Elevated CO2 and O3 Levels Influence the Uptake and Leaf Concentration of Mineral N, P, K in Phyllostachys edulis (Carrière) J.Houz. and Oligostachyum lubricum (wen) King f.
Abstract
:1. Introduction
2. Materials and Methods
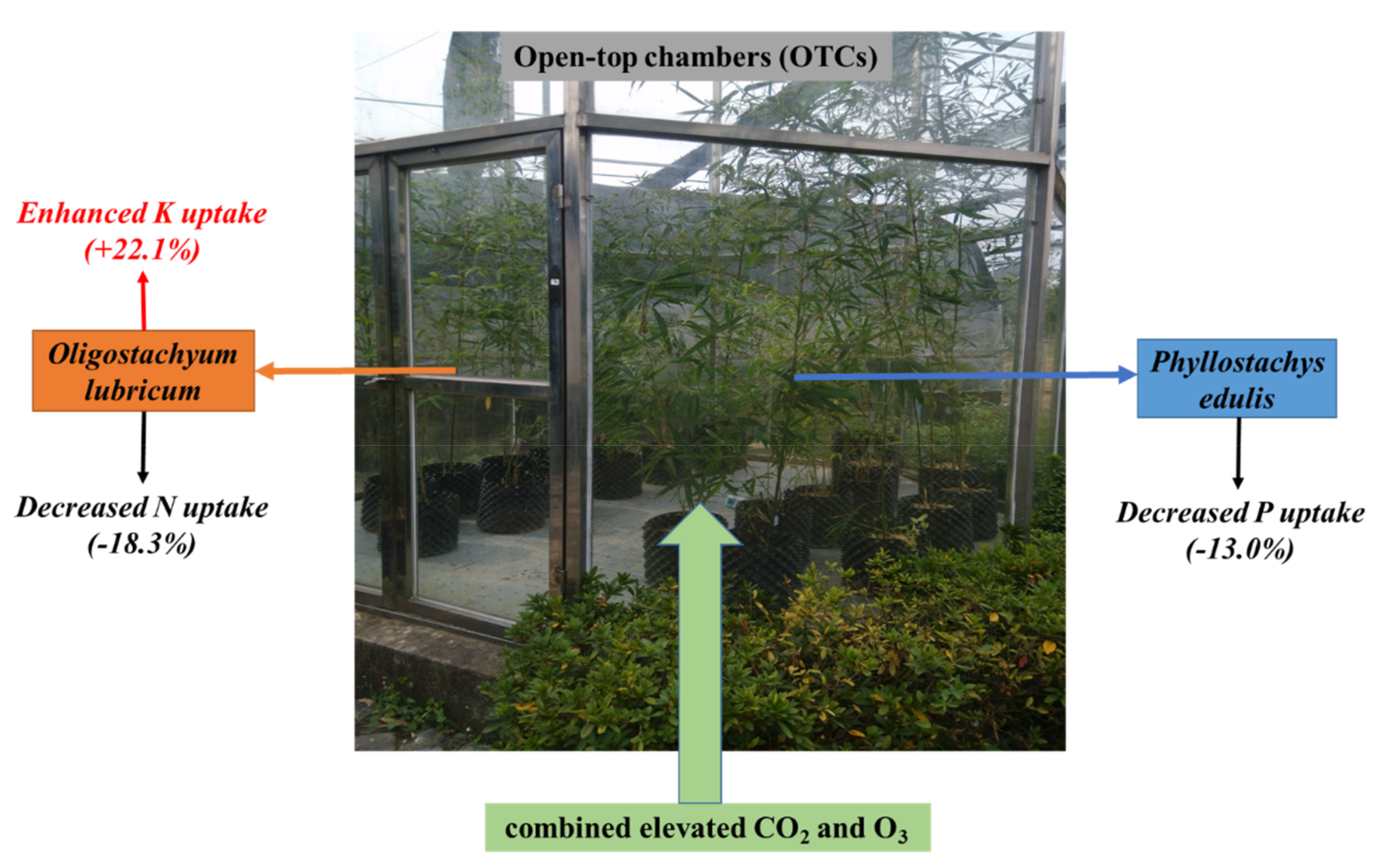
2.1. Research Site and Experimental Design
2.2. Plant Materials
2.3. Biomass and N, P, and K Measurements
2.4. Statistical Analysis
3. Results and Discussion
3.1. Leaf N, P, and K Concentrations
3.2. Leaf Biomass
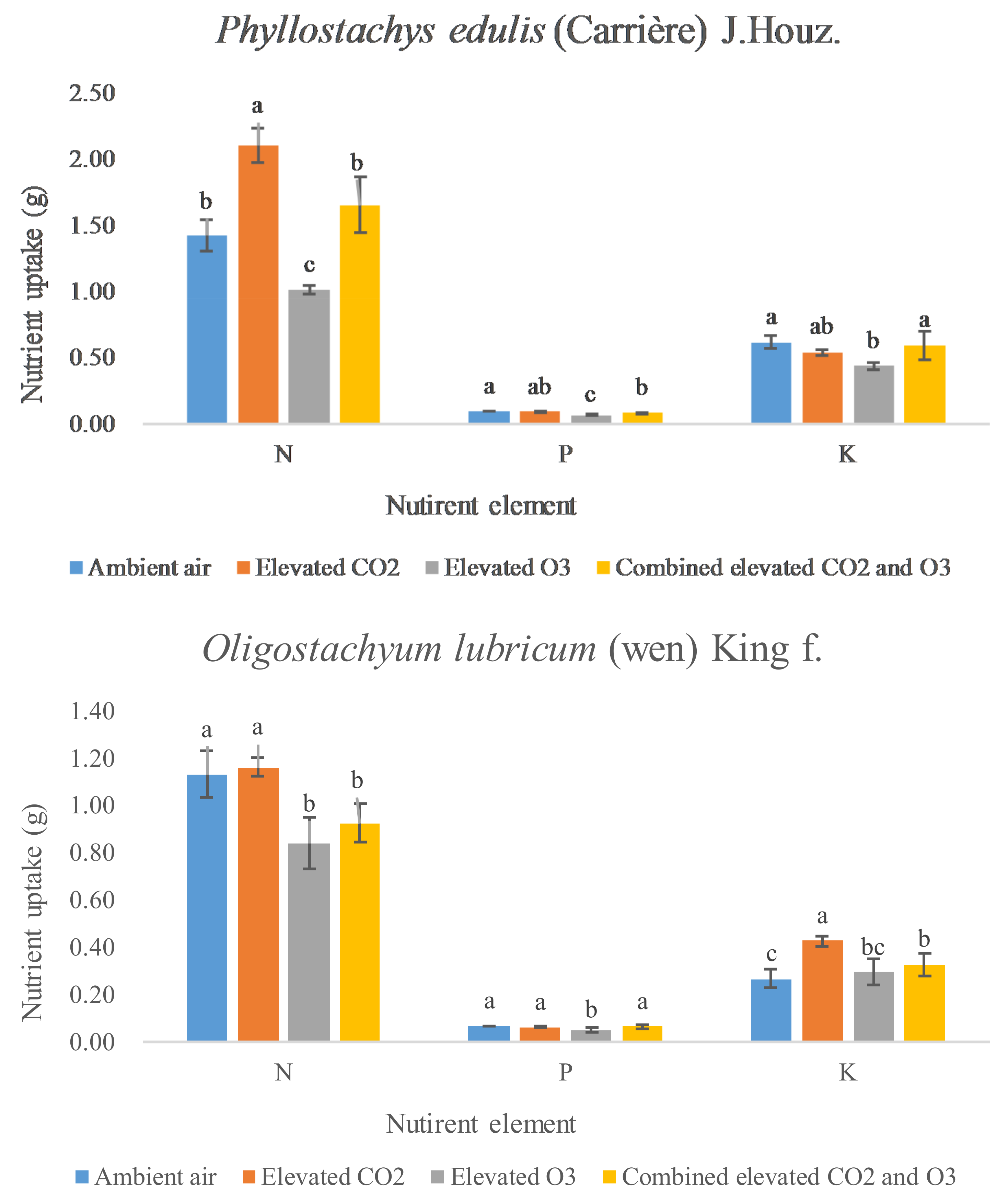
3.3. N, P, and K Uptake
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marschner, H.; Marschner, P. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: London, UK, 2012. [Google Scholar]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.M.; Zhang, Y.Q.W.; Zheng, S.X.; Shangguan, Z.P. Linking plant leaf nutrient/stoichimetry to water use efficiency on the Losee Plateau in China. Ecol. Eng. 2016, 87, 124–131. [Google Scholar] [CrossRef]
- IPCC Climate Change. The Physical Science Basis: Summary for Policymakers; Stocker, T., Ed.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Cao, J.X.; Shang, H.; Chen, Z.; Tian, Y.; Yu, H. Effects of Elevated Ozone on Stoichiometry and Nutrient Pools of Phoebe Bournei (Hemsl.) Yang and Phoebe Zhennan S. Lee et F. N. Wei Seedlings in Subtropical China. Forests 2016, 7, 78. [Google Scholar] [CrossRef]
- Percy, K.E.; Manninen, S.; Haberle, K.H.; Heerdt, C.; Werner, H.; Henderson, G.W.; Matyssek, R. Effect of 3 years’ free-air exposure to elevated ozone on mature Norway spruce (Picea abies L. Karst) needle epicuticular wax physicochemical characteristics. Environ. Pollut. 2009, 157, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Rivas-Ubach, A.; Penuelas, J. The C:N:P stoichiometry of organisms and ecosystems in a changing world: A review and perspectives. Perspect. Plant Ecol. 2012, 14, 33–47. [Google Scholar] [CrossRef]
- Piikki, K.; Vorne, V.; Ojanper, K.; Pleijel, H. Impact of elevated O3 and CO2 exposure on potato (Solanum tuberosum L. cv. Bintje) tuber macronutrients (N, P, K, Mg, Ca). Agric. Ecosyst. Environ. 2007, 118, 55–64. [Google Scholar] [CrossRef]
- Yang, Y.H.; Luo, Y.Q.; Lu, M.; Schädel, C.; Han, W.X. Terrestrial C:N stoichiometry in response to elevated CO2 and N addition: A synthesis of two meta-analyses. Plant Soil 2011, 343, 393–400. [Google Scholar] [CrossRef]
