Provenance Variation in Phenology and Frost Tolerance in Subalpine Fir (Abies lasiocarpa) Planted in Denmark and Iceland †
Abstract
:1. Introduction
- Provenance phenology for flushing and bud set
- Provenance ranking in frost sensitivity in late spring and early autumn based on tests in freezing chambers
- Relationships between date of flushing, spring frost tolerance, survival rates and the geographical origin of provenances
- Relationships between bud set, autumn frost tolerance, survival rates and the geographical origin of provenances.
2. Materials and Methods
2.1. Provenances and Sites
2.2. Phenology
2.3. Survival
2.4. Freezing Tests
2.5. Statistical Analysis
3. Results
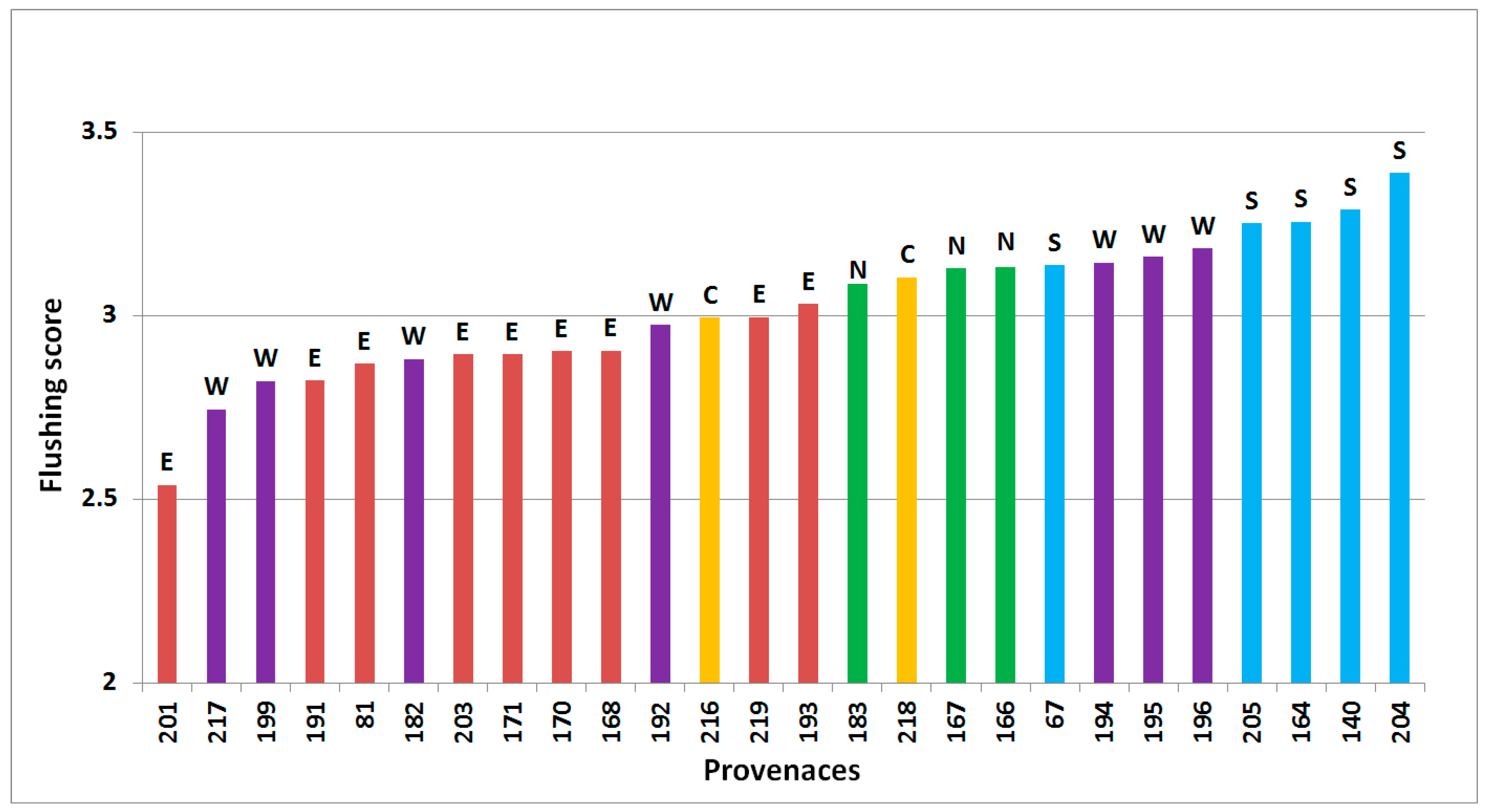
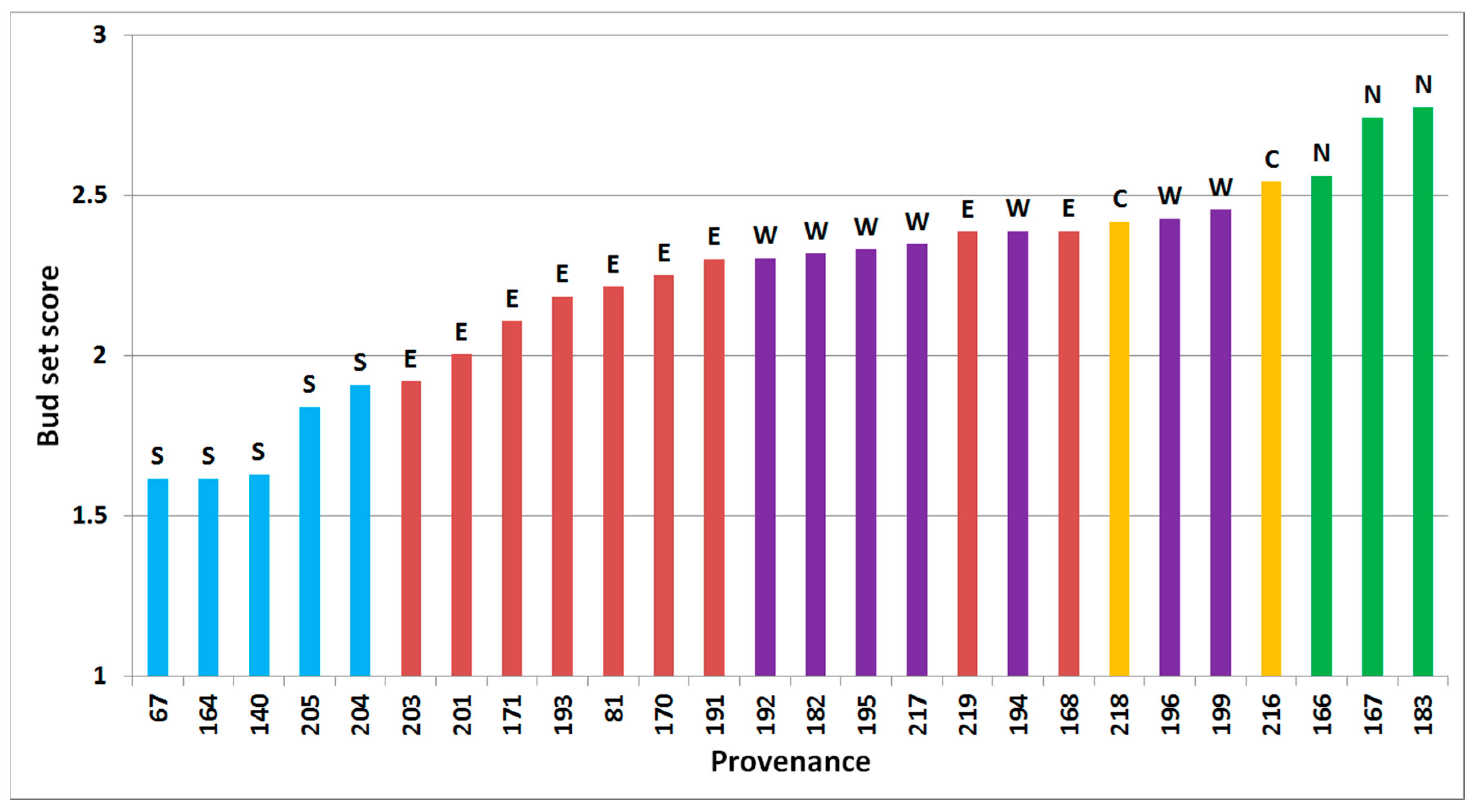
3.1. Phenology
