Climatic Factors Shape the Spatial Distribution of Concentrations of Triterpenoids in Barks of White Birch (Betula Platyphylla Suk.) Trees in Northeast China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Sites and Sample Collection
2.3. Climatic Data Collection
2.4. Extraction and Determination of Triterpenoids
2.5. Statistical Analysis
3. Results
3.1. General Description of Data About Geographical and Climatic Factors
3.2. Spatial Distribution of Climatic Factors
3.3. Spatial Distribution of Concentrations of Three Triterpenoids
3.4. The Relationship Between Climatic Factors and Concentrations of BTB Triterpenoids
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, G.L.; Yan, W.D.; Cao, D. Simultaneous determination of betulin and betulinic acid in white birch bark using RP-HPLC. J. Pharmaceut. Biomed. 2007, 43, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Popov, S.A.; Sheremet, O.P.; Kornaukhova, L.M.; Grazhdannikov, A.E.; Shults, E.E. An approach to effective green extraction of triterpenoids from outer birch bark using ethyl acetate with extractant recycle. Ind. Crop. Prod. 2017, 102, 122–132. [Google Scholar] [CrossRef]
- Drąg-Zalesińska, M.; Drąg, M.; Poręba, M.; Borska, S.; Kulbacka, J.; Saczko, J. Anticancer properties of ester derivatives of botulin in human metastatic melanoma cells (Me-45). Cancer Cell Int. 2017, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Fuchino, H.; Konishi, S.; Satoh, T.; Yagi, A.; Saitsu, K.; Tatsumi, T.; Tanaka, N. Chemical evaluation of Betula species in Japan. II. Constituents of Betula platyphylla var japonica. Chem. Pharm. Bull. 1996, 44, 1033–1038. [Google Scholar] [CrossRef]
- Patočka, J. Biologically active pentacyclic triterpenes and their current medicine significance. J. Appl. Biomed. 2003, 1, 7–12. [Google Scholar]
- Wang, S.; Zhao, H.; Jiang, J.; Liu, G.F.; Yang, C.P. Analysis of three types of triterpenoids in tetraploid white birches (Betula platyphylla Suk.) and selection of plus trees. J. For. Res. 2015, 26, 623–633. [Google Scholar] [CrossRef]