- Broberg, M.C.; Högy, P.; Pleijel, H. CO2-Induced Changes in Wheat Grain Composition: Meta-Analysis and Response Functions. Agron. J. 2017, 7, 32. [Google Scholar] [CrossRef]
- Hao, X.Y.; Li, P.; Han, X.; Norton, R.M.; Lam, S.K.; Zong, Y.Z.; Sun, M.; Lin, E.; Gao, Z.Q. Effects of free-air CO2 enrichment (FACE) on N, P and K uptake of soybean in northern China. Agric. For. Meteorol. 2016, 218–219, 261–266. [Google Scholar] [CrossRef]
- Huang, W.J.; Houlton, B.Z.; Marklein, A.R.; Liu, J.X.; Zhou, G.Y. Plant stoichiometric responses to elevated CO2 vary with nitrogen and phosphorus inputs: Evidence from a global-scale meta-analysis. Sci. Rep. 2015, 5, 18225. [Google Scholar] [CrossRef] [PubMed]
- Gifford, R.M.; Barrett, D.J.; Lutze, J.L. The effects of elevated CO2 on the C:N and C:P mass ratios of plant tissues. Plant Soil 2000, 224, 1–14. [Google Scholar] [CrossRef]
- Taub, D.R.; Wang, X.Z. Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. J. Integr. Plant Biol. 2008, 50, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- De Graaff, M.A.; van Groenigen, K.J.; Six, J.; Hungate, B.; van Kessel, C. Interactions between plant growth and soil nutrient cycling under elevated CO2: A meta-analysis. Glob. Chang. Biol. 2006, 12, 2077–2091. [Google Scholar] [CrossRef]
- Feng, Z.Z.; Rütting, T.; Pleijel, H.; Wallin, G.; Reich, P.B.; Kammann, C.I.; Newton, P.C.; Kobayashi, K.; Luo, Y.Q.; Uddling, J. Constraints to Nitrogen Acquisition of Terrestrial Plants under Elevated CO2. Glob. Chang. Biol. 2015, 21, 3152–3168. [Google Scholar] [CrossRef] [PubMed]
- Bloom, A.J.; Burger, M.; Rubio-Asensio, J.S.; Cousins, A.B. Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science 2010, 328, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Bloom, A.J.; Burger, M.; Kimball, B.A.; Pinter, P.J.J. Nitrate assimilation is inhibited by elevated CO2 in field-grown wheat. Nat. Clim. Chang. 2014, 4, 477–480. [Google Scholar] [CrossRef]
- Cheng, L.; Booker, F.L.; Tu, C.; Burkey, K.O.; Zhou, L.; Shen, H.D.; Rufty, T.W.; Hu, S. Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science 2012, 337, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Houlton, B.Z.; Wang, Y.P.; Vitousek, P.M.; Field, C.B. A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature 2008, 454, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.H.; Kou, T.J.; Xu, X.F.; Zhu, J.G.; Zhu, X.K. Effects of ozone pollution on phosphorus absorption and distribution in different varieties of wheat. J. Plant Nutr. Fertil. 2012, 18, 1319–1325. (In Chinese) [Google Scholar]