3.2. Survival
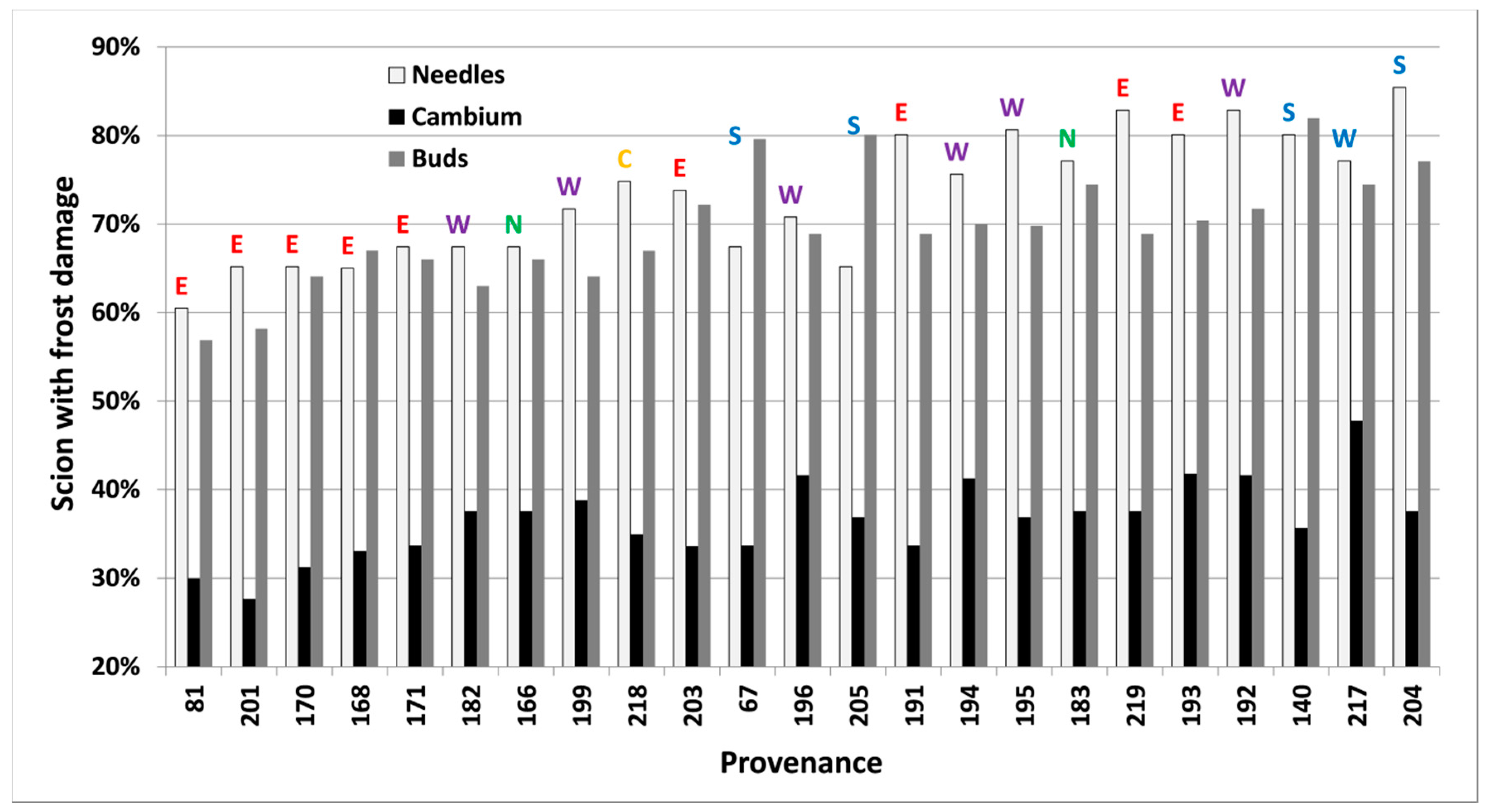
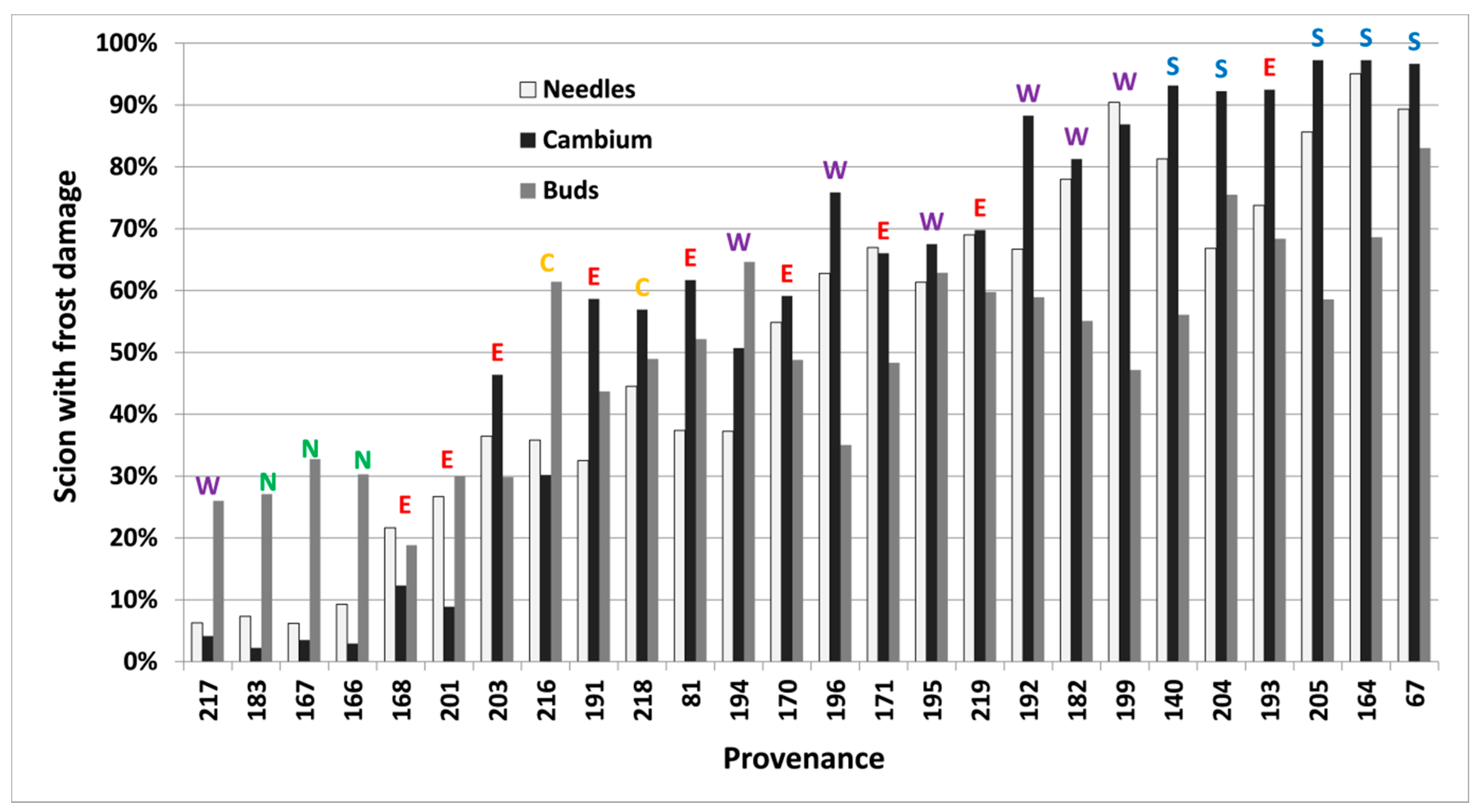
3.3. Freezing Tests
3.4. Correlations
4. Discussion
4.1. Methods and Assessment
4.2. Bud Set and Autumn Frost Hardiness
4.3. Flushing and Spring Frost Hardiness
4.4. Frost Hardiness and Survival
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lange, J. Kulturplanternes Indførselshistorie i Danmark: Indtil Midten af 1900-Tallet; DSR Forlag: Fredriksberg, Denmark, 1999; ISBN 9788774324041. (In Danish) [Google Scholar]
- Christensen, K.I. Nåletræer I Danmark og Norden-en Bestemmelseshåndbog; Dansk Dendrologisk Forening: Copenhagen, Denmark, 2009; ISBN 9788799354801. (In Danish) [Google Scholar]
- Blöndal, S.; Gunnarsson, S.B. Íslandsskógar: Hundrað ára; Mál og mynd: Reykjavík, Iceland, 1999; ISBN 9979939982. (In Icelandic) [Google Scholar]
