Drought Decreases Growth and Increases Mortality of Coexisting Native and Introduced Tree Species in a Temperate Floodplain Forest
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Area and Tree Species
2.2. Climate, Drought, and Hydrology Data
2.3. Field Sampling and Tree-Ring Data (Growth and Wood Anatomy)
2.4. Carbon Isotopes in Wood
2.5. Comparing Living and Recently Dead Trees: Features, Mortality, and Growth Models
2.6. Detection of Phytophthora Presence and Soil Features
3. Results
3.1. Climate, Drought, and Water Table Depth: Trends and Temporal Variability
3.2. Characteristics of Living and Recently Dead Trees
3.3. Mortality and Growth Models
3.4. Wood Anatomy and Isotope Discrimination in Living and Dead Oaks
3.5. Phytophthora Presence and Soil Characteristics
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lugo, A.E. Forestry in the Anthropocene. Science 2015, 349, 771. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Kane, J.M.; Anderegg, L.D.L. Consequences of widespread tree mortality triggered by drought and temperature stress. Nat. Clim. Chang. 2012, 3, 30–36. [Google Scholar] [CrossRef]
- Stojanović, D.B.; Levanič, T.; Matović, B.; Orlovic, S. Growth decrease and mortality of oak floodplain forests as a response to change of water regime and climate. Eur. J. For. Res. 2015, 134, 555–567. [Google Scholar] [CrossRef]
- Lockaby, B.G. Floodplain ecosystems of the Southeast: Linkages between forest and people. Wetlands 2009, 29, 407–412. [Google Scholar] [CrossRef]
- Lageard, J.G.A.; Drew, I.B. Hydrogeomorphic control on tree growth responses in the Elton area of the Cheshire Saltfield, UK. Geomorphology 2008, 95, 158–171. [Google Scholar] [CrossRef]
- Horáček, P.; Šlezingerová, J.; Gandelová, L. Analysis of cambial activity and formation of wood in Quercus robur L. under conditions of a floodplain forest. J. For. Sci. 2003, 49, 412–418. [Google Scholar] [CrossRef]
- Levanič, T.; Čater, M.; McDowell, N.G. Associations between growth, wood anatomy, carbon isotope discrimination and mortality in a Quercus robur forest. Tree Physiol. 2011, 31, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Tessier, L.; Nola, P.; Serre-Bachet, F. Deciduous Quercus in the Mediterranean region–tree-ring/climate relationships. New Phytol. 1994, 126, 355–367. [Google Scholar] [CrossRef]
- Bréda, N.; Badeau, V. Forest tree responses to extreme drought and some biotic events: Towards a selection according to hazard tolerance? Comp. Rend. Geosci. 2008, 340, 651–662. [Google Scholar] [CrossRef]