- Jin, J.; Tang, C.X.; Hogarth, T.W.; Armstrong, R.; Sale, P. Nitrogen form but not elevated CO2 alters plant phosphorus acquisition from sparingly soluble phosphorus sources. Plant Soil 2014, 374, 109–119. [Google Scholar] [CrossRef]
- Johnson, D.W.; Cheng, W.; Joslin, J.D.; Norby, R.J.; Edwards, N.T.; Todd, D.E. Effects of elevated CO2 on nutrient cycling in a sweetgum plantation. Biogeochemistry 2004, 69, 379–403. [Google Scholar] [CrossRef]
- Teng, N.J.; Wang, J.; Chen, T.; Wu, X.Q.; Wang, Y.H.; Lin, J.X. Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytol. 2006, 172, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Treseder, K.K. A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol. 2004, 164, 347–355. [Google Scholar] [CrossRef]
- Broberg, M.C.; Feng, Z.; Xin, Y.; Pleijel, H. Ozone effects on wheat grain quality—A summary. Environ. Pollut. 2015, 197, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Couture, J.J.; Meehan, T.D.; Lindroth, R.L. Atmospheric change alters foliar quality of host trees and performance of two outbreak insect species. Oecologia 2012, 168, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.L.; King, J.S.; Giardina, C.P. Effects of elevated atmospheric CO2 and tropospheric O3 on nutrient dynamics: Decomposition of leaf litter in trembling aspen and paper birch communities. Plant Soil 2007, 299, 65–82. [Google Scholar] [CrossRef]
- Weigt, R.B.; Haberle, K.H.; Millard, P.; Metzger, U.; Ritter, W.; Blaschke, H.; Göttlein, A.; Matyssek, R. Ground-level ozone differentially affects nitrogen acquisition and allocation in mature European beech (Fagus sylvatica) and Norway spruce (Picea abies) trees. Tree Physiol. 2012, 32, 1259–1273. [Google Scholar] [CrossRef] [PubMed]
- King, J.; Liu, L.L.; Aspinwall, M. Tree and Forest Responses to Interacting Elevated Atmospheric CO2 and Tropospheric O3: A Synthesis of Experimental Evidence. Dev. Environ. Sci. 2013, 13, 179–208. [Google Scholar]
- Zhang, W.W.; Feng, Z.Z.; Wang, X.K.; Niu, J.F. Impacts of elevated ozone on growth and photosynthesis of Metasequoia glyptostroboides Hu et Cheng. Plant Sci. 2014, 226, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Plessl, M.; Elstner, E.F.; Rennenberg, H.; Habermeyer, J.; Heiser, I. Influence of elevated CO2 and ozone concentrations on lateblight resistance and growth of potato plants. Environ. Exp. Bot. 2007, 60, 447–457. [Google Scholar] [CrossRef]
