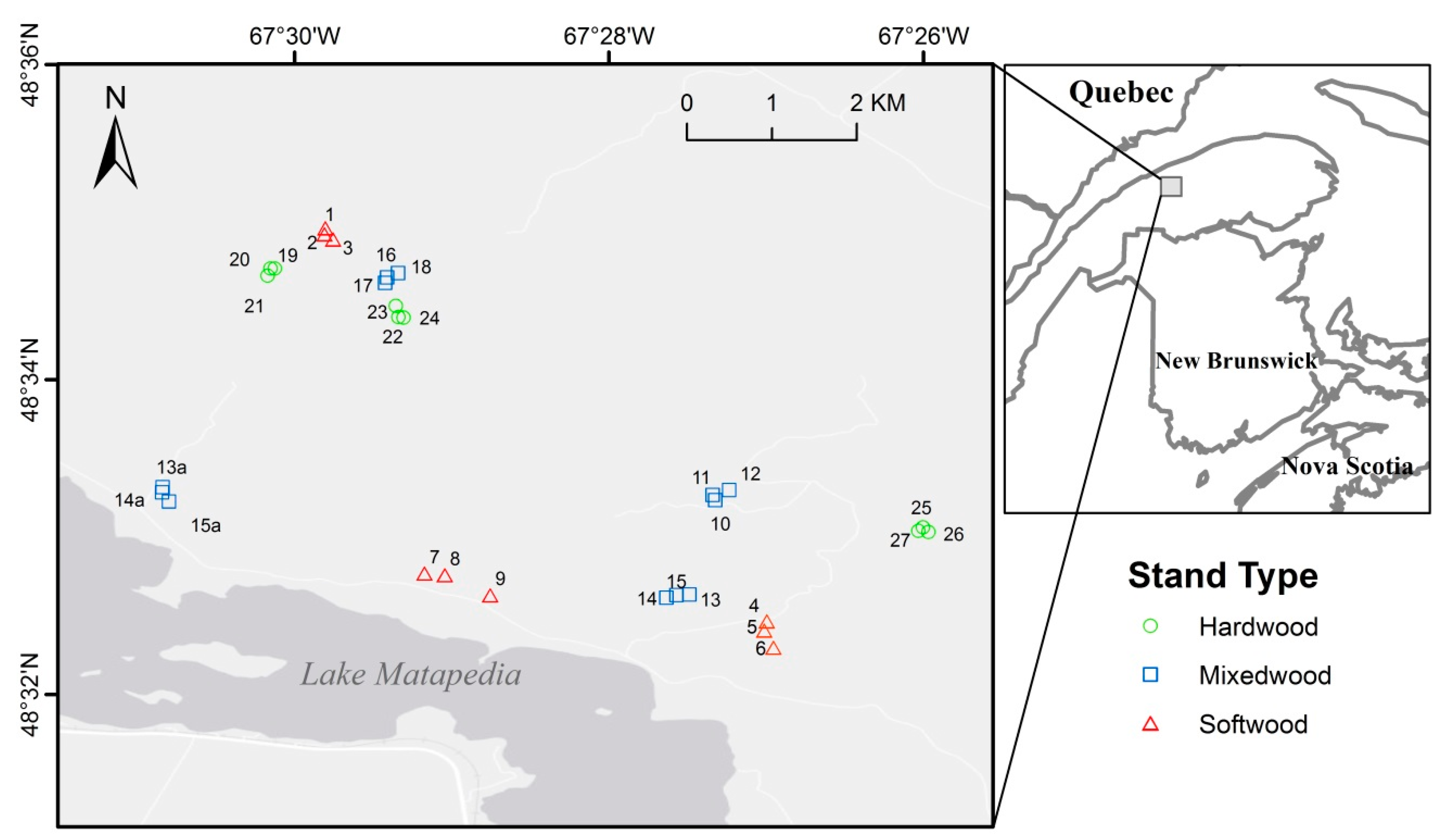



| Plot No. b | Density (stems/ha) | DBH c (cm) | Ht c (m) | DBHbF c (cm) | BA c (m2/ha) | Species Composition % Basal Area d | Total HW d (%BA) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| bF | wS | OS | sM | yB | IH | |||||||
| Softwood a | ||||||||||||
| 1 | 1020 | 20.0 ± 1.1 | 16.3 ± 0.6 | 20.7 ± 1.1 | 37 | 78 | 4 | 9 | ‒ | 2 | 8 | 9 |
| 2 | 2800 | 13.6 ± 0.5 | 12.8 ± 0.4 | 14.1 ± 0.6 | 49 | 78 | 3 | ‒ | ‒ | 4 | 15 | 19 |
| 3 | 920 | 21.5 ± 1.4 | 15.7 ± 0.8 | 23.4 ± 1.3 | 39 | 64 | 17 | 16 | ‒ | ‒ | 3 | 3 |
| 4 | 2975 | 13.1 ± 0.5 | 12.9 ± 0.3 | 13.1 ± 0.5 | 47 | 84 | 3 | 12 | ‒ | ‒ | 1 | 1 |
| 5 | 1650 | 16.1 ± 0.9 | 14.6 ± 0.7 | 15.8 ± 1.0 | 40 | 81 | ‒ | 5 | ‒ | ‒ | 14 | 14 |
| 6 | 2125 | 15.6 ± 0.5 | 15.8 ± 0.3 | 15.8 ± 0.6 | 44 | 95 | ‒ | 2 | ‒ | ‒ | 3 | 3 |
| 7 | 2100 | 15.6 ± 0.8 | 13.9 ± 0.4 | 16.0 ± 0.8 | 50 | 69 | 7 | 8 | ‒ | 1 | 16 | 16 |
| 8 | 1100 | 16.5 ± 1.7 | 12.7 ± 0.8 | 21.5 ± 2.2 | 38 | 81 | ‒ | ‒ | ‒ | ‒ | 19 | 19 |
| 9 | 1380 | 16.4 ± 1.1 | 14.1 ± 0.7 | 22.5 ± 1.4 | 39 | 79 | ‒ | ‒ | ‒ | 8 | 13 | 21 |
| Mixedwood | ||||||||||||
| 10 | 2540 | 11.2 ± 0.7 | 9.7 ± 0.5 | 7.3 ± 0.7 | 36 | 20 | 10 | 7 | ‒ | 34 | 30 | 64 |
| 11 | 760 | 19.2 ± 2.1 | 12.5 ± 1.0 | 20.8 ± 2.6 | 32 | 36 | 2 | ‒ | 29 | 19 | 13 | 62 |
| 12 | 1620 | 14.1 ± 1.1 | 10.7 ± 0.6 | 14.1 ± 1.3 | 37 | 45 | ‒ | ‒ | 1 | 23 | 31 | 55 |