- Christensen, M.; Emborg, J. Biodiversity in natural versus managed forest in Denmark. For. Ecol. Manag. 1996, 85, 47–51. [Google Scholar] [CrossRef]
- Kjær, E.D.; Lobo, A.; Myking, T. The role of exotic tree species in Nordic forestry. Scand. J. For. Res. 2014, 29, 323–332. [Google Scholar] [CrossRef]
- Jakobsen, F. Juletræer og klippegrønt. In Skoven og Dens Dyrkning; Henriksen, H.A., Ed.; Dansk Skovforening: Copenhagen, Denmark, 1988; pp. 607–636. ISBN 9788717059375. (In Danish) [Google Scholar]
- Larsen, J.B.; Nielsen, U.B.; Møller, I.S. Nordmannsgran—Proveniensvariation, forædling og frøkildevalg. In Træarts—og Proveniensvalget i et Bæredygtigt Skovbrug; Larsen, J.B., Ed.; Dansk Skovforening: Copenhagen, Denmark, 1997; pp. 203–213. (In Danish) [Google Scholar]
- Nielsen, U.B. Genetic variation in characters important for noble fir greenery production. Scand. J. For. Res. 2007, 22, 99–109. [Google Scholar] [CrossRef]
- Madsen, S.F.; Sigurgeirsson, A. Abies lasiocarpa/a promising species for Christmas tree production. In Inter-Nordic Research Programme; A report by the SNS Nordic-Network-Group for research and development of subalpine fir, Abies lasiocarpa, for the production of Christmas trees and greenery in the Nordic countries; SNS/Nordic Forest Research Co-operation Committee: Alnarp, Sweden, 1998. [Google Scholar]
- Alexander, R.R.; Shearer, R.C.; Shepperd, W.D. Silvical Characteristics of Subalpine Fir; General Technical Reports; RM-115; USDA Forest Service: Fort Collins, CO, USA, 1984.