- Thomas, V.F.D.; Braun, S.; Fliickiger, W. Effects of simultaneous ozone exposure and nitrogen loads on carbohydrate concentrations, biomass, growth, and nutrient concentrations of young beech trees (Fagus sylvatica). Environ. Pollut. 2006, 143, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Thomas, V.F.D.; Braun, S.; Fliickiger, W. Effects of simultaneous ozone exposure and nitrogen loads on carbohydrate concentrations, biomass, and growth of young spruce trees (Picea abies). Environ. Pollut. 2005, 137, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Dios, V.R.D.; Mereed, T.E.; Ferrio, J.P.; Tissue, D.T.; Voltas, J. Intraspecific variation in juvenile tree growth under elevated CO2 alone and with O3: A meta-analysis. Tree Physiol. 2016, 36, 682. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, R.I.; Elzawahry, Y.A.; Mulchi, C.L. Influence of atmospheric CO2, O3 and soil moisture regimes on carbohydrate and nitrogen contents in soybean plants. Egypt. J. Bot. 2015, 40, 115–131. [Google Scholar]
- Couture, J.J.; Meehan, T.D.; Rubert-Nason, K.F.; Lindroth, R.L. Effects of Elevated Atmospheric Carbon Dioxide and Tropospheric Ozone on Phytochemical Composition of Trembling Aspen (Populus tremuloides) and Paper Birch (Betula papyrifera). J. Chem. Ecol. 2016, 43, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Valkama, E.; Koricheva, J.; Oksanen, E. Effects of elevated O3, alone and in combination with elevated CO2, on tree leaf chemistry and insect herbivore performance: A meta-analysis. Glob. Chang. Biol. 2007, 13, 184–201. [Google Scholar] [CrossRef]
- Liu, L.L.; King, J.S.; Giardina, C.P. Effects of elevated atmospheric CO2 and tropospheric O3 on leaf litter production and chemistry in trembling aspen and paper birch ecosystems. Tree Physiol. 2005, 15, 1511–1522. [Google Scholar] [CrossRef]
- Minden, V.; Kleyer, M. Internal and external regulation of plant organ stoichiometry. Plant Biol. 2014, 16, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.P.; Ouyang, M.; Yang, G.Y.; Song, Q.N.; Guo, C.L.; Fang, X.M.; Chen, X.; Huang, L.; Chen, F.S. Research on ecological stoichiometry in bamboos: From biological basis to applications in silviculture of bamboo forests. Chin. J. Plant Ecol. 2016, 40, 264–278. (In Chinese) [Google Scholar]