- Yin, J.; Ren, C.L.; Zhan, Y.G.; Li, C.X.; Xiao, J.L.; Qiu, W.; Li, X.Y.; Peng, H.M. Distribution and expression characteristics of triterpenoids and OSC genes in white birch (Betula platyphylla Suk.). Mol. Biol. Rep. 2012, 39, 2321–2328. [Google Scholar] [CrossRef] [PubMed]
- Krasutsky, P.A. Birch bark research and development. Nat. Prod. Rep. 2006, 23, 919–942. [Google Scholar] [CrossRef] [PubMed]
- Szuster-Ciesielska, A.; Kandefer-Szerszeń, M. Protective effects of betulin and betulinic acid against ethanol-induced cytotoxicity in HepG2 cells. Pharmacol. Rep. 2005, 57, 588–595. [Google Scholar] [PubMed]
- Recio, M.C.; Giner, R.M.; Mánez, S.; Ríos, J.L. Structural requirements for the anti-inflammatory activity of natural triterpenoids. Planta Med. 1995, 61, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.H.; Raj, K.M.; Luscombe, C.A.; Gadawski, I.; Tam, T.; Chu, J.H.; Gibson, D.; Carlson, R.; Sacks, S.L. The synergistic effects of betulin with acyclovir against herpes simplex viruses. Antivir. Res. 2004, 64, 127–130. [Google Scholar] [CrossRef]
- Pavlova, N.I.; Savinova, O.V.; Nikolaeva, S.N.; Boreko, E.I.; Flekhter, O.B. Antiviral activity of betulin, betulinic and betulonic acids against some enveloped and non-enveloped viruses. Fitoterapia 2003, 74, 489–492. [Google Scholar] [CrossRef]
- Muceniece, R.; Saleniece, K.; Rumaks, J.; Krigere, L.; Dzirkale, Z.; Mezhapuke, R.; Zharkova, O.; Klusa, V. Betulin binds to γ-aminobutyric acid receptors and exerts anticonvulsant action in mice. Pharmacol. Biochem. Behav. 2008, 90, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Holonec, L.; Ranga, F.; Crainic, D.; Truta, A.; Socaciu, C. Evaluation of betulin and betulinnic acid content in birch bark from different forestry areas of Western Carpathians. Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 99–105. [Google Scholar]
