The Impact of Effective Microorganisms (EM) and Organic and Mineral Fertilizers on the Growth and Mycorrhizal Colonization of Fagus sylvatica and Quercus robur Seedlings in a Bare-Root Nursery Experiment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Survey Area and Experimental Design
2.2. Growth Parameters and Ectomycorrhizal Assessment
2.3. Molecular Identification of Mycorrhizal Root Tips
2.4. Data Analysis
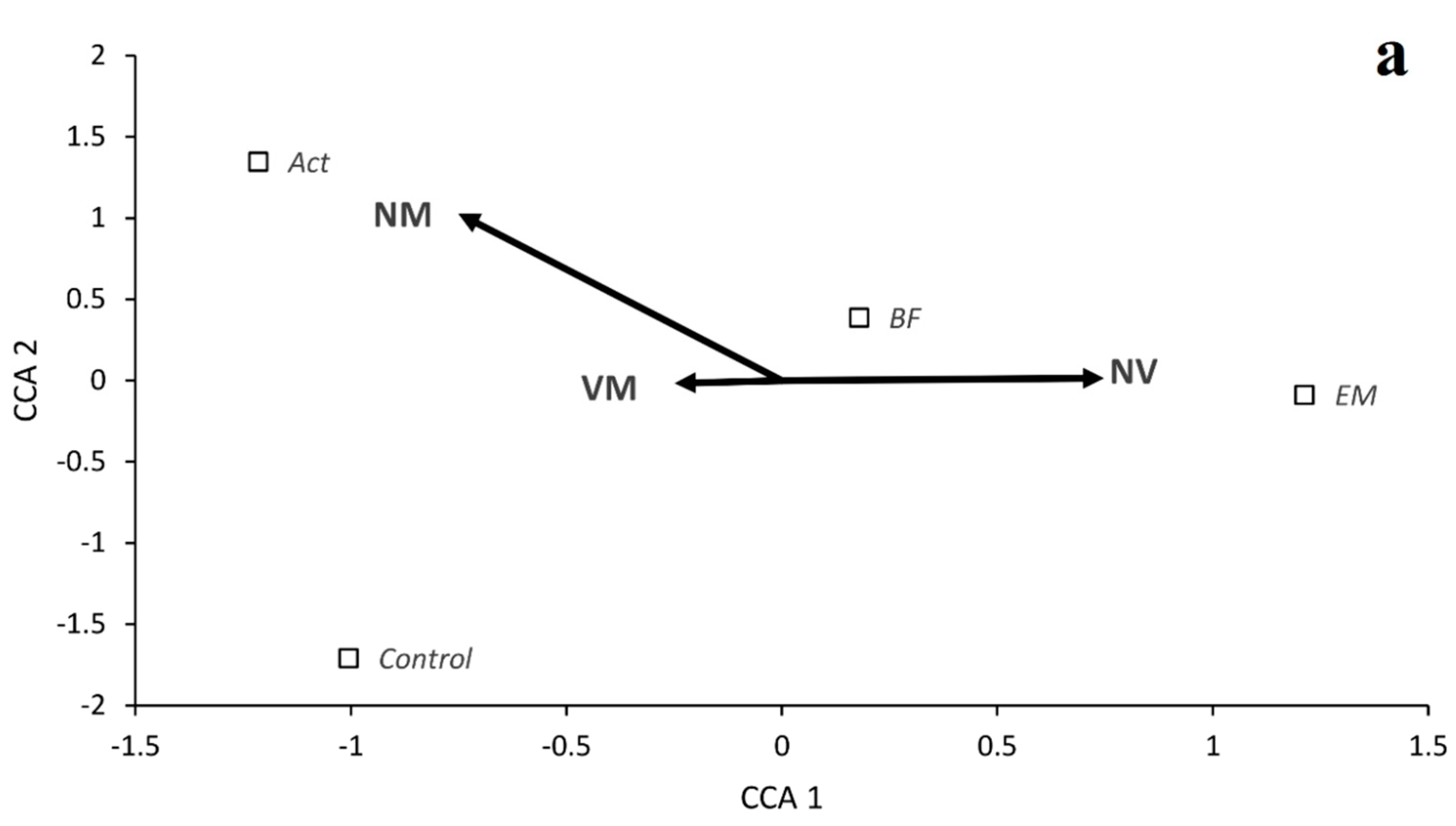
3. Results
Growth of Seedlings and Mycorrhizal Colonization
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fao.org. Available online: http:// www.fao.org/ (accessed on 15 November 2017).