- Liu, G.H.; Shi, P.J.; Xu, Q.; Dong, X.B.; Wang, F.S.; Wang, G.G.; Hui, C. Does the size–density relationship developed for bamboo species conform to the self-thinning rule? For. Ecol. Manag. 2016, 361, 339–345. [Google Scholar] [CrossRef]
- Zhuang, M.H.; Chen, S.L.; Li, Y.C.; Guo, Z.W.; Yang, Q.P. Effects of elevated O3 and CO2 concentrations on the membrane lipid peroxidation and antioxidant system of Oligostachyum lubricum leaves. J. Ecol. 2012, 31, 2184–2190. (In Chinese) [Google Scholar]
- Zhuang, M.H.; Li, Y.C.; Guo, Z.W.; Yang, Q.P.; Gu, L.J.; Chen, S.L.; Deng, Z.F. Effects of elevated O3 and/or CO2 n the membrane lipid peroxidation and antioxidant system in Phyllostachys eduis leaves. Acta Bot. Boteal. Occident. Sin. 2013, 33, 322–328. (In Chinese) [Google Scholar]
- Zhuang, M.H.; Li, Y.C.; Chen, S.L. Differences of O3 stress tolerance between Phyllostachys edulis and Oligostachyum lubricum. J. Ecol. 2011, 30, 2191–2196. (In Chinese) [Google Scholar]
- Zhuang, M.H.; Lam, S.K.; Li, Y.C.; Chen, S.L. Elevated tropospheric ozone affects the concentration and allocation of mineral nutrients of two bamboo species. Sci. Total Environ. 2017, 577, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Page, A.L. Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; American Society of Agronomy; Soil Science Society of America: Madison, WI, USA, 1982; p. 1159. [Google Scholar]
- Couture, J.J.; Holeski, L.M.; Lindroth, R.L. Long-term exposure to elevated CO2 and O3 alters aspen foliar chemistry across developmental stages. Plant Cell Environ. 2014, 37, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.Q.; Hui, D.F.; Zhang, D.Q. Elevated CO2 stimulates net accumulations of carbon and nitrogen in land ecosystems: A meta-analysis. Ecology 2006, 87, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Loladze, L. Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. eLife 2014, 3, e02245. [Google Scholar] [CrossRef] [PubMed]
- Usue, P.L.; Jon, M.A.; Maite, L.; Amaia, M.P.; Alberto, M.R. Growth and nutritional quality improvement in two differently pigmented lettuce cultivars grown under elevated CO2 and/or salinity. Sci. Hortic. 2015, 195, 56–66. [Google Scholar]
- Penny, M.G.; Bowling, D.J.F. A study of potassium gradients in the epidermis of intact levels of Commelina communis L. in relation to stomatal opening. Planta 1974, 119, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Kanowski, J. Effects of elevated CO2 on the foliar chemistry of seedlings of two rainforest trees from north-east Australia: Implications for folivorous marsupials. Austral Ecol. 2001, 26, 165–172. [Google Scholar] [CrossRef]