- Friedrichs, D.A.; Büntgen, U.; Frank, D.C.; Esper, J.; Neuwirth, B.; Löffler, J. Complex climate controls on 20th century oak growth in Central-West Germany. Tree Physiol. 2009, 29, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Tumajer, J.; Treml, V. Response of floodplain pedunculate oak (Quercus robur L.) tree ring width and vessel anatomy to climatic trends and extreme hydroclimatic events. For. Ecol. Manag. 2016, 379, 185–194. [Google Scholar] [CrossRef]
- Čejková, A.; Poláková, S. Growth responses of sessile oak to climate and hydrological regime in the Zbytka Nature Reserve, Czech Republic. Geochronometria 2012, 39, 285–294. [Google Scholar] [CrossRef]
- Rozas, V.; García-González, I. Too wet for oaks? Inter-tree competition and recent persistent wetness predispose oaks to rainfall-induced dieback in Atlantic rainy forest. Glob. Planet. Chang. 2012, 94, 62–71. [Google Scholar] [CrossRef] [Green Version]
- Scharnweber, T.; Couwenberg, J.; Heinrich, I.; Wilmking, M. New insights for the interpretation of ancient bog oak chronologies? Reactions of oak (Quercus robur L.) to a sudden peatland rewetting. Palaeogeogr. Palaeoclim. Paleoecol. 2015, 417, 534–543. [Google Scholar] [CrossRef]
- Drobyshev, I.; Niklasson, M.; Eggertsson, O.; Linderson, H. and Sonesson, K. Influence of annual weather on growth of pedunculate oak in southern Sweden. Ann. For. Sci. 2008, 65, 512. [Google Scholar] [CrossRef]
- Andersson, M.; Milberg, P.; Bergman, K.-O. Low pre-death growth rates of oak (Quercus robur L.)—Is oak death a long-term process induced by dry years? Ann. For. Sci. 2011, 68, 159–168. [Google Scholar] [CrossRef]
- Walther, G.R.; Roques, A.; Hulme, P.E.; Sykes, M.T.; Pyšek, P.; Kühn, I.; Zobel, M.; Bacher, S.; Botta-Dukát, Z.; Bugmann, H.; et al. Alien species in a warmer world: Risks and opportunities. Trends Ecol. Evol. 2009, 24, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.M.; Blank, R.; Hartmann, G. Abiotic and biotic factors and their interactions as causes of oak decline in Central Europe. For. Pathol. 2002, 32, 277–307. [Google Scholar] [CrossRef]
- Brasier, C.M. Phytophthora cinnamomi and oak decline in southern Europe. Environmental constraints including climate change. Ann. Sci. For. 1996, 53, 347–358. [Google Scholar] [CrossRef]
- Führer, E. Oak decline in central Europe: A synopsis of hypotheses. In Proceedings: Population Dynamics, Impacts, and Integrated Management of Forest Defoliating Insects; USDA Forestry Service, General Technical Report NE-247; U.S. Department of Agriculture, Forest Service, Northeastern Research Station: Radnor, PA, USA, 1998; pp. 7–24. [Google Scholar]
- Helama, S.; Sohar, K.; Läänelaid, A.; Mäkelä, H.M.; Raisio, J. Oak decline as illustrated through plant-climate interactions near the northern edge of species range. Bot. Rev. 2016, 82, 1–23. [Google Scholar] [CrossRef]