- Payn, T.; Carnus, J.-M.; Freer-Smith, P.; Kimberley, M.; Kollert, W.; Liu, S.; Orazio, C.; Rodriguez, L.; Neves Silva, L.; Wingfield, M.J. Changes in planted forests and future global implications. For. Ecol. Manag. 2015, 352, 57–67. [Google Scholar] [CrossRef]
- Central Statistical Office. Forestry 2017. Warsaw 2017. Available online: https://stat.gov.pl/en/topics/agriculture-forestry/forestry/forestry-2017,1,8.html (accessed on 25 September 2018).
- Central Statistical Office. Report on the Condition of Forests in Poland 2017. Warsaw 2017. Available online: http://www.lasy.gov.pl/pl/informacje/publikacje/in-english/forests-in-poland/lasy-w-polsce-2017-en.pdf (accessed on 25 September 2018).
- Ducousso, A.; Bordacs, S. EUFORGEN Technical Guidelines for Genetic Conservation and Use for Pedunculate and Sessile oaks (Quercus robur and Q. petraea); International Plant Genetic Resources Institute: Rome, Italy, 2004. [Google Scholar]
- Von Wuehlisch, G. EUFORGEN Technical Guidelines for Genetic Conservation and Use for European Beech (Fagus sylvatica); Bioversity International: Rome, Italy, 2008. [Google Scholar]
- Wang, B.; Qiu, Y.L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Myzorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.; Pausch, M.; Urban, A. Diversity of ectomycorrhizal morphotypes and oak decline. Phyton Ann. Rei Bot. A 2000, 40, 109–116. [Google Scholar]
- Mosca, E.; Montecchio, L.; Sella, L.; Garbaye, J. Short-term effect of removing tree competition on the ectomycorrhizal status of a declining pedunculate oak forest (Quercus robur L.). For. Ecol. Manag. 2007, 244, 129–140. [Google Scholar] [CrossRef]
- Leski, T.; Pietras, M.; Rudawska, M. Ectomycorrhizal fungal communities of pedunculate and sessile oak seedlings from bare-root forest nurseries. Mycorrhiza 2010, 20, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Pena, R.; Offermann, C.; Simon, J.; Naumann, P.S.; Geßler, A.; Holst, J.; Dannenmann, M.; Mayer, H.; Kögel-Knabner, I.; Rennenberg, H.; et al. Girdling affects ectomycorrhizal fungal (EMF) diversity and reveals functional differences in EMF community composition in a beech forest. Appl. Environ. Microbiol. 2010, 76, 1831–1841. [Google Scholar] [CrossRef] [PubMed]
- Pietras, M.; Rudawska, M.; Leski, T.; Karliński, L. Diversity of ectomycorrhizal fungus assemblages on nursery grown European beech seedlings. Ann. For. Sci. 2013, 70, 115–121. [Google Scholar] [CrossRef]
- Pena, R.; Polle, A. Attributing functions to ectomycorrhizal fungal identities in assemblages for nitrogen acquisition under stress. ISME J. 2014, 8, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Leberecht, M.; Dannenmann, M.; Gschwendtner, S.; Bilela, S.; Meier, R.; Simon, J.; Rennenberg, H.; Schloter, M.; Polle, A. Ectomycorrhizal communities on the roots of two beech (Fagus sylvatica) populations from contrasting climates differ in nitrogen acquisition in a common environment. Appl. Environ. Microbiol. 2015, 81, 5957–5967. [Google Scholar] [CrossRef] [PubMed]
- Brundrett, M.C. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002, 154, 275–304. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: Cambridge, UK, 2008. [Google Scholar]
- Dahm, H.; Strzelczyk, E.; Cisielska, A.; Redlak, K. The effect of mycorrhizal fungi and bacteria on pine seedlings. Acta Mycol. 1988, 33, 25–36. [Google Scholar] [CrossRef]
- Vaario, L.; Tervonen, A.; Haukioja, K.; Haukioja, M.; Pennanen, T.; Timonen, S. The effect of nursery substrate and fertilization on the growth and ectomycorrhizal status of containerized and outplanted seedlings of Picea abies. Can. J. For. Res. 2009, 39, 64–75. [Google Scholar] [CrossRef]
- Sousa, N.R.; Franco, A.R.; Oliveira, R.S.; Castro, P.M.L. Ectomycorrhizal fungi as an alternative to the use of chemical fertilisers in nursery production of Pinus pinaster. J. Environ. Manag. 2012, 95, S269–S274. [Google Scholar] [CrossRef] [PubMed]
- Wesoły, W.; Hauke, M. Szkółkarstwo Leśne od A do Z; CILP: Warszawa, Poland, 2009; pp. 118–254.