- Laghari, A.H.; Memon, S.; Nelofar, A.; Khan, K.M. Alhagi maurorum: A convenient source of lupeol. Ind. Crop. Prod. 2011, 34, 1141–1145. [Google Scholar] [CrossRef]
- Hassegawa, M.; Stevanovic, T.; Achim, A. Relationship between ethanolic extracts of yellow birch and tree characteristics. Ind. Crop. Prod. 2016, 94, 1–8. [Google Scholar] [CrossRef]
- Yin, J.; Liang, T.; Wang, S.Y.; Zhang, M.Y.; Xiao, J.L.; Zhan, Y.G.; Li, C.X. Effect of drought and nitrogen on betulin and oleanolic acid accumulation and OSC gene expression in white birch saplings. Plant Mol. Biol. Rep. 2015, 33, 705–715. [Google Scholar] [CrossRef]
- Schuster, A.C.; Burghaardt, M.; Alfarhan, A.; Bueno, A.; Hedrich, R.; Leide, J.; Thomas, J.; Riederer, M. Effectiveness of cuticular transpiration barriers in a desert plant at controlling water loss at high temperature. AOB Plant 2016, 8, plw027. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.J.; Song, Y.G.; Liu, Y.L.; Qiao, M.; Zhai, X.L.; Xiang, F.N. The effect of elicitors on oleanolic acid accumulation and expression of triterpenoid synthesis genes in Gentiana straminea. Biol. Plantraum 2013, 57, 139–143. [Google Scholar] [CrossRef]
- Nasrollahi, V.; Mirzaie-asl, A.; Piri, K.; Nazeri, S.; Mehrabi, R. The effect of drought stress on the expression of key genes involved in the biosynthesis of triterpenoid saponins in liquorice (Glycyrrhiza glabra). Phytochemistry 2014, 103, 32–37. [Google Scholar] [CrossRef] [PubMed]
- China Meteorological Data Service Center. Available online: http://data.cma.cn/en/?r=site/index (accessed on 5 June 2017).
- Wu, H.B.; Guo, Z.T.; Gao, Q.; Peng, C.H. Distribution of soil inorganic carbon storage and its changes due to agricultural land use activity in China. Agric. Ecosyst. Environ. 2009, 129, 413–421. [Google Scholar] [CrossRef]
- Zhou, R.; Pan, X.Z.; Wei, H.X.; Xie, X.L.; Wang, C.K.; Liu, Y.; Li, Y.L.; Shi, R.J. Soil organic carbon stocks in terrestrial ecosystems of China: Revised estimation on three-dimensional surfaces. Sustainability 2016, 8, 1003. [Google Scholar] [CrossRef]