- Wargo, P.M. Consequences of environmental stress on oak: Predisposition to pathogens. Ann. For. Sci. 1996, 53, 359–368. [Google Scholar] [CrossRef]
- Sangüesa-Barreda, G.; Camarero, J.J.; Oliva, J.; Montes, F.; Gazol, A. Past logging, drought and pathogens interact and contribute to forest dieback. Agric. For. Meteorol. 2015, 208, 85–94. [Google Scholar] [CrossRef]
- DEPFAR Project. Indagini Diagnostiche sul Deperimento Della Farina nei Boschi Della Valle del Ticino; Consorzio Parco Lombardo della Valle del Ticino: Pontevecchio di Magenta, Italy, 2006. [Google Scholar]
- Motta, R.; Nola, P.; Berretti, R. The rise and fall of the black locust (Robinia pseudoacacia L.) in the “Siro Negri” Forest Reserve (Lombardy, Italy): Lessons learned and future uncertainties. Ann. For. Sci. 2009, 66, 410. [Google Scholar] [CrossRef]
- Stefanelli, S.; Della Rocca, F.; Bogliani, G. Saproxylic beetles of the Po plain woodlands, Italy. Biodivers. Data J. 2014, 2, e1106. [Google Scholar] [CrossRef] [PubMed]
- Schmull, M.; Thomas, M.F. Morphological and physiological reactions of young deciduous trees (Quercus robur L., Q. petraea [Matt.] Liebl., Fagus sylvatica L.) to waterlogging. Plant Soil 2000, 225, 227–242. [Google Scholar] [CrossRef]
- Glenz, C.; Schlaepfer, R.; Iorgulescu, I.; Kienast, F. Flooding tolerance of Central European tree and shrub species. For. Ecol. Manag. 2006, 235, 1–13. [Google Scholar] [CrossRef]
- Ferner, E.; Rennenberg, H.; Kreuzwieser, J. Effect of flooding on C metabolism of flood tolerant (Quercus robur) and non-tolerant (Fagus sylvatica) tree species. Tree Physiol. 2012, 32, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, E.; Bousquet, F.; Ducrey, M. Use of pressure volume curves in water relation analysis on woody shoots: Influence of rehydration and comparison in four European oak species. Ann. For. Sci. 1990, 47, 285–297. [Google Scholar] [CrossRef]
- Saracchi, M.; Bottigiola, C.; Lanticina, M.; Vailati, A.M.; Parco, V.; Caronni, F. Further studies on oak decline distribution in Lombardy. J. Plant Pathol. 2005, 87, 305. [Google Scholar]
- Huntley, J.C. Robinia pseudoacacia. In Hardwoods: Silvics of North America; Burns, R.M., Honkala, B.H., Eds.; USDA: Washington, DC, USA, 1990; pp. 755–761. [Google Scholar]
- Vítková, M.; Tonika, J.; Müllerová, J. Black locust–successful invader of a wide range of soil conditions. Sci. Total Environ. 2015, 505, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dai, Y.; Sun, J.; Wan, X. Differential hydric deficit responses of Robinia pseudoacacia and Platycladus orientalis in pure and mixed stands in northern China and the species interactions under drought. Trees 2017, 31, 2011–2021. [Google Scholar] [CrossRef]