- Walker, R.F. Growth and nutritional responses of containerized sugar and Jeffrey pine seedlings to controlled release fertilization and induced mycorrhization. For. Ecol. Manag. 2001, 149, 163–179. [Google Scholar] [CrossRef]
- Rincón, A.; Parladé, J.; Pera, J. Influence of the fertilisation method in controlled ectomycorrhizal inoculation of two Mediterranean pines. Ann. For. Sci. 2007, 64, 577–583. [Google Scholar] [CrossRef] [Green Version]
- Edmonds, R.L. General Strategies of Forest Disease Management. In Infectious Forest Diseases; Lilja, A., Poteri, M., Gonthier, P., Nicolotti, G., Eds.; CBA International: Wallingford, UK, 2013; pp. 29–49. [Google Scholar]
- Arnebrant, K. Nitrogen amendments reduce the growth of extramatical mycelium. Mycorrhiza 1994, 5, 7–15. [Google Scholar] [CrossRef]
- Liu, Q.; Loganathan, P.; Hedley, M.J.; Grace, L.J. Effect of mycorrhizal inoculationon rhizosphere properties, phosphorus uptake and growth of pine seedlings treated with and without a phosphate rock fertilizer. J. Plant Nutr. 2008, 31, 137–156. [Google Scholar] [CrossRef]
- Van Wambeke, E.; Van Assche, C.; Vanachter, A. Long term risks or benefits from soil fumigant use? Med. Fac. Landbouww. Univ. Gent. 1992, 57, 1261–1268. [Google Scholar]
- Cordell, C.E.; Marx, D.H. Effects of nursery cultural practices on management of specific ectomycorrhizae on bareroot tree seedlings. In Mycorrhizas and Plant Health; Pfleger, F.L., Linderman, R.G., Eds.; APS Press: St-Paul, MN, USA, 1994; pp. 133–151. [Google Scholar]
- Higa, T. Effective microorganisms: A biotechnology for mankind. In Proceedings of the First International Conference on Kyusei Nature Farming, Khon Kaen, Thailand, 17–21 October 1989; Parr, J.F., Hornick, S.B., Eds.; U.S. Department of Agriculture: Washington, DC, USA, 1991; pp. 8–14. [Google Scholar]
- Higa, T.; Parr, J.F. Beneficial and Effective Microorganisms for a Sustainable Agriculture and Environment; International Nature Farming Research Center: Atami, Japan, 1994. [Google Scholar]
- Curtis, H.; Noll, U.; Störmann, J.; Slusarenko, A.J. Broad-spectrum activity of the volatile phytoanticipin allicin in extracts of garlic (Allium sativum L.) against plant pathogenic bacteria, fungi and Oomycetes. Physiol. Mol. Plant Pathol. 2004, 65, 79–89. [Google Scholar] [CrossRef]
- Slusarenko, A.J.; Patel, A.; Portz, D. Control of plant diseases by natural products: Allicin from garlic as a case study. Eur. J. Plant Pathol. 2008, 121, 313–322. [Google Scholar] [CrossRef]
- Higa, T. Effective microorganisms, concept and recent advances in technology. In Proceedings of the 4th International Conference on Kyusei Nature Farming in Bellingham, Washington, DC, USA, 19–21 June 1995; Parr, J.F., Ed.; Department of Agriculture: Washingtod, DC, USA, 1998; pp. 247–248. [Google Scholar]
- Montecchio, L.; Causin, R.; Rossi, S.; Mutto Accordi, S. Changes in ectomycorrhizal diversity in a declining Quercus ilex coastal forest. Phytopathol. Mediterr. 2004, 43, 26–34. [Google Scholar]
- Agerer, R. Colour Atlas of Ectomycorrhizae, 1st–15th ed.; Einhorn: Munich, Germany, 1987–2008. [Google Scholar]
- Agerer, R.; Rambold, G. DEEMY—An Information System for Characterization and Determination of Ectomycorrhizae, 2004–2015. Available online: http://www.deemy.de (accessed on 12 December 2015).
- Thermofisher.com. Available online: https://www.thermofisher.com/ (accessed on 15 November 2017).