- Thompson, R.S.; Anderson, K.H.; Bartlein, P.J. Atlas of Relations between Climatic Parameters and Distributions of Important Trees and Shrubs in North America. U.S. Available online: http://pubs.usgs.gov/pp/p1650-a/ (accessed on 5 March 2016).
- Langlet, O. Patterns and Terms of Intra-Specific Ecological Variability. Nature 1963, 200, 347–348. [Google Scholar] [CrossRef]
- Alberto, F.J.; Aitken, S.N.; Alia, R.; Gonzalez-Martinez, S.C.; Hänninen, H.; Kremer, A.; Lefevre, F.; Lenormand, T.; Yeaman, S.; Whetten, R.; et al. Potential for evolutionary responses to climate change evidence from tree populations. Glob. Chang. Biol. 2013, 19, 1645–1661. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.R.; Hamann, A. Glacial refugia and modern genetic diversity of 22 western North America tree species. Proc. R. Soc. B. 2015. [Google Scholar] [CrossRef] [PubMed]
- Hansen, O.K.; Nielsen, U.B.; Edvardsen, Ø.M.; Skulason, B.; Skage, J.-O. Nordic provenance trials with Abies lasiocarpa and Abies lasiocarpa var. arizonica: Three-year results. Scand. J. For. Res. 2004, 19, 112–126. [Google Scholar] [CrossRef]
- Bannister, P.; Neuner, G. Frost Resistance and the Distribution of Conifers. In Conifer Cold Hardiness; Bigras, F.J., Colombo, S.J., Eds.; Kluwer Academic Publisher: Boston, MA, USA, 2001; pp. 3–11. ISBN 9780792366362. [Google Scholar]
- Cannell, M.G.R.; Smith, R.I. Spring frost damage on young Picea sitchensis. 2. Predicted dates of budburst and probability of frost damage. Forestry 1984, 57, 177–197. [Google Scholar] [CrossRef]
- Cannell, M.G.R.; Sheppard, L.J.; Smith, R.I.; Murray, M.B. Autumn frost damage on young Picea sitchensis. 2. Shoot frost hardening, and the probability of frost damage in Scotland. Forestry 1985, 58, 145–166. [Google Scholar] [CrossRef]
- Timmis, R.; Flewelling, J.; Talbert, C. Frost injury prediction model for Douglas-fir seedlings in the Pacific Northwest. Tree Physiol. 1994, 14, 855–869. [Google Scholar] [CrossRef] [PubMed]
- Einarsson, M.A. Climate of Iceland. In World Survey of Climatology. Climates of the Oceans; van Loon, H., Ed.; Elsevier: Amsterdam, The Netherlands, 1984; Volume 15, pp. 673–697. [Google Scholar]
- Eysteinsson, T.; Skulason, B. Adaptation of Siberian and Russian larch provenances to spring frosts and cold summers. Icel. Agric. Sci. 1995, 9, 91–97. [Google Scholar]
- Rasmussen, H.N.; Nielsen, C.C.N. Klima og frostskader i nobilis og nordmannsgran. Pyntegrønt Videnblade 2007, 5, 8–10. (In Danish) [Google Scholar]
- Nielsen, C.C.N.; Rasmussen, H.N. Frost hardening and dehardening in Abies procera and other conifers under differing temperature regimes and warm-spell treatments. Forestry 2009, 8, 43–59. [Google Scholar] [CrossRef]
- Langlet, O. Mellaneuropeiska granprovenienser i svenskt skogsbruk. Kungl. Skogs-Lantbruksakad. Tidskr. 1960, 5–6, 259–329. (In Norwegian) [Google Scholar]
- Johnsen, Ø.; Apeland, I. Screening early autumn frost hardiness among progenies from Norway spruce seed orchards. Silva Fenn. 1988, 22, 203–212. [Google Scholar] [CrossRef]
- Skrøppa, T. Within-population variation in autumn frost hardiness and its relationship to bud-set and height growth in Picea abies. Scand. J. For. Res. 1991, 6, 353–363. [Google Scholar] [CrossRef]
- Astronomical Applications Department of the U.S. Naval Observatory. Complete Sun and Moon Data for One Day. Available online: http://aa.usno.navy.mil/data/docs/RS_OneDay.php (accessed on 7 January 2016).