- Li, P.; Han, X.; Zong, Y.; Li, P.; Lin, E.D.; Han, Y.H.; Hao, X.Y. Effects of free-air CO2 enrichment (FACE) on the uptake and utilization of N, P and K in Vigna radiate. Agric. Ecosyst. Environ. 2015, 202, 120–125. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Watanabe, M.; Iwasaki, M.; Tabe, C.; Matsumura, H.; Kohno, Y.; Izuta, T. Growth and photosynthetic responses of Fagus crenata seedlings to O3 under different nitrogen loads. Trees 2007, 21, 707–718. [Google Scholar] [CrossRef]
- Li, S.; Zheng, Y.F.; Wu, R.J.; Yin, J.F.; Xu, J.X. Research progress on plant vegetable growth and protection under ozone pollution stress. J. Agric. Sci. Technol. 2014, 16, 117–124. (In Chinese) [Google Scholar]
- Li, P.; Feng, Z.Z.; Catalayud, V.; Yuan, X.Y.; Xu, Y.S.; Paoletti, E. A meta-analysis on growth, physiological, and biochemical responses of woody species to ground-level ozone highlights the role of plant functional types. Plant Cell Environ. 2017, 40, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Ashmore, M.R. Assessing the future global impacts of ozone on vegetation. Plant Cell Environ. 2005, 28, 949–964. [Google Scholar] [CrossRef]
- Li, P.; Calatayud, V.; Gao, F.; Uddling, J.; Feng, Z.Z. Differences in ozone sensitivity among woody species are related to leaf morphology and antioxidant levels. Tree Physiol. 2016, 36, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Pinkard, E.A.; Beadle, C.L.; Mendham, D.S.; Carter, J.; Glen, M. Determining photosynthetic responses of forest species to elevated [CO2]: Alternatives to FACE. For. Ecol. Manag. 2010, 260, 1251–1261. [Google Scholar] [CrossRef]
- Lu, T.; He, X.Y.; Chen, W.; Yan, K.; Zhao, T. Effects of elevated O3 and/or elevated CO2 on lipid peroxidation and antioxidant systems in Ginkgo biloba leaves. Bull. Environ. Contam. Toxicol. 2009, 83, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Chen, W.; Zhang, G.Y.; Xu, S.; Liu, Z.L.; He, X.Y.; Wang, L.L. Elevated CO2 ameliorated oxidative stress induced by elevated O3 in Quercus mongolica. Acta Physiol. Plant. 2010, 69, 198–204. [Google Scholar] [CrossRef]
- Zeng, Q.; Liu, B.; Gilna, B.; Zhang, Y.L.; Zhu, C.W.; Ma, H.L.; Pang, J.; Chen, G.P.; Zhu, J.G. Elevated CO2 effects on nutrient competition between a C3 crop (Oryza sativa L.) and a C4 weed (Echinochloa crusgalli L.). Nutr. Cycl. Agroecosyst. 2011, 89, 3–4. [Google Scholar] [CrossRef]