- Ncbi.nlm.nih.gov. Available online: http://www.ncbi.nlm.nih.gov/ (accessed on 15 November 2017).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.B. Vegan: Community Ecology Package. R Package Version 2.4-2. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 24 August 2017).
- Shokouhian, A.A.; Davarynejad, G.H.; Tehranifar, A.; Imani, A.; Rasoulzadeh, A. Investigation of Effective Microorganisms (EM) impact in water stress condition on growth of almond (Prunus dulcis mill) seedling. J. Basic. Appl. Sci. Res. 2013, 3, 86–92. [Google Scholar]
- Khan, B.M.; Hossain, M.K.; Mridha, M.A.U. Effect of microbial inoculants on Albizia saman germination and seedling growth. J. For. Res. 2007, 17, 99–102. [Google Scholar] [CrossRef]
- Mohamed, F.; Sahain, M.; Elham, Z.; Motty, A.; Mohamed, H.; Shiekh, El.; Laila, F. Effect of some biostimulant on growth and fruiting of Anna apple trees in newly reclaimed areas. Res. J. Agric. Biol. Sci. 2007, 3, 422–429. [Google Scholar]
- Olle, M.; Williams, I.H. Effective microorganisms and their influence on vegetable production—A review. J. Hortic. Sci. Biotechnol. 2015, 88, 380–386. [Google Scholar] [CrossRef]
- Higa, T.; Wididana, G.N. The concept and theories of effective microorganisms. In Proceedings of the First International Conference on Kyusei Nature Farming, Khon Kaen, Tailand, 17–21 October 1989; Parr, J.F., Hornick, S.B., Whitman, C.E., Eds.; Department of Agriculture: Washington, DC, USA, 1991; pp. 118–124. [Google Scholar]
- Javaid, A. Foliar application of effective microorganisms on pea as an alternative fertilizer. Agron. Sustain. Dev. 2006, 26, 257–262. [Google Scholar] [CrossRef]
- Pati, B.R.; Chandra, A.K. Effect of spraying nitrogen fixing phyllospheric bacterial isolates on wheat plants. Plant Soil 1981, 61, 419–427. [Google Scholar] [CrossRef]
- Górski, R.; Kleiber, T. Effect of Effective Microorganisms (EM) on nutrient contents in substrate and development and yielding of Rose (Rosa x hybrida) and Gerbera (Gerbera jamesonii). Ecol. Chem. Eng. S 2010, 17, 505–513. [Google Scholar]
- Mayera, J.; Scheida, S.; Widmera, F.; Fliessbach, A.; Oberholzer, H.R. How effective are effective microorganisms (EM)? Results from a field study in temperate climate. Appl. Soil Ecol. 2010, 46, 230–239. [Google Scholar]
- Araújo, G.C.; Sousa, N.R.; Ramos, M.; Vega, A.; Castro, P.M.L. Performance of Quercus suber L. at nursery stage—Application of two bio-inoculants under two distinct environments. Ann. For. Sci. 2018, 75. [Google Scholar] [CrossRef]
- Castellano, S.M.; Gorchov, D.L. Reduced ectomycorrhizae on oak near invasive garlic mustard. Northeast. Nat. 2012, 19, 1–24. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Wolfe, B.E.; Rodgers, V.L.; Stinson, K.A.; Pringle, A. The invasive plant Alliaria petiolata (Garlic Mustard) inhibits ectomycorrhizal fungi in its introduced range. J. Ecol. 2008, 96, 777–783. [Google Scholar] [CrossRef]
- Perry, D.A.; Molina, R.; Amaranthus, M.P. Mycorrhizae, mycorrhizospheres and reforestation: Current knowledge and research needs. Can. J. For. Res. 1987, 17, 929–940. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Qi, Y. Long-term effective microorganisms application promote growth and increase yields and nutrition of wheat in China. Eur. J. Agron. 2013, 46, 3–67. [Google Scholar] [CrossRef]