- Johnsen, Ø. Freeze-testing young Picea abies plants. A methodological study. Scand. J. For. Res. 1989, 4, 351–367. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. Available online: http://www.R-project.org/ (accessed on 3 March 2015).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LmerTest: Tests in Linear Mixed Effects Models. R package version 2.0-29. 2015. Available online: http://CRAN.R-project.org/package=lmerTest (accessed on 3 May 2016).
- R Core Team. Stats: The R Stats Package. R package version 3.1.3. Available online: http://www.R-project.org/ (accessed on 3 March 2015).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 2nd ed.; Sage Publications: Thousand Oaks, CA, USA, 2011; ISBN 9781412975148. [Google Scholar]
- Bigras, F.J.; Ryyppö, A.; Lindström, A.; Stattin, E. Cold acclimation and deacclimation of shoots and roots of conifer seedlings. In Conifer Cold Hardiness; Bigras, F.J., Colombo, S.J., Eds.; Kluwer Academic Publisher: Boston, MA, USA, 2001; pp. 57–88. ISBN 9780792366362. [Google Scholar]
- Vitasse, Y.; Basler, D. Is the use of cuttings a good proxy to explore phenological responses of temperate forests in warming and photoperiod experiments? Tree Physiol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Aitken, S.N.; Hannerz, M. Genecology and Gene Resource Management Strategies for Conifer Cold Hardiness. In Conifer Cold Hardiness; Bigras, F.J., Colombo, S.J., Eds.; Kluwer Academic Publisher: Boston, MA, USA, 2001; pp. 23–53. ISBN 9780792366362. [Google Scholar]
- Basler, D.; Körner, C. Photoperiod sensitivity of bud burst in 14 temperate forest tree species. Agric. For. Meteorol. 2012, 165, 73–81. [Google Scholar] [CrossRef]
- Eysteinsson, T.; Karlman, L.; Fries, A.; Martinsson, O.; Skulason, B. Variation in spring and autumn frost tolerance among provenances of Russian larches (Larix Mill.). Scand. J. For. Res. 2009, 24, 100–110. [Google Scholar] [CrossRef]
- Beuker, E.; Valtonen, E.; Repo, T. Seasonal variation in the frost hardiness of Scots pine and Norway spruce in old provenance experiments in Finland. For. Ecol. Manag. 1998, 107, 87–98. [Google Scholar] [CrossRef]
- Nielsen, U.B. Frysetest af Nordmannsgran Provenienser og Plustræafkom; Forskningscentret for Skov & Landskab: Copenhangen, Denmark, 2001. (In Danish) [Google Scholar]
- Sakai, A.; Weiser, D.J. Freezing resistance of trees in North America with reference to tree regions. Ecology 1973, 54, 118–126. [Google Scholar] [CrossRef]
- Campbell, R.K.; Sorensen, F.C. Cold-acclimation in seedling Douglas-fir related to phenology and provenance. Ecology 1973, 54, 1148–1151. [Google Scholar] [CrossRef]
- Lindgren, K.; Nilsson, J.-E. Cold acclimation of Pinus sylvestris and Pinus contorta provenances as measured by freezing tolerance of detached needles. Scand. J. For. Res. 1992, 7, 309–315. [Google Scholar] [CrossRef]
- Burr, K.E.; Tinus, R.W.; Wallner, S.J.; King, R.M. Comparison of three cold hardiness tests for conifer seedlings. Tree Physiol. 1990, 6, 351–369. [Google Scholar] [CrossRef] [PubMed]
- Glerum, C. Annual trends in frost hardiness and electrical impedance for seven coniferous species. Can. J. Plant Sci. 1973, 53, 881–889. [Google Scholar] [CrossRef]