- Harris, I.; Jones, P.D.; Osborn, T.J.; Lister, D.H. Updated high-resolution grids of monthly climatic observations–the CRU TS3.10 Dataset. Int. J. Climatol. 2014, 34, 623–642. [Google Scholar] [CrossRef] [Green Version]
- Vicente-Serrano, S.M.; Beguería, S.; López-Moreno, J.I. A multi-scalar drought index sensitive to global warming: The Standardized Precipitation Evapotranspiration Index-SPEI. J. Clim. 2010, 23, 1696–1718. [Google Scholar] [CrossRef]
- Rodionov, S.N. A sequential algorithm for testing climate regime shifts. Geophys. Res. Lett. 2004, 31, L09204. [Google Scholar] [CrossRef]
- Dobbertin, M. Tree growth as indicator of tree vitality and of tree reaction to environmental stress: A review. Eur. J. For. Res. 2005, 124, 319–333. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Oliva, J.; Vicente-Serrano, S.M. To die or not to die: Early-warning signals of dieback in response to a severe drought. J. Ecol. 2015, 103, 44–57. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate; Academic Press: New York, NY, USA, 1976. [Google Scholar]
- Gärtner, H.; Nievergelt, D. The core-microtome: A new tool for surface preparation on cores and time series analysis of varying cell parameters. Dendrochronologia 2010, 28, 85–92. [Google Scholar] [CrossRef]
- Holmes, R.L. Computer—Assisted quality control in tree—Ring dating and measurement. Tree-Ring Res. 1983, 43, 69–78. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, M.; Camarero, J.J.; Battipaglia, G.; Borghetti, M.; De Micco, V.; Gentilesca, T.; Ripullone, F. A multi-proxy assessment of dieback causes in a Mediterranean oak species. Tree Physiol. 2017, 37, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Sperry, J.S.; Nichols, K.L.; Sullivan, J.E.M.; Eastlack, S.E. Xylem embolism in ring-porous, diffuse-porous, and coniferous trees of Northern Utah and Interior Alaska. Ecology 1994, 75, 1736–1752. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Richards, R.A. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust. J. Plant Physiol. 1984, 11, 539–552. [Google Scholar] [CrossRef]
- Borella, S.; Leuenberger, M.; Saurer, M.; Siegwolf, R. Reducing uncertainties in δ13C analysis of tree rings: Pooling, milling, and cellulose extraction. J. Geophys. Res. 1998, 103, 19519–19526. [Google Scholar] [CrossRef]
- Gibbons, J.D.; Chakraborti, S. Nonparametric Statistical Inference; CRC Press: New York, NY, USA, 2011. [Google Scholar]