- Chen, C.H.; Chen, X.J.; Niu, J.L.; He, Z.Y. Analysis of spatial distribution and main controlling factors of soil organic carbon content and total nitrogen content in Northeast China. J. Arid Land Resour. Environ. 2015, 29, 3, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Liu, S.Y.; Xu, F.; Liu, L.J.; Ma, D.Y. A convenient synthesis of lupeal from betulin. Ind. Crop. Prod. 2015, 74, 494–496. [Google Scholar] [CrossRef]
- Kozlov, M.V. Losses of birch foliage due to insect herbivory along geographical gradients in Europe: A climate-driven pattern? Clim. Chang. 2008, 87, 107–117. [Google Scholar] [CrossRef]
- Stark, S.; Julkunen-Tiitto, R.; Holappa, E.; Mikkola, K.; Nikula, A. Concentrations of foliar quercetin in natural populations of white birch (Betula pubescens) increase with latitude. J. Chem. Ecol. 2008, 34, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Kylin, H.; Söderkvist, K.; Undeman, A.; Franich, R. Seasonal variation of the terpene content, an overlooked factor in the determination of environmental pollutants in pine needles. Bull. Environ. Contam. Toxicol. 2002, 68, 155–160. [Google Scholar] [CrossRef] [PubMed]
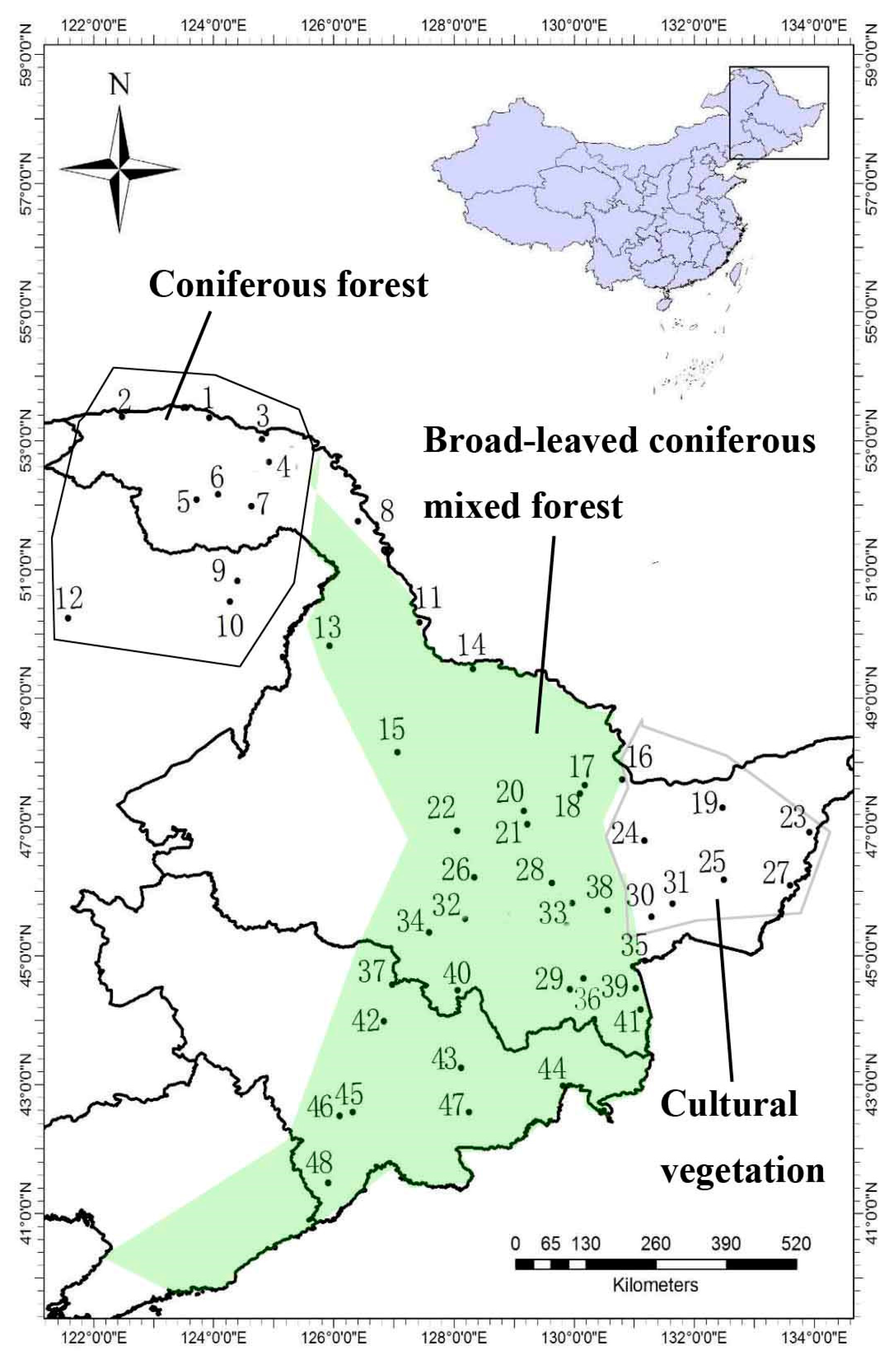
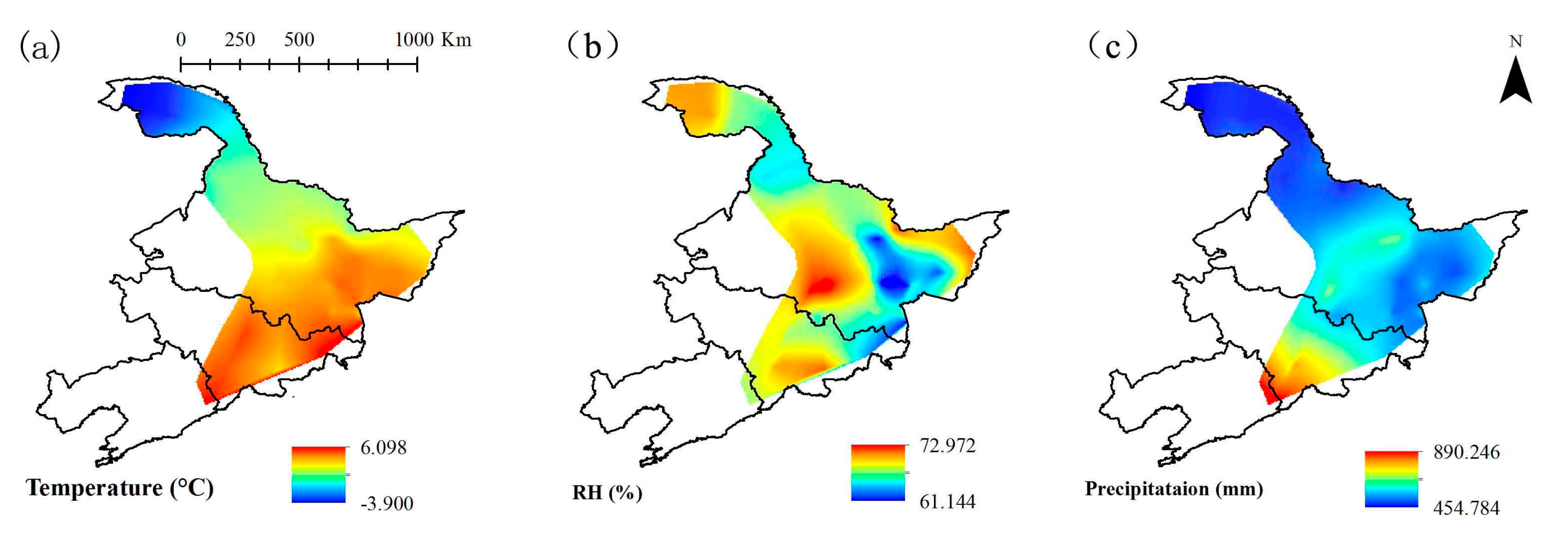
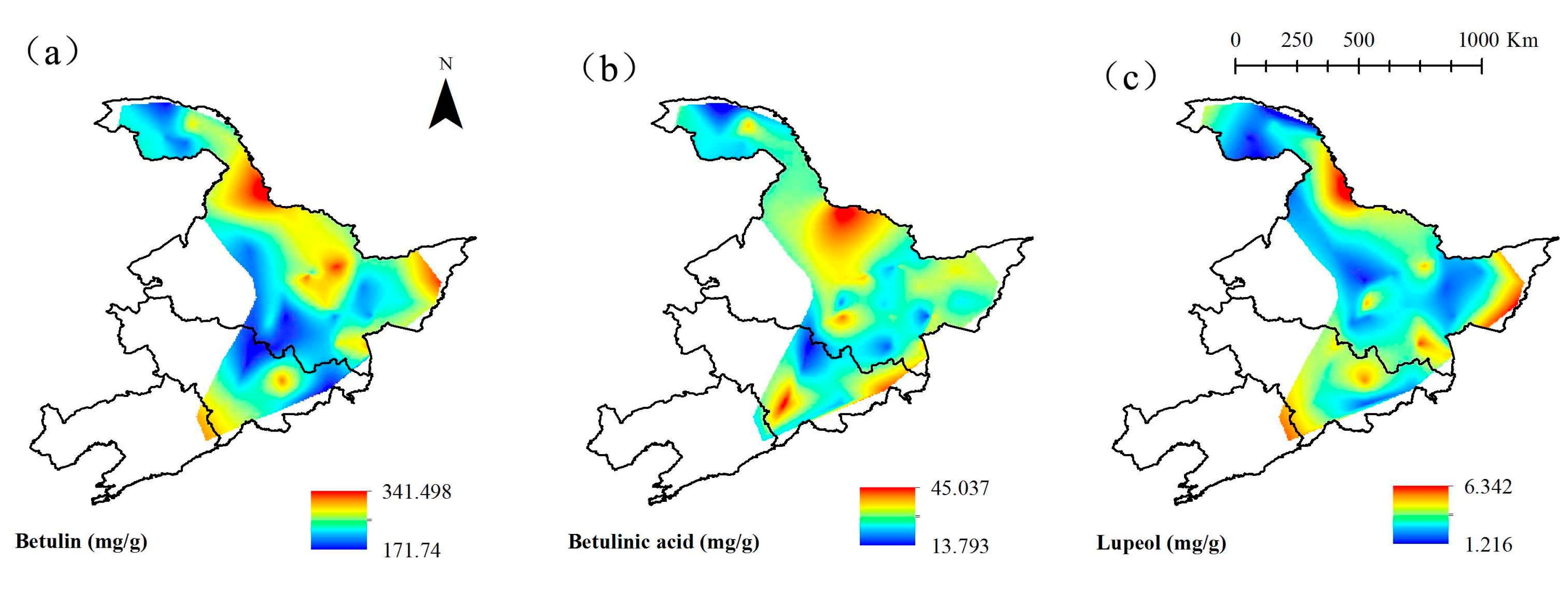

| No. | Plot Name | Coordinates | Altitude (m) | No. | Plot Name | Coordinates | Altitude (m) |
|---|---|---|---|---|---|---|---|
| 1 | Amuer | 53°22′ N, 123°50′ E | 424 | 25 | Baoqing | 46°7′ N, 132°26′ E | 271 |
| 2 | Mohe | 53°14′ N, 122°13′ E | 705 | 26 | Mulan | 46°4′ N, 128°6′ E | 143 |
| 3 | Kaikukang | 53°6′ N, 124°43′ E | 284 | 27 | Hulin | 45°59′ N, 133°35′ E | 62 |
| 4 | Tahe | 52°32′ N, 124°44′ E | 657 | 28 | Yilan | 45°59′ N, 129°38′ E | 118 |
| 5 | Huzhong | 52°5′ N, 123°35′ E | 529 | 29 | Boli | 45°41′ N, 130°19′ E | 542 |