- Khaliq, A.; Kaleem Abbasi, M.; Hussain, T. Effects of integrated use of organic and inorganic nutrient sources with effective microorganisms (EM) on seed cotton yield in Pakistan. Bioresour. Technol. 2006, 97, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Quoreshi, A.M. Nutritional preconditioning and ectomycorrhizal formation of Picea mariana (Mill.) B.S.P. seedlings. Eurasian J. For. Res. 2003, 6, 1–63. [Google Scholar]


| Abundance (%) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fagus sylvatica | Quercus robur | |||||||||||||||
| Indices | Control | Act | BF | EM | Control | Act | BF | EM | ||||||||
| VM | 99.4 | a | 99.2 | a | 83.3 | b | 55.2 | c | 90.5 | a | 99.3 | a | 96.5 | a | 52.2 | b |
| NV | 0.6 | c | 0.8 | c | 16.6 | b | 44.8 | a | 1.4 | b | 0.7 | b | 3.5 | b | 47.8 | a |
| NM | 0.0 | a | 0.0 | a | 0.0 | a | 0.0 | a | 8.1 | a | 0.0 | b | 0.0 | b | 0.0 | b |
| BLAST Top-Hit | Relative Abundance/Frequency | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fagus sylvatica | Quercus robur | |||||||||
| Identification | Closest Match | NCBI | Control | Act | BF | EM | Control | Act | BF | EM |
| Ascomycota | ||||||||||
| Pezizaceae sp. | Pezizaceae | MH040299 | 53/72 | 85/100 | 43.7/60 | 0.9/8 | ||||
| Peziza ostracoderma | Peziza sp. | MH040300 | 5.2/24 | 3.4/32 | 24/67 | 6/33 | 43.3/93 | 20.4/67 | ||
| Tuber maculatum | Tuber maculatum | MH040280 | 16/67 | 9.6/47 | 7.3/67 | 13.4/73 | ||||
| Tuber sp. | Tuber sp. | MH040296 | 5.3/68 | 6.5/44 | 7.2/92 | 6.2/100 | ||||
| Cenococcum-like | Cenococcum geophilum | 1.2/24 | 0.2/16 | 0.8/36 | 4.2/28 | 2.7/27 | 7.8/27 | |||
| Basidiomycota | ||||||||||
| Hebeloma sp. | Hebeloma sacchariolens | MH040294 | 40.1/32 | 3.2/44 | 42.4/32 | 88.8/32 | ||||
| Naucoria salicis | Naucoria salicis | MH040281 | 28.5/93 | 26/73 | 16.8/67 | 13.2/60 | ||||
| Scleroderma areolatum | Scleroderma areolatum | MH040287 | 0.04/4 | 10.5/40 | 8.6/47 | 29/73 | 11.1/40 | |||
| Laccaria tortilis | Laccaria tortilis | MH040289 | 3.4/27 | 5.7/40 | 4.3/53 | |||||
| Inocybe curviceps | Inocybe curviceps | MH040283 | 1.9/20 | 36.2/87 | 4.5/20 | |||||
| Clavulina sp. | Clavulina sp. | MH040298 | 0.4/4 | 2.5/4 | ||||||
| Observed species richness | 5 | 6 | 6 | 4 | 7 | 7 | 6 | 4 | ||
| Mean species richness per seedling | 4 | 4 | 4 | 3 | 6 | 5 | 6 | 3 | ||
| Shannon diversity index (Hʹ) | 0.55 ab | 0.55 ab | 0.66 a | 0.44 b | 1.03 a | 1.11 a | 1.04 a | 0.70 b | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bzdyk, R.M.; Olchowik, J.; Studnicki, M.; Oszako, T.; Sikora, K.; Szmidla, H.; Hilszczańska, D. The Impact of Effective Microorganisms (EM) and Organic and Mineral Fertilizers on the Growth and Mycorrhizal Colonization of Fagus sylvatica and Quercus robur Seedlings in a Bare-Root Nursery Experiment. Forests 2018, 9, 597. https://doi.org/10.3390/f9100597
Bzdyk RM, Olchowik J, Studnicki M, Oszako T, Sikora K, Szmidla H, Hilszczańska D. The Impact of Effective Microorganisms (EM) and Organic and Mineral Fertilizers on the Growth and Mycorrhizal Colonization of Fagus sylvatica and Quercus robur Seedlings in a Bare-Root Nursery Experiment. Forests. 2018; 9(10):597. https://doi.org/10.3390/f9100597
Chicago/Turabian StyleBzdyk, Roman Mariusz, Jacek Olchowik, Marcin Studnicki, Tomasz Oszako, Katarzyna Sikora, Hanna Szmidla, and Dorota Hilszczańska. 2018. "The Impact of Effective Microorganisms (EM) and Organic and Mineral Fertilizers on the Growth and Mycorrhizal Colonization of Fagus sylvatica and Quercus robur Seedlings in a Bare-Root Nursery Experiment" Forests 9, no. 10: 597. https://doi.org/10.3390/f9100597