| 13 | 960 | 20.1 ± 1.5 | 18.7 ± 1.0 | 20.9 ± 1.7 | 39 | 33 | 11 | 7 | ‒ | 1 | 49 | 49 |
| 14 | 980 | 21.5 ± 1.9 | 17.5 ± 1.2 | 19.0 ± 1.8 | 49 | 27 | 1 | 17 | ‒ | 7 | 55 | 55 |
| 15 | 1280 | 17.8 ± 1.0 | 17.5 ± 0.9 | 18.1 ± 1.5 | 39 | 42 | 5 | 2 | ‒ | 2 | 53 | 53 |
| 13a | 1980 | 11.0 ± 0.7 | 9.7 ± 0.5 | 10.0 ± 1.3 | 26 | 40 | ‒ | ‒ | ‒ | ‒ | 59 | 60 |
| 14a | 1620 | 13.7 ± 0.9 | 11.0 ± 0.6 | 13.3 ± 1.3 | 32 | 44 | ‒ | ‒ | ‒ | ‒ | 56 | 56 |
| 15a | 1780 | 15.1 ± 0.8 | 12.0 ± 0.5 | 14.4 ± 0.9 | 40 | 57 | 4 | ‒ | ‒ | 5 | 35 | 40 |
| 16 | 1260 | 17.7 ± 1.1 | 15.0 ± 0.5 | 19.0 ± 1.2 | 38 | 48 | ‒ | ‒ | 9 | 16 | 28 | 52 |
| 17 | 1200 | 16.9 ± 1.1 | 14.5 ± 0.6 | 18.9 ± 2.3 | 33 | 43 | ‒ | ‒ | 8 | 22 | 28 | 57 |
| 18 | 1200 | 17.3 ± 1.4 | 14.0 ± 0.6 | 16.7 ± 1.9 | 40 | 33 | 4 | ‒ | 6 | 17 | 40 | 63 |
| Hardwood | ||||||||||||
| 19 | 800 | 18.2 ± 1.8 | 16.7 ± 0.8 | 26.9 ± 7.3 | 28 | 10 | ‒ | ‒ | 90 | ‒ | ‒ | 90 |
| 20 | 1080 | 16.8 ± 1.4 | 14.5 ± 0.8 | 21.3 ± 3.7 | 33 | 24 | ‒ | ‒ | 35 | 9 | 31 | 76 |
| 21 | 520 | 22.8 ± 2.3 | 16.5 ± 1.0 | 20.0 ± 2.3 | 27 | 12 | ‒ | ‒ | 51 | 8 | 29 | 88 |
| 22 | 1000 | 18.5 ± 1.7 | 12.0 ± 0.7 | 11.4 ± 2.0 | 38 | 12 | 3 | ‒ | 34 | 24 | 26 | 84 |
| 23 | 620 | 22.6 ± 2.7 | 17.2 ± 1.4 | 26.8 ± 4.1 | 35 | 14 | ‒ | ‒ | 86 | ‒ | ‒ | 86 |
| 24 | 640 | 18.7 ± 2.4 | 12.6 ± 1.0 | 25.7 ± 1.2 | 27 | 8 | ‒ | ‒ | 16 | 28 | 49 | 92 |