- Colangelo, M.; Camarero, J.J.; Borghetti, M.; Gazol, A.; Gentilesca, T.; Ripullone, F. Size matters a lot: Drought-affected Italian oaks are smaller and show lower growth prior to tree death. Front. Plant Sci. 2017, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Firth, D. Bias reduction of maximum likelihood estimates. Biometrika 1993, 80, 27–38. [Google Scholar] [CrossRef]
- Heinze, G. A comparative investigation of methods for logistic regression with separated or nearly separated data. Stat. Med. 2006, 25, 4216–4226. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.N. Generalized Additive Models: An Introduction with R; Chapman and Hall/CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Mudelsee, M. Climate Time Series Analysis: Classical Statistical and Bootstrap Methods; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Jung, T.; Cooke, D.E. L.; Blaschke, H.; Duncan, J.M.; Oßwald, W. Phytophthora quercina sp. nov., causing root rot of European oaks. Mycol. Res. 1999, 103, 785–798. [Google Scholar] [CrossRef]
- Jung, T.; Blaschke, H.; Oßwald, W. Involvement of soilborne Phytophthora species in Central European oak decline and the effect of site factors on the disease. Plant Pathol. 2000, 49, 706–718. [Google Scholar] [CrossRef]
- Jung, T. Beech decline in Central Europe driven by the interaction between Phytophthora infections and climatic extremes. For. Pathol. 2009, 39, 73–94. [Google Scholar] [CrossRef]
- Jeffers, S.N.; Martin, S.B. Comparison of two media selective for Phytophthora and Pythium species. Plant Dis. 1986, 70, 1038–1043. [Google Scholar] [CrossRef]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; The American Phytopathological Society: St. Paul, MN, USA, 1996. [Google Scholar]
- Taubner, H.; Roth, B.; Tippkotter, R. Determination of soil texture: Comparison of the sedimentation method and the laser-diffraction analysis. J. Plant Nutr. Soil Sci. 2009, 172, 161–171. [Google Scholar] [CrossRef]
- Rozas, V. Dendrochronology of pedunculate oak (Quercus robur L.) in an old-growth pollarded woodland in northern Spain: Tree-ring growth responses to climate. Ann. For. Sci. 2005, 62, 209–218. [Google Scholar] [CrossRef]
- Alla, A.Q.; Camarero, J.J. Contrasting responses of radial growth and wood anatomy to climate in a Mediterranean ring-porous oak: Implications for its future persistence or why the variance matters more than the mean. Eur. J. For. Res. 2012, 131, 1537–1550. [Google Scholar] [CrossRef]
- Pilcher, J.R.; Gray, B. The relationships between oak tree growth and climate in Britain. J. Ecol. 1982, 70, 297–304. [Google Scholar] [CrossRef]
- Helama, S.; Läänelaid, A.; Raisio, J.; Tuomenvirta, H. Oak decline in Helsinki portrayed by tree-rings, climate and soil data. Plant Soil 2009, 319, 163–174. [Google Scholar] [CrossRef]