| 6 | Hanjiayuan | 52°4′ N, 123°50′ E | 851 | 30 | Qitaihe | 45°36′ N, 131°17′ E | 611 |
| 7 | Xinlin | 51°53′ N, 124°31′ E | 538 | 31 | Mishan | 45°35′ N, 131°27′ E | 273 |
| 8 | Huma | 51°49′ N, 126°29′ E | 309 | 32 | Yanshou | 45°34′ N, 128° E | 223 |
| 9 | Songling | 50°50′ N, 124°13′ E | 464 | 33 | Linkou | 45°34′ N, 129°56′ E | 445 |
| 10 | Cuifeng | 50°20′ N, 124°7′ E | 415 | 34 | Maoershan | 45°22′ N, 127°33′ E | 687 |
| 11 | Heihe | 50°5′ N, 127°31′ E | 126 | 35 | Jixi | 44°54′ N, 131°1′ E | 657 |
| 12 | Kuduer | 50°1′ N, 121°28′ E | 1082 | 36 | Daimagou | 44°34′ N, 130°2′ E | 712 |
| 13 | Nenjiang | 49°38′ N, 125°49′ E | 418 | 37 | Yushu | 44°32′ N, 126°56′ E | 216 |
| 14 | Xunke | 49°27′ N, 128°8′ E | 170 | 38 | Sidao | 44°28′ N, 129°49′ E | 467 |
| 15 | Bei′an | 47°59′ N, 126°50′ E | 286 | 39 | Suifenhe | 44°28′ N, 131°8′ E | 724 |
| 16 | Luobei | 47°45′ N, 130°48′ E | 281 | 40 | Shanhetun | 44°24′ N, 127°51′ E | 392 |
| 17 | Hegang | 47°25′ N, 130°10′ E | 193 | 41 | Dongning | 44°11′ N, 131°12′ E | 682 |
| 18 | Helin | 47°23′ N, 130°1′ E | 290 | 42 | Jilin | 43°46′ N, 126°43′ E | 428 |
| 19 | Fujin | 47°13′ N, 132°16′ E | 418 | 43 | Dunhua | 43°14′ N, 128°2′ E | 663 |
| 20 | Nancha | 47°8′ N, 129°5′ E | 210 | 44 | Tumen | 42°53′ N, 129°43′ E | 378 |
| 21 | Dailing | 47°3′ N, 129°0′ E | 324 | 45 | Huadian | 42°31′ N, 126°14′ E | 361 |
| 22 | Tieli | 46°52′ N, 128°2′ E | 225 | 46 | Jingyu | 42°26′ N, 126°4′ E | 434 |
| 23 | Raohe | 46°47′ N, 133°51′ E | 192 | 47 | Changbai | 42°24′ N, 128°6′ E | 774 |
| 24 | Jixian | 46°38′ N, 130°59′ E | 296 | 48 | Tonghua | 41°2′ N, 125°16′ E | 325 |
| Parameters | Max | Min | Mean | S.E. |
|---|---|---|---|---|
| Geographical indices | ||||
| Latitude (°) | 53.24 | 41.04 | 47.07 | 3.25 |
| Longitude (°) | 133.9 | 121.5 | 128.07 | 2.98 |
| Elevation (m) | 1082 | 62 | 422.39 | 220.31 |
| Climatic factors | ||||
| Temperature (°C) | 6 | −3.9 | 2.15 | 2.68 |
| Relative humidity (%) | 80 | 55 | 66.89 | 4.69 |