- Jones, G.E.; Cregg, B.M. Budbreak and winter injury in exotic firs. HortScience 2006, 41, 143–148. [Google Scholar]
- Worrall, J. Temperature-bud-burst relationships in amabilis and subalpine fir provenance tests replicated at different elevations. Silvae Genet. 1983, 32, 203–209. [Google Scholar]
- Barney, D.L.; Beuer, M.; Jensen, J. Survival, frost susceptibility, growth, and disease resistance of corkbark and subalpine fir grown for landscape and Christmas trees. Horttechnology 2013, 23, 194–200. [Google Scholar]
- Howe, G.T.; Aitken, S.N.; Neale, D.B.; Jermstad, K.D.; Wheeler, N.C.; Chen, T.H.H. From genotype to phenotype: Unraveling the complexities of cold adaptation in forest trees. Can. J. Bot. 2003, 81, 1247–1266. [Google Scholar] [CrossRef]
- Savolainen, O.; Pyhajarvi, T.; Knurr, T. Gene flow and local adaptation in trees. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 595–619. [Google Scholar] [CrossRef]
- Aitken, S.N.; Bemmels, J.B. Time to get moving: Assisted gene flow of forest trees. Evol. Appl. 2016, 9, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Hänninen, H.; Tanino, K. Tree seasonality in a warming climate. Trends Plant Sci. 2011, 16, 312–416. [Google Scholar] [CrossRef] [PubMed]
- Green, D.S. Controls of growth phenology vary in seedlings of three, co-occurring ecologically distinct northern conifers. Tree Physiol. 2007, 27, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Green, D.S. Adaptive strategies in seedlings of three co-occurring, ecologically distinct northern coniferous tree species across an elevational gradient. Can. J. For. Res. 2005, 35, 910–917. [Google Scholar] [CrossRef]
- Cannell, M.G.R.; Smith, R.I. Climatic warming, spring budburst and frost damage on trees. J. Appl. Ecol. 1986, 23, 177–191. [Google Scholar] [CrossRef]
- Harrington, C.A.; Gould, P.J. Tradeoffs between chilling and forcing in satisfying dormancy requirements for Pacific Northwest tree species. Front. Plant Sci. 2015, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]

| Provenance No. | State/Province | Regional Division | Latitude/Longitude | Elevation m a.s.l. | Locality/Provenance |
|---|---|---|---|---|---|
| 167 | Yukon | North | 61°50′/133°00′ | 900 | Canol Road |
| 183 | Alaska | North | 59°50′/135°30′ | 800 | Seed zone A16, Skagway |
| 166 | Br. Col. | North | 59°20′/133°45′ | 800 | Atlin |
| 217 | Br. Col. | West | 55°48′/129°10′ | 320 | White River |
| 168 | Br. Col. | East | 50°50′/119°45′ | 1400 | Adam’s Plateau |
| 218 | Br. Col. | Central | 50°43′/115°27′ | 1525 | Albert River |
| 216 | Br. Col | Central | 49°33′/118°31′ | 1970 | Blue Joint |
| 219 | Br. Col. | East | 49°14′/118°30′ | 1300 | (Name unknown) |
| 196 1 | Wash. | West | 48°37′/120°36′ | 2000 2 | Okanogan N.F., Mile Marker 172, Rt. 20 |
| 194 1 | Wash. | West | 48°29′/119°58′ | 1300 | Okanogan N.F., For. Rd. 42, Looploop Campg Rd. |
| 195 1 | Wash. | West | 48°26′/119°50′ | 1500 | Okanogan N.F., For. Rd. 42, Rt..20 |
| 191 1 | Montana | East | 48°10′/113°30′ | 1200 | Flathead N.F., West of Marias Pass |
| 170 | Montana | East | 48°00′/115°30′ | 1200 2 | Kootenai N.F. |