- Yan, W.; Zhong, Y.; Shangguan, Z. Rapid response of the carbon balance strategy in Robinia pseudoacacia and Amorpha fruticosa to recurrent drought. Environ. Exp. Bot. 2017, 138, 46–56. [Google Scholar] [CrossRef]
- Rizzo, D.M.; Garbelotto, M.; Davidson, J.M.; Slaughter, G.W.; Koike, S.T. Phytopththora ramorum as the cause of extensive mortality of Quercus spp. and Lithocarpus densiflorus in California. Plant Dis. 2002, 86, 205–214. [Google Scholar] [CrossRef]
- Rice, S.K.; Westerman, B.; Federici, R. Impacts of the exotic, nitrogen-fixing black locust (Robinia pseudoacacia) on nitrogen-cycling in a pine-oak ecosystem. Plant Ecol. 2004, 174, 97–107. [Google Scholar] [CrossRef]
- Elliott, K.J.; Swank, W.T. Impacts of drought on tree mortality and growth in a mixed hardwood forest. J. Veg. Sci. 1994, 5, 229–236. [Google Scholar] [CrossRef]
- Mantovani, D.; Veste, M.; Boldt-Burisch, K.; Fritsch, S.; Koning, L.A.; Freese, D. Carbon allocation, nodulation, and biological nitrogen fixation of black locust (Robinia pseudoacacia L.) under soil water limitation. Ann. For. Res. 2015, 58, 259–274. [Google Scholar] [CrossRef]
- Mantovani, D.; Veste, M.; Böhm, C.; Vignudelli, M.; Freese, D. Spatial and temporal variation of drought impact on black locust (Robinia pseudoacacia L.) water status and growth. iForest Biogeosci. For. 2015, 8, 743–747. [Google Scholar] [CrossRef]
- Wurzburger, N.; Miniat, C.F. Drought enhances symbiotic dinitrogen fixation and competitive ability of a temperate forest tree. Oecologia 2014, 174, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Von Holle, B.; Neill, C.; Largay, E.F.; Budreski, K.A.; Ozimec, B.; Clark, S.A.; Lee, K. Ecosystem legacy of the introduced N2-fixing tree Robinia pseudoacacia in a coastal forest. Oecologia 2013, 172, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Tainter, F.H.; Retzlaff, W.A.; Starkey, D.A.; Oak, S.W. Decline of radial growth in red oaks is associated with short-term changes in climate. Eur. J. For. Pathol. 1990, 20, 95–105. [Google Scholar] [CrossRef]
- Drobyshev, I.; Linderson, H.; Sonesson, K. Relationship between crown condition and tree diameter growth in southern Swedish oaks. Environ. Monit. Assess. 2007, 128, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Sohar, K.; Helama, S.; Läänelaid, A.; Raisio, J.; Tuomenvirta, H. Oak decline in a southern Finnish forest as affected by a drought sequence. Geochronometria 2014, 41, 92–103. [Google Scholar] [CrossRef] [Green Version]