- Nie, G.Y.; Tomasevic, M.; Baker, N.R. Effects of ozone on the photosynthetic apparatus and leaf proteins during leaf development in plants. Plant Cell Environ. 1993, 16, 643–651. [Google Scholar] [CrossRef]
| O3 (nmol mL−1) | CO2 (μmol mol−1) | |
|---|---|---|
| 360~380 | 685~730 | |
| 40~45 | Ambient air | Elevated CO2 |
| 92~106 | Elevated O3 | Combined elevated CO2 and O3 |
| Bamboo Species | Determination Index | Treatments | |||
|---|---|---|---|---|---|
| Ambient Air | Elevated CO2 | Elevated O3 | Combined Elevated CO2 and O3 | ||
| Phyllostachys edulis (Carrière) J.Houz. | N | 19.37 ± 0.46b | 22.96 ± 0.41a | 21.20 ± 0.58ab | 20.50 ± 0.61b |
| P | 1.27 ± 0.04a | 0.97 ± 0.02b | 1.35 ± 0.04a | 1.01 ± 0.05b | |
| K | 8.41 ± 0.10ab | 5.88 ± 0.82c | 9.17 ± 0.89a | 7.34 ± 0.96bc | |
| Oligostachyum lubricum (wen) King f. | N | 17.97 ± 1.07a | 15.37 ± 0.45b | 18.30 ± 0.40a | 15.90 ± 0.89b |
| P | 1.05 ± 0.08a | 0.81 ± 0.05b | 1.07 ± 0.42a | 1.06 ± 0.16a | |
| K | 4.25 ± 0.45b | 5.62 ± 0.20a | 6.35 ± 0.39a | 5.60 ± 0.63a | |
| Bamboo Species | Treatments | |||
|---|---|---|---|---|
| Ambient Air | Elevated CO2 | Elevated O3 | Combined Elevated CO2 and O3 | |
| Phyllostachys edulis | 73.17 ± 6.01b | 91.39 ± 7.14a | 47.54 ± 3.53c | 80.34 ± 4.84b |
| Oligostachyum lubricum | 62.67 ± 2.28b | 75.77 ± 1.87a | 45.97 ± 2.79c | 58.07 ± 2.24b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, M.; Li, Y.; Guo, Z.; Li, Y.; Pan, W.; Chen, S. Elevated CO2 and O3 Levels Influence the Uptake and Leaf Concentration of Mineral N, P, K in Phyllostachys edulis (Carrière) J.Houz. and Oligostachyum lubricum (wen) King f. Forests 2018, 9, 195. https://doi.org/10.3390/f9040195
Zhuang M, Li Y, Guo Z, Li Y, Pan W, Chen S. Elevated CO2 and O3 Levels Influence the Uptake and Leaf Concentration of Mineral N, P, K in Phyllostachys edulis (Carrière) J.Houz. and Oligostachyum lubricum (wen) King f. Forests. 2018; 9(4):195. https://doi.org/10.3390/f9040195
Chicago/Turabian StyleZhuang, Minghao, Yingchun Li, Ziwu Guo, Yueqiao Li, Wenting Pan, and Shuanglin Chen. 2018. "Elevated CO2 and O3 Levels Influence the Uptake and Leaf Concentration of Mineral N, P, K in Phyllostachys edulis (Carrière) J.Houz. and Oligostachyum lubricum (wen) King f." Forests 9, no. 4: 195. https://doi.org/10.3390/f9040195