| 25 | 1120 | 13.7 ± 1.0 | 15.2 ± 0.7 | 22.9 ± 3.9 | 22 | 8 | ‒ | ‒ | 81 | 2 | 10 | 92 |
| 26 | 800 | 17.2 ± 1.7 | 16.5 ± 0.8 | 19.7 ± 4.4 | 25 | 5 | ‒ | ‒ | 79 | 14 | 2 | 95 |
| 27 | 560 | 24.8 ± 2.3 | 17.2 ± 0.6 | 19.6 ± 2.8 | 33 | 10 | ‒ | ‒ | 44 | 28 | 18 | 90 |
| Fixed Effects | Parameter Estimates | Analysis of Deviance | |||
|---|---|---|---|---|---|
| Par. | Est. | SE | X2 | p | |
| Intercept | β0 | −2.1225 | 0.3434 | ||
| HW% | β1 | −0.0089 | 0.0061 | 46.68 | <0.001 |
| D0 | β2 | 0.0490 | 0.0055 | 175.70 | <0.001 |
| HW% × D0 | β4 | −0.0002 | 0.0001 | 6.51 | 0.011 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; MacLean, D.A.; Johns, R.C.; Eveleigh, E.S. Effects of Hardwood Content on Balsam Fir Defoliation during the Building Phase of a Spruce Budworm Outbreak. Forests 2018, 9, 530. https://doi.org/10.3390/f9090530
Zhang B, MacLean DA, Johns RC, Eveleigh ES. Effects of Hardwood Content on Balsam Fir Defoliation during the Building Phase of a Spruce Budworm Outbreak. Forests. 2018; 9(9):530. https://doi.org/10.3390/f9090530
Chicago/Turabian StyleZhang, Bo, David A. MacLean, Rob C. Johns, and Eldon S. Eveleigh. 2018. "Effects of Hardwood Content on Balsam Fir Defoliation during the Building Phase of a Spruce Budworm Outbreak" Forests 9, no. 9: 530. https://doi.org/10.3390/f9090530