- Cailleret, M.; Jansen, S.; Robert, E.M.R.; Desoto, L.; Aakala, T.; Antos, J.A.; Beikircher, B.; Bigler, C.; Bugmann, H.; Caccianiga, M.; et al. A synthesis of radial growth patterns preceding tree mortality. Glob. Chang. Biol. 2017, 23, 1675–1690. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.S. The role of stress in the mortality of Midwestern oaks as indicated by growth prior to death. Ecology 1998, 79, 79–93. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Meinzer, F.C.; Hao, G.Y.; Scholz, F.G.; Bucci, S.J.; Takahashi, F.S.C.; Villalobos-Vega, R.; Giraldo, J.P.; Cao, K.F.; Hoffmann, W.A.; et al. Size-dependent mortality in a Neotropical savanna tree: The role of height-related adjustments in hydraulic architecture and carbon allocation. Plant Cell Environ. 2009, 32, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.; Dragoni, D.; Phillips, R.P.; Roman, D.T.; Novick, K.A. Dynamics of stem water uptake among isohydric and anisohydric species experiencing a severe drought. Tree Physiol. 2017, 37, 1379–1392. [Google Scholar] [CrossRef] [PubMed]
- Voltas, J.; Camarero, J.J.; Carulla, D.; Aguilera, M.; Oriz, A.; Ferrio, J.P. A retrospective, dual-isotope approach reveals individual predispositions to winter-drought induced tree dieback in the southernmost distribution limit of Scots pine. Plant Cell Environ. 2013, 36, 1435–1448. [Google Scholar] [CrossRef] [PubMed]
- Gaylord, M.L.; Kolb, T.E.; McDowell, N.G. Mechanisms of piñon pine mortality after severe drought: A retrospective study of mature trees. Tree Physiol. 2015, 35, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Granda, E.; Alla, A.Q.; Laskurain, N.A.; Loidi, J.; Sánchez-Lorenzo, A.; Camarero, J.J. Coexisting oak species, including rear-edge populations, buffer climate stress through xylem adjustments. Tree Physiol. 2018, 38, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Petrucco, L.; Nardini, A.; Von Arx, G.; Saurer, M.; Cherubini, P. Isotope signals and anatomical features in tree rings suggest a role for hydraulic strategies in diffuse drought-induced die-back of Pinus nigra. Tree Physiol. 2017, 37, 523–535. [Google Scholar] [PubMed]
- Corcuera, L.; Camarero, J.J.; Gil-Pelegrín, E. Effects of a severe drought on growth and wood-anatomical properties of Quercus faginea. IAWA J. 2004, 25, 185–204. [Google Scholar] [CrossRef]
- Schume, H.; Grabner, M.; Eckmüllner, O. The influence of an altered groundwater regime on vessel properties of hybrid poplar. Trees 2004, 18, 184–194. [Google Scholar] [CrossRef]
- Corcuera, L.; Camarero, J.J.; Sisó, S.; Gil-Pelegrín, E. Radial-growth and wood-anatomical changes in overaged Quercus pyrenaica coppice stands: Functional responses in a new Mediterranean landscape. Trees 2006, 20, 91–98. [Google Scholar] [CrossRef]

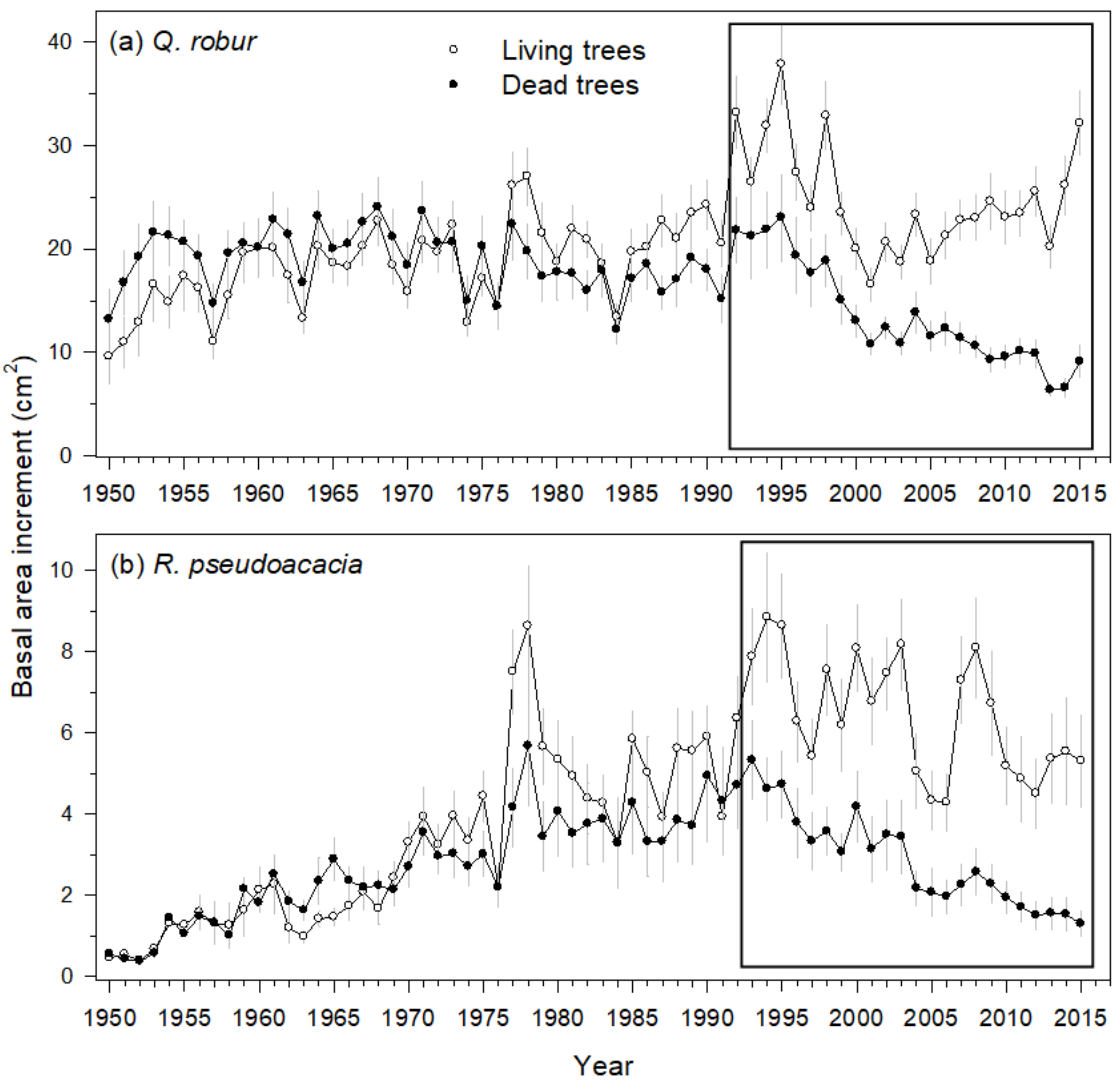
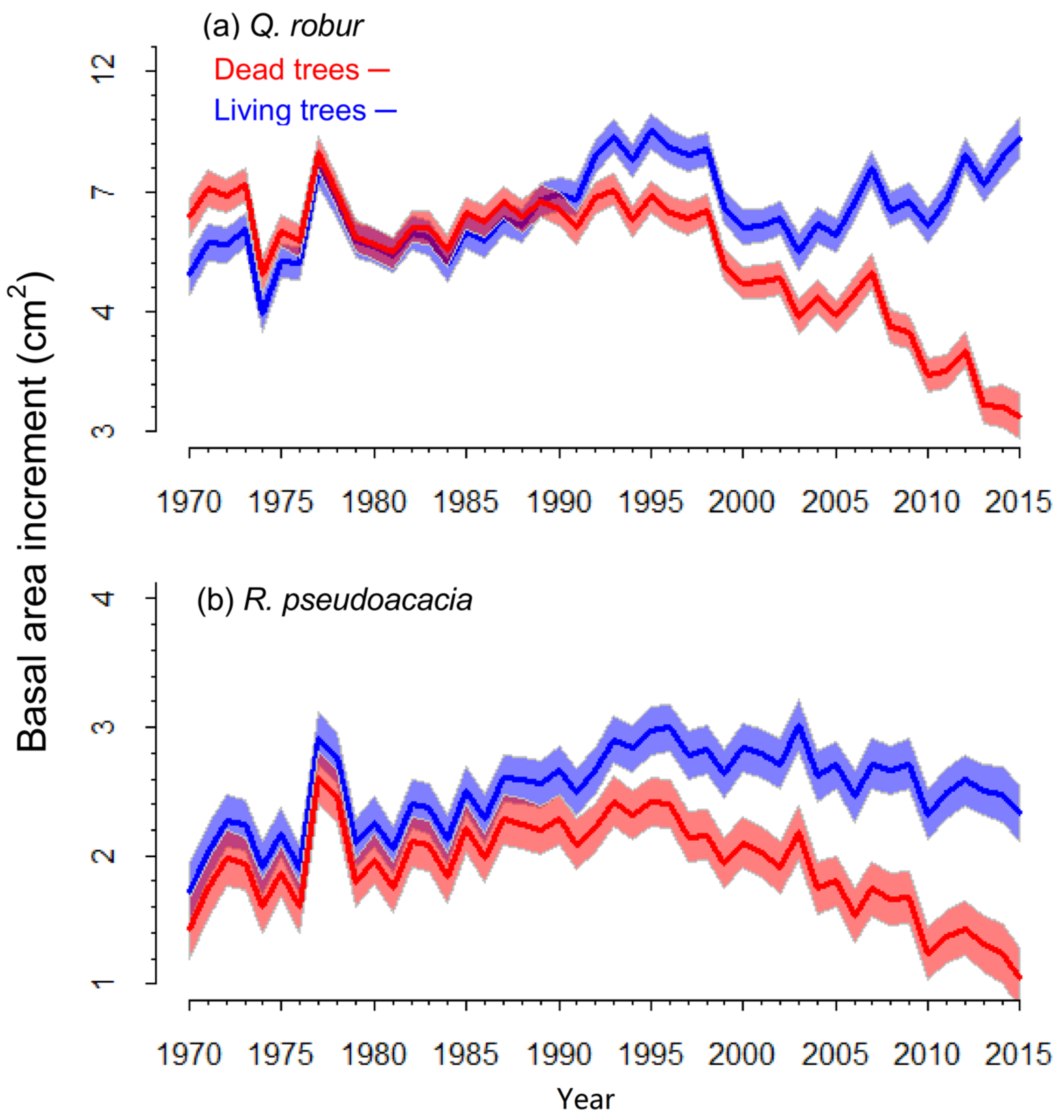