| 171 | Montana | East | 48°00′/114°15′ | 1100 2 | Flathead N.F. |
| 192 1 | Wash. | West | 47°55′/117°07′ | 1400 | Mount Spokane |
| 193 1 | Wash. | East | 47°55′/117°07′ | 1500 | Mount Spokane |
| 81 | Montana | East | 47°30′/113°30′ | 1800 2 | Flathead N.F., Hungry Horse |
| 199 1 | Wash. | West | 46°47′/121°40′ | 1200 2 | Mt. Rainier N.F., Before Stevens Creek Cross. |
| 182 | Wash. | West | 46°10′/122°15′ | 1300 | Seed zone 440, South-west of Mount St. Helens |
| 203 1 | Wyoming | East | 41°21′/106°32′ | 2400 | Medicine Bow N.F., East of F.S. Work Camp |
| 201 1 | Utah | East | 40°15′/112°00′ | 1500 2 | Wahsatch N.F., Rt. Cottonwood Canyon |
| 204 1 | New Mexico | South | 35°11′/106°25′ | 2900 | Cibola N.F., Sandria Crest |
| 205 1 | New Mexico | South | 35°15′/107°40′ | 2900 | Cibola N.F., Mount Taylor |
| 67 | Arizona | South | 34°00′/109°38′ | 3000 2 | Apache N.F., Hannagan Meadows |
| 140 | Arizona | South | 34°00′/109°38′ | 3000 2 | Apache N.F., Hannagan Meadows + Big Lake |
| 164 | Arizona | South | 34°00′/109°38′ | 3000 2 | Apache N.F., Hannagan Meadows + Big Lake |
| Freezing Date | Temperature | Needles % | Cambium % | Buds % |
|---|---|---|---|---|
| Spring 2001 7 May Iceland | −4 | 9.5 | 2.4 | 14.6 |
| −8 | 14.0 | 3.0 | 21.5 | |
| −12 | 22.2 | 3.5 | 28.9 | |
| −16 | 73.2 | 36.7 | 69.7 | |
| Autumn 2012 20 October Denmark | 4 | 1.0 | 1.0 | 1.2 |
| −6 | 0.5 | 0.5 | 0.5 | |
| −12 | 2.9 | 3.9 | 9.5 | |
| −18 | 21.5 | 29.8 | 50.2 | |
| −24 | 52.7 | 58.3 | 99.8 |
| Needles −16 °C | Cambium −16 °C | Buds −16 °C | Flushing | |
|---|---|---|---|---|
| Cambium, −16 °C | 0.52 | |||
| Buds, −16 °C | 0.49 | 0.40 | ||
| Flushing | 0.35 | 0.27 | 0.67 | |
| Survival in Hallormsstadur | 0.11 | 0.04 | 0.63 | 0.50 |
| Survival in Hønning | −0.06 | 0.45 | 0.23 | 0.23 |
| Latitude | 0.01 | 0.32 | −0.40 | −0.27 |
| Longitude | 0.02 | 0.47 | −0.20 | −0.13 |
| Elevation | 0.01 | −0.26 | 0.54 | 0.56 |
| Needles −24 °C | Cambium −24 °C | Buds −18 °C | Bud Set | Survival Hall. | Survival Hønning | |
|---|---|---|---|---|---|---|
| Cambium, −24 °C | 0.95 | |||||
| Buds, −18 °C | 0.73 | 0.78 | ||||
| Bud set | −0.63 | −0.62 | −0.50 | |||
| Survival in Hall. | 0.43 | 0.37 | 0.38 | −0.62 | ||
| Survival in Hønning | 0.42 | 0.42 | 0.38 | −0.19 | 0.24 | |
| Latitude | −0.74 | −0.72 | −0.59 | 0.93 | −0.68 | −0.22 |
| Longitude | −0.61 | −0.66 | −0.52 | 0.84 | −0.40 | −0.05 |
| Elevation | 0.63 | 0.64 | 0.57 | −0.82 | 0.75 | 0.17 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skulason, B.; Hansen, O.K.; Nielsen, U.B. Provenance Variation in Phenology and Frost Tolerance in Subalpine Fir (Abies lasiocarpa) Planted in Denmark and Iceland. Forests 2018, 9, 17. https://doi.org/10.3390/f9010017
Skulason B, Hansen OK, Nielsen UB. Provenance Variation in Phenology and Frost Tolerance in Subalpine Fir (Abies lasiocarpa) Planted in Denmark and Iceland. Forests. 2018; 9(1):17. https://doi.org/10.3390/f9010017
Chicago/Turabian StyleSkulason, Brynjar, Ole Kim Hansen, and Ulrik Braüner Nielsen. 2018. "Provenance Variation in Phenology and Frost Tolerance in Subalpine Fir (Abies lasiocarpa) Planted in Denmark and Iceland" Forests 9, no. 1: 17. https://doi.org/10.3390/f9010017