| Precipitation (mm) | 893.6 | 384.7 | 567.5 | 103.98 |
| Climatic factors | ||||
| Betulin (mg g−1) | 343.05 | 169.77 | 239.114 | 6.267 |
| Betulinic acid (mg g−1) | 45.36 | 13.07 | 28.905 | 0.973 |
| Lupeol (mg g−1) | 6.4 | 1.13 | 3.354 | 0.166 |
| Geographical and Climatic Indices | Betulin (mg g−1) | Betulinic Acid (mg g−1) | Lupeol (mg g−1) |
|---|---|---|---|
| Pearson correlation | |||
| Latitude (°) | 0.001 | −0.135 | −0.230 |
| Longitude (°) | 0.220 | 0.127 | 0.220 |
| Elevation (m) | −0.103 | −0.079 | −0.221 |
| Temperature (°C) | 0.332 * | 0.243 | 0.540 *** |
| Humidity (%) | −0.323 * | 0.066 | −0.493 *** |
| Precipitation (mm) | 0.347 * | 0.031 | 0.495 *** |
| Spearman correlation | |||
| Latitude | 0.046 | −0.064 | −0.220 |
| Longitude | 0.214 | 0.140 | 0.181 |
| Elevation (m) | −0.138 | −0.051 | −0.215 |
| Temperature (°C) | 0.314 * | 0.146 | 0.541 *** |
| Humidity (%) | −0.238 | 0.047 | −0.394 ** |
| Precipitation (mm) | 0.192 | −0.031 | 0.342 * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Zhang, D.; Wei, H.; Zhao, Y.; Cao, Y.; Yu, T.; Wang, Y.; Yan, X. Climatic Factors Shape the Spatial Distribution of Concentrations of Triterpenoids in Barks of White Birch (Betula Platyphylla Suk.) Trees in Northeast China. Forests 2017, 8, 334. https://doi.org/10.3390/f8090334
Guo S, Zhang D, Wei H, Zhao Y, Cao Y, Yu T, Wang Y, Yan X. Climatic Factors Shape the Spatial Distribution of Concentrations of Triterpenoids in Barks of White Birch (Betula Platyphylla Suk.) Trees in Northeast China. Forests. 2017; 8(9):334. https://doi.org/10.3390/f8090334
Chicago/Turabian StyleGuo, Shenglei, Dehui Zhang, Huanyong Wei, Yinan Zhao, Yibo Cao, Tao Yu, Yang Wang, and Xiufeng Yan. 2017. "Climatic Factors Shape the Spatial Distribution of Concentrations of Triterpenoids in Barks of White Birch (Betula Platyphylla Suk.) Trees in Northeast China" Forests 8, no. 9: 334. https://doi.org/10.3390/f8090334