| Tree Species | Type of Tree | Dbh (cm) | Height (m) | Age at 1.3 m (Years) |
|---|---|---|---|---|
| Oak (Quercus robur) | Living tree | 52.0 ± 2.4 | 25.9 ± 1.1b | 80 ± 4 |
| Dead tree | 51.0 ± 3.1 | 20.7 ± 0.8a | 85 ± 4 | |
| Black locust (Robinia pseudoacacia) | Living tree | 25.9 ± 2.0 | 13.0 ± 0.6 | 53 ± 2 |
| Dead tree | 23.8 ± 1.6 | 12.2 ± 0.5 | 56 ± 2 |
| Tree Species | Variable | Coefficient | edf | Statistic (t or F) |
|---|---|---|---|---|
| Quercus robur | Dbh | 0.02 ± 0.01 | 4.37 | |
| Height | 0.03 ± 0.01 | 2.70 | ||
| Living tree | 7.23 | 8.15 | ||
| Dead tree | 4.92 | 32.32 | ||
| Tsum | 8.78 | 18.42 | ||
| Psum | 5.63 | 14.84 | ||
| Robinia pseudoacacia | Dbh | 0.05 ± 0.01 | 4.66 | |
| Living tree | 3.28 | 7.19 | ||
| Dead tree | 3.23 | 11.31 | ||
| Tsum | 8.30 | 10.59 | ||
| Psum | 6.37 | 6.25 |
| Tree Species | Type of Tree | Earlywood Dh (µm) | δ13C (‰) |
|---|---|---|---|
| Quercus robur | Living tree | 400 ± 5 b | −25.3 ± 0.3 b |
| Dead tree | 320 ± 6 a | −26.5 ± 0.1 a | |
| Robinia pseudoacacia | Living tree | 375 ± 8 b | −25.4 ± 0.4 b |
| Dead tree | 337 ± 7 a | −27.6 ± 0.5 a |
| Tree Species | Type of Tree | Soil pH | Soil C (%) | Soil N (%) | Sand (%) | Loam (%) | Soil Electrical Conductivity (µS cm−1) |
|---|---|---|---|---|---|---|---|
| Quercus robur | Living tree | 3.9 ± 0.1 | 7.77 ± 1.91 | 0.37 ± 0.11 | 78.0 ± 1.8 | 21.8 ± 1.8 | 88.7 ± 5.3 |
| Dead tree | 4.0 ± 0.1 | 7.81 ± 1.19 | 0.36 ± 0.06 | 80.8 ± 1.6 | 19.1 ± 1.5 | 85.9 ± 4.0 | |
| Robinia pseudoacacia | Living tree | 4.1 ± 0.1 | 5.37 ± 0.67 | 0.36 ± 0.04b | 81.6 ± 1.1 | 19.8 ± 0.8 | 72.2 ± 3.6 |
| Dead tree | 4.2 ± 0.1 | 4.19 ± 0.42 | 0.25 ± 0.02a | 81.6 ± 2.3 | 19.9 ± 1.7 | 65.6 ± 5.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colangelo, M.; Camarero, J.J.; Ripullone, F.; Gazol, A.; Sánchez-Salguero, R.; Oliva, J.; Redondo, M.A. Drought Decreases Growth and Increases Mortality of Coexisting Native and Introduced Tree Species in a Temperate Floodplain Forest. Forests 2018, 9, 205. https://doi.org/10.3390/f9040205
Colangelo M, Camarero JJ, Ripullone F, Gazol A, Sánchez-Salguero R, Oliva J, Redondo MA. Drought Decreases Growth and Increases Mortality of Coexisting Native and Introduced Tree Species in a Temperate Floodplain Forest. Forests. 2018; 9(4):205. https://doi.org/10.3390/f9040205
Chicago/Turabian StyleColangelo, Michele, Jesús J. Camarero, Francesco Ripullone, Antonio Gazol, Raúl Sánchez-Salguero, Jonàs Oliva, and Miguel A. Redondo. 2018. "Drought Decreases Growth and Increases Mortality of Coexisting Native and Introduced Tree Species in a Temperate Floodplain Forest" Forests 9, no. 4: 205. https://doi.org/10.3390/f9040205






